Abstract
Most enterococcal endocarditis is caused by Enterococcus faecalis and Enterococcus faecium. Enterococcus durans is a rare member of non-faecalis, non-faecium enterococcal species and is found in the intestines of animals. E durans endocarditis is a very rare infection—only two cases of endocarditis in humans have been reported in the literature—and usually associated with good outcomes when treated with appropriate antibiotics. We report the first case of fatal E durans endocarditis. This patient had end-stage liver disease with associated compromised immune status that likely contributed to the progression of disease in spite of appropriate antibiotic coverage and clearance of bacteraemia.
Background
Endocarditis with enterococcus is a potentially devastating infection and most often requires prolonged intravenous antibiotics with associated risks. Enterococcus durans endocarditis is very rare. Host factors including liver disease contribute to morbidity and mortality, as was seen in this patient. We could not find any other case in the literature with E durans endocarditis associated with fatal outcome.
Case presentation
A 61-year-old Caucasian man presented with complaints of generalised weakness lasting 1 month and a fever on the day of admission to the hospital. He had a history of alcoholic liver disease, portal hypertension, anaemia, thrombocytopaenia and severe aortic stenosis. The patient had no history of recent dental, gastrointestinal or genito-urinary procedure and no history of intravenous access lines or catheters prior to presentation. At the time of admission, he had a temperature of 100.6°F (38.1 °C). His physical examination was significant for a grade 4 systolic murmur in the aortic area with radiation to the carotids. The abdomen was distended, with dullness to percussion in the flanks suggestive of ascites; the liver was not palpable and the spleen tip was just palpable below the left costal margin. He also had cutaneous telangiectasias consistent with cirrhosis and bilateral lower extremity pitting oedema. He was awake, alert and had no evidence of hepatic encephalopathy. He had no Janeway lesions, Osler nodes or Roth spots.
Investigations
Laboratory workup revealed a normal leucocyte count (5.1 × 10−9/l), anaemia (haemoglobin 7.9 g/dl) and thrombocytopaenia (platelets 43 000/μl). Alanine transaminase was 11 u/l, aspartate transaminase 41 u/l, alkaline phosphatase 72 u/l, albumin 1.7 g/dl, international normalised ratio (INR) 1.5. Creatinine was 1.08 mg/dl. Beta natriuretic peptide (BNP) was 1122 pg/ml (normal: 0–100 pg/ml). C-reactive protein was elevated (69.67 mg/l; normal: 0–4.9 mg/l). Urine analysis was positive for few squamous epithelial cells, red blood cells, white blood cells and few bacteria. It was negative for casts, and urine culture was negative. Chest radiography revealed pulmonary oedema with bilateral pleural effusions and no evidence of pneumonia. An ultrasound of the abdomen revealed an irregular and nodular liver consistent with cirrhosis, large collaterals seen adjacent to the spleen, splenomegally and ascites—consistent with portal hypertension. Two sets of blood cultures (BacT/Alert 3D, bioMérieux Vitek, Inc., Durham, North Carolina, USA) drawn on admission were positive for gram-positive cocci in clusters in all four bottles in less than 24 h. Preliminary identification of the isolates over the next few days was based on gram stain, milky white colonies, negative catalase and pyrrolidonyl arylamidase (PYR) reaction (LifeSign, LLC, Skillman, New Jersey, USA). The predominant colony was PYR negative and was identified as E. durans (Vitek1 (bioMérieux Vitek, Inc., Durham, North Carolina, USA) and API 20 Strep (bioMérieux SA, Marcy l'Etoille France)). Minimum inhibitory concentrations by the Kerby Bauer diffusion method revealed: penicillin 22 mm, ampicillin 26 mm, vancomycin 22 mm, with synergy to gentamicin and streptomycin interpreted as ‘sensitive’ according to the Clinical and Laboratory Standards Institute guidelines. A transthoracic echocardiogram showed mobile vegetation on the aortic valve without any adjacent abscess (figure 1). A transoesophageal echocardiogram was deferred because of the risk of bleeding secondary to high-grade oesophageal varices. Colonoscopy revealed an ulcer at the splenic flexure. It was assumed to be ischaemic and biopsy was not done.
Figure 1.
