Abstract
Acute myelitis is an aetiologically heterogeneous inflammatory disorder of the spinal cord. We report on a 71-year-old woman with a recurrent cervical and thoracic myelitis who presented with a new relapse of the disease. Neuromyelitis optica was ruled out such as other possible causes of acute and/or recurrent myelopathy. Serum immunoglobulin levels and specific antibody responses were consistent with the diagnosis of common variable immunodeficiency (CVID). She was treated with high-dose methylprednisolone and intravenous immunoglobulin. As a remission-maintaining drug, we decided to treat her with subcutaneous immunoglobulin (CSL Behring) at 0.2 g/kg/week at doses higher than usually employed in replacement therapy in CVID. At 3-year follow-up, the response to treatment was good. No relapses occurred. Our case suggests the effectiveness and safety of subcutaneous immunoglobulin in maintaining remission and in sparing prednisone in a woman with recurrent myelitis associated with CVID.
Background
Common variable immunodeficiency (CVID) is a primary immunodeficiency characterised by a low level of serum immunoglobulin and an increased susceptibility to infections. Autoimmune manifestations are described in about 20% of the cases.1 2 Autoimmune myelitis is an inflammatory demyelinating disease of the central nervous system. Recurrent myelitis is rare and it had been known to occur in systemic autoimmune diseases, multiple sclerosis, neuromyelitis optica and in idiopathic cases sparing the cerebral hemispheres and the optic nerves.3–5
Case presentation
In November 2008, a 68-year-old woman with a history of recurrent myelitis of unknown origin was admitted to the Neurological Department. Her history revealed upper respiratory tract infections. In 2007, she developed myelitis for the first time and subsequently presented other three myelitis relapses during the following 2 years. At that time, cerebrospinal fluid (CSF) examination was normal, except for mild mononuclear pleocytosis (22 cells/μl), including a normal IgG index and negative oligoclonal bands. Figure 1 shows the spinal cord MRI performed in 2007. On examination in 2008, she presented with spastic gait and sensory ataxia, loss of vibration and position sense in lower limbs, moderate spastic paraparesis and mild weakness of her right hand. Her upper- and lower-limb reflexes were brisk and the plantar responses were extensor. Perception of pain, temperature, pinprick and touch was decreased below T5 level and bladder neurogenic dysfunction was present.
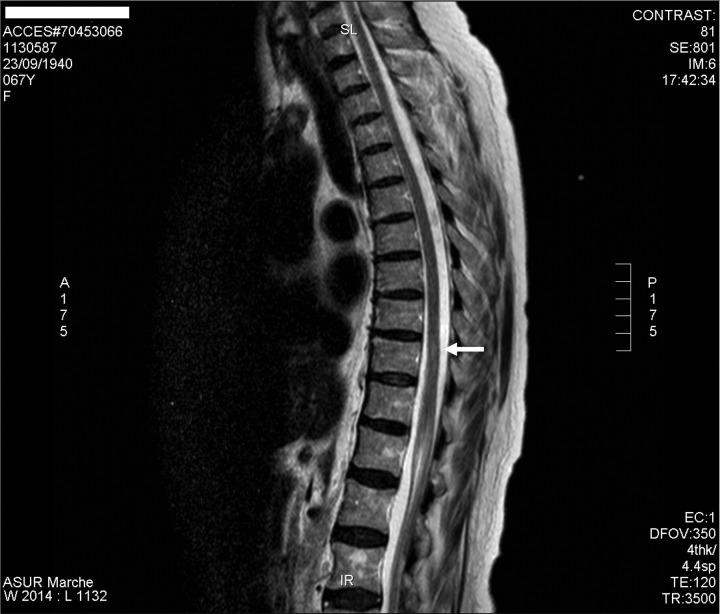
Figure 1.
Sagittal T2-weighted MRI of the thoracic spine showed at T10 a hyperintense lesion with spinal swelling extending for 8 mm (2007).