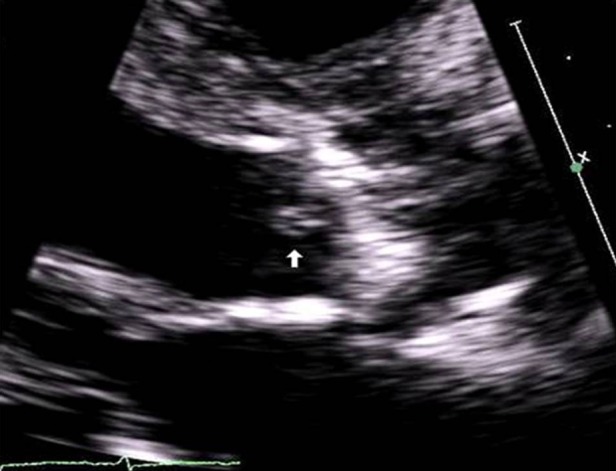
Transthoracic echocardiogram (parasternal long-axis view) showing aortic valve with leaflet vegetation (white arrow).
Differential diagnosis
Spontaneous bacterial peritonitis, endocarditis, urinary tract infection.
Treatment
On admission, the patient was initiated on empiric antibiotic therapy with intravenous ceftriaxone 2 g every 24 h. This was secondary to clinical suspicion of spontaneous bacterial peritonitis that is most often associated with enteric gram-negative organisms. However, when blood cultures revealed gram-positive cocci in clusters, antibiotics were switched to intravenous vancomycin (20 mg/kg every 12 h). On account of high-grade bacteraemia, a transthoracic echocardiogram was requested to evaluate for endocarditis. When the organism was identified as enterococcus—sensitive to ampicillin with synergy for gentamicin—therapy was changed to a combination of ampicillin (2 g every 4 h) and gentamicin (1 mg/kg every 8 h).
Outcome and follow-up
The patient had no more fevers in hospital. Five days after admission, the blood cultures turned negative. Two weeks after the initial presentation, the patient was discharged on combination antibiotics delivered through a peripherally inserted central catheter with a planned duration of 6 weeks from first negative blood culture. Unfortunately, inflammatory markers like C-reactive protein were not rechecked before the patient was discharged from hospital. Creatinine and gentamicin levels were monitored twice a week, and gentamicin dose adjusted appropriately to keep troughs <1 μg/ml.
The patient required readmission to the hospital 2 weeks later for treatment of decompensated congestive heart failure (BNP 2376 pg/ml) and worsening renal failure (creatinine 1.67 mg/dl). INR had increased to 1.5 and platelet count had fallen to 25 000/μl. Repeat blood cultures were done from both peripheral sites as well as from the central catheter to determine whether there was evidence of line infection. The results of all blood cultures were negative. The gold standard for testing for line infection—removal and culture of the line—was not possible because it was very risky to remove the line and then to place a new line in the presence of low platelets and high INR. A repeat echocardiogram demonstrated multiple mobile densities on the aortic valve suggestive of vegetations and multiple abscesses in the aortic sinuses (figure 2). The patient was evaluated by two cardiothoracic surgeons and was considered to have high mortality risk if he underwent cardiac surgery. Liver cirrhosis with clinical and laboratory evidence of liver failure, thrombocytopaenia and nutritional status were all considered in the decision-making process. Gentamicin was discontinued on account of increased creatinine and substituted with ceftriaxone. Ampicillin was continued. He completed the recommended 6-week course of antibiotics. Surveillance blood cultures on days 7, 12 (before first discharge from hospital), 17, 45 (3 days after completing antibiotic course) and 54 (12 days after completing antibiotic course) remained negative.
Figure 2.
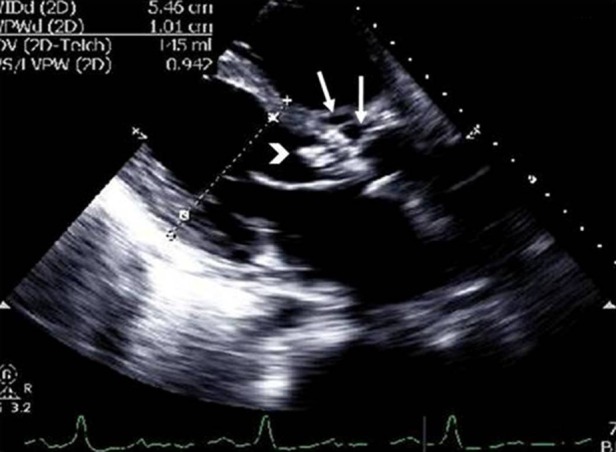
Transthoracic echocardiogram (parasternal long-axis view) showing aortic valve with increased size of vegetation (white arrowhead) and multiple abscesses in the aortic sinus (white arrows).
Unfortunately, he developed progressive heart failure (BNP 4892 pg/ml), renal failure (creatinine 3.56 mg/dl), thrombocytopaenia (platelets 20 000/μl) and epistaxis. Once again, he was deemed to be at very high risk for aortic valve surgery. After detailed discussion of his disease and prognosis, the patient made an informed decision declining further aggressive treatment in favour of primary palliation of symptoms. He died in a few days.
Discussion
The incidence of infective endocarditis is approximately 1.7–6.2 cases per 100 000 patient years.1 Enterococci are the third most common organism (approximately 8% of cases) causing endocarditis, after streptococci and staphylococci,2 and are emerging as an increasingly important cause of infection, especially in the elderly. They are gram-positive, catalase-negative, facultative anaerobes that are seen under direct microscopy as diplococci in short chains. They can be differentiated from other species of catalase-negative gram-positive cocci by their ability to produce PYR reaction. Although staphylococci and streptococci are established as the most common causes of bacterial endocarditis, enterococcal species were detected more frequently in a large surveillance study in Italy.3 This was attributed to a high percentage of elderly patients in that study population. Anderson et al4 also found enterococcal endocarditis more common in the elderly. In their study, intracardiac abscess was more common in prosthetic valves when compared to native valve involvement. In another collaborative study involving 107 patients with enterococcal endocarditis, it was found that enterococcal endocarditis occurred in older patients, predominantly affected the aortic valve and led to more heart failure compared to other streptococcus species.2 5 Peripheral signs of endocarditis such as Osler nodes, Roth spots and petechial lesions are found less frequently in enterococcal endocarditis than in other species.
Among all enterococcal infections, E faecalis is isolated in 63–81%, . faecium in 13–23% and other species (Enterococcus avium, Enterococcus durans, Enterococcus casseliflavus, Enterococcus gallinarum, Enterococcus hirae, and Enterococcus raffinosus) in the remaining.6 Non-faecalis, non-faecium enterococci are a group of diverse enterococcal species that have rarely been associated with human infections. Greater attention is now focused on these rarer species because of their potential to express low-level resistance to antibiotics. Tan et al7 analysed 182 patients with non-faecalis, non-faecium enterococcal bacteraemia, of which 5% were found to have infective endocarditis. In their study, malignancy was found to be the most common underlying disease, followed by biliary tract disease. de Perio et al8 studied 36 cases of non-faecalis, non-faecium enterococcal bacteraemia and found no difference in death rates compared to E faecalis. Again, the gastrointestinal tract was the most likely source of infection. Patients with non-faecalis, non-faecium enterococcal infection were more likely to have underlying disease that compromised the immune system than those with E faecalis infection.
E durans is a very rare species of non-faecalis, non-faecium enterococci. E durans has been isolated from clinical specimens (usually from the intestine of animals and less frequently in humans) but rarely causes human infections as it is known to have low virulence.9 Devriese et al10 reviewed the literature on E durans and noted this organism to be an inhabitant of the gut of young chickens and calves. It was also associated with enteropathies in domesticated animals including dogs, kittens and piglets. E durans was a rare cause of enterococcal bacteraemia (0.1%) in a large series (1887 cases) from a 9- year database from Taiwan.7 They reported 182 cases of non-faecalis, non-faecium endocarditis, of which only two cases were E durans. In their 36 cases of non-faecalis, non-faecium bacteraemia, de Perio8 observed only five cases of E durans.
Our 61-year-old patient with end-stage liver cirrhosis developed enterococcal endocarditis complicated by an abscess of the native aortic valve. Peripheral stigmata of endocarditis were absent. The ulcer seen at the splenic flexure of the colon could have served as the portal of entry for enterococcus from the gastrointestinal tract into the blood stream.
When initial blood cultures turned positive, the organism was reported as gram-positive cocci in clusters and hence treatment was changed from ceftriaxone to vancomycin to cover Staphylococcus aureus—of which methicillin-resistant Staphylococcus aureus has worse prognosis. A change to ampicillin + gentamicin was made as soon as it became evident that the organism was an enterococcus susceptible to these antibiotics.
The recommended treatment regimen for enterococcus endocarditis is a combination of a β-lactam along with an aminoglycoside for synergistic action. Patients treated with a single agent have severe complications and hence combination therapy is always preferred.5 In our patient, owing to renal failure, gentamicin had to be discontinued and we opted for an alternate treatment regimen. In vitro studies have demonstrated good synergism with combinations of cell-wall active antibiotics like teicoplanin–imipenem, ampicillin–ceftriaxone and ampicillin–imipenem.11 Typically, cephalosporins have weak activity against enterococcal species with the exception of ceftriaxone or cefotaxim in combination with ampicillin12 which was used in our patient.
In this case, we feel that the antibiotic choice was very appropriate at every step. Vancomycin is the recommended drug of choice for suspected methicillin-resistant Staphylococcus aureus endocarditis (gram-positive cocci in clusters—as per initial report from the microbiology laboratory) and a combination of ampicillin + gentamicin is recommended once enterococcus is identified. The only broader coverage would have been to add gentamicin to the vancomycin initially and it has been recommended to avoid this, secondary to risk for nephrotoxicity. In this patient with liver disease and congestive heart failure, it would have been inappropriate to add gentamicin to vancomycin to broaden the coverage and risk precipitating acute renal failure. Our antibiotic choice was appropriate as evidenced by sustained clearance of the bacteraemia not only after initiation but even after completion of the antibiotic course.
The initial echocardiogram showed only aortic valve vegetation, but the follow-up imaging documented multiple abscesses indicating progression of the disease in spite of successful clearance of organism from the blood. This calls attention to host factors that are required to clear any infection.
All host defence mechanisms are compromised in patients with cirrhosis, and mortality from infection is increased more than 20 times compared to the general population.13 In vivo studies in patients with cirrhotic liver disease have demonstrated deficient neutrophil recruitment and impaired phagocytic activity, and defects in mucosal barriers and lymphocyte activation.14 This impaired innate and adaptive immunity in our patient could have contributed to the development of endocarditis with valve abscess caused by this low-virulence species of enterococcus.
Learning points.
Enterococci are emerging as very important aetiological agents causing endocarditis, especially in the elderly and in those with underlying immune compromising conditions.
Enterococcus durans is a very rare, low-virulence species of enterococcus.
End-stage liver disease is associated with multiple defects in host immune response.
Even low-virulence organisms like E durans may cause a fatal outcome in a patient with advanced liver disease in spite of optimised antibiotic therapy.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications. Circulation 2005;111:e394–434. [DOI] [PubMed] [Google Scholar]
- 2.McDonald JR, Olaison L, Anderson DJ, et al. Enterococcal endocarditis: 107 cases from the international collaboration on endocarditis merged database. Am J Med 2005;118:759–66. [DOI] [PubMed] [Google Scholar]
- 3.Scudeller L, Badano L, Crapis M, et al. Population-based surveillance of infectious endocarditis in an Italian region. Arch Intern Med 2009;169:1720–3. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DJ, Olaison L, McDonald JR, et al. Enterococcal prosthetic valve infective endocarditis: report of 45 episodes from the International Collaboration on Endocarditis-merged database. Eur J Clin Microbiol Infect Dis 2005;24:665–70. [DOI] [PubMed] [Google Scholar]
- 5.Martínez Odriozola P, Muñoz Sánchez J, Arriola Martínez P, et al. Enterococcal infective endocarditis: description of 12 cases. Ann Med Intern 2007;24:539–42. [DOI] [PubMed] [Google Scholar]
- 6.Ruoff KL, de la Maza L, Murtagh MJ, et al. Species identities of enterococci isolated from clinical specimens. J Clin Microbiol 1990;28:435–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan CK, Lai CC, Wang JY, et al. Bacteremia caused by non-faecalis and non-faecium enterococcus species at a Medical center in Taiwan, 2000 to 2008. J Infect 2010;61:34–43. [DOI] [PubMed] [Google Scholar]
- 8.de Perio MA, Yarnold PR, Warren J, et al. Risk factors and outcomes associated with non-Enterococcus faecalis, non-Enterococcus faecium enterococcal bacteremia. Infect Control Hosp Epidemiol 2006;27:28–33. [DOI] [PubMed] [Google Scholar]
- 9.Stepanović S, Jovanović M, Lavadinović L, et al. Enterococcus durans endocarditis in a patient with transposition of the great vessels. J Med Microbiol 2004;53:259–61. [DOI] [PubMed] [Google Scholar]
- 10.Devriese LA, Vancanneyt M, Descheemaeker P, et al. Differentiation and identification of Enterococcus durans, E. hirae and E. villorum. J Appl Microbiol 2002;92:821–7. [DOI] [PubMed] [Google Scholar]
- 11.Hoen B. Epidemiology and antibiotic treatment of infective endocarditis: an update. Heart 2006;92:1694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavaldà J, Len O, Miró JM, et al. Brief communication: treatment of Enterococcus faecalis endocarditis with ampicillin plus ceftriaxone. Ann Intern Med 2007;146:574–9. [DOI] [PubMed] [Google Scholar]
- 13.Vilstrup H. Cirrhosis and bacterial infections. Rom J Gastroenterol 2003;12:297–302. [PubMed] [Google Scholar]
- 14.Fiuza C, Salcedo M, Clemente G, et al. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J Infect Dis 2000;182:526–33. [DOI] [PubMed] [Google Scholar]