Investigations
In November 2008, routine laboratory tests, serum copper, vitamin E and B12 and folate, tumour markers (carcinoembryonic antigen, α-fetoprotein, carbohydrate antigen (Ca) 19-9, Ca 125, Ca 15-3, cytokeratin 19 fragment antigen 21-1, neuron-specific enolase) were all normal. Nephelometry revealed a low level of serum immunoglobulin (IgG 384 mg/dl, IgA10 mg/dl, IgM 11 mg/dl). The diagnosis of CVID was confirmed by the absence of isohaemagglutinins and the impaired response to a booster of tetanus vaccination. Moreover, her daughter had CVID with chronic lung disease and rheumatoid arthritis and a nephew with autoimmune thyroiditis (without CVID). She was screened for antinuclear, anti-dsDNA, antineutrophil cytoplasm, antimicrosomal, antismooth muscle, antithyroperoxidase, antigliadin, which were all negative. Anti-Ro/SSA antibodies titres were elevated. No clinical and other laboratory features (including minor salivary gland biopsy) of Sjögren syndrome were present. Antibodies to aquaporin-4 were absent. An extensive search (including CT of thorax and abdomen and CSF angiotensin-converting enzyme) for sarcoidosis and for fungal, viral and bacterial agents was negative. Lumbar puncture demonstrated normal glucose and cell count, the protein content was 23 mg/dl (normal values: 15–50 mg/dl), IgG levels was 2.8 mg/dl (normal values: 2.00–4.00 mg/dl) and IgG index was 0.39 (normal values: <0.65). Oligoclonal bands were absent. The patient's CSF was negative for routine Gram stain, bacterial, fungal and viral culture, India ink microscopy and cryptococcal antigen assay. Enterovirus nucleic acid detection by PCR was not done. Spinal cord MRI showed multiple lesions in T2-weighted images at C3–C4 and T5–T10 segments (figure 2), mainly located in the posterior columns in the cervical spinal cord, where the lesions were also enhanced by gadolinium. Brain MRI was normal. Visual-evoked potential were normal, whereas somatosensory-evoked potentials of the median and tibial nerve were abnormal.
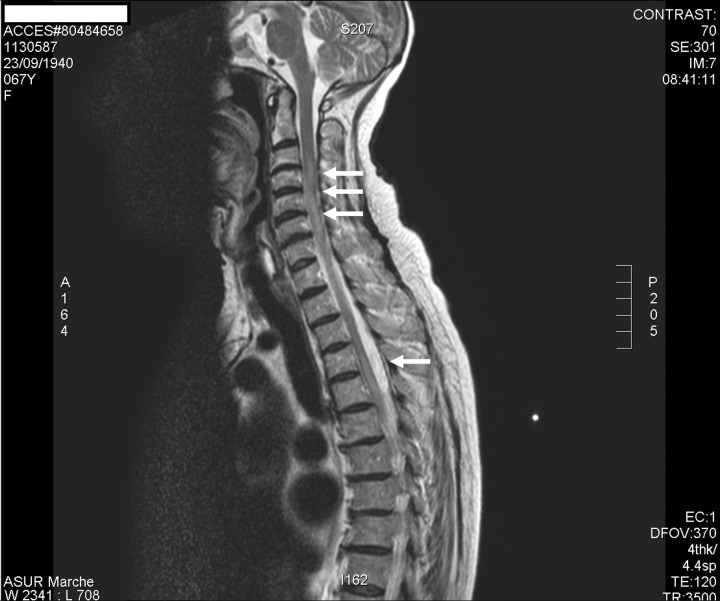
Figure 2.
Sagittal T2-weighted MRI of the cervical and thoracic cord showed multiple hyperintense lesions (2008).
Treatment
All previous relapses had been treated with glucocorticoids and a high dose of intravenous immunoglobulin (IVIg) infusions. We thus treated her with intravenous methylprednisolone (1 g for 3 consecutive days) with amelioration of her paraparesis and IVIg 2 g/kg on two consecutive days monthly for 3 months. Once the remission was obtained, we decided to treat her with subcutaneous immunoglobulin (SCIg). SCIg (Vivaglobin, CSL Behring GmbH Marburg, Germany, 160 mg/ml; then switched to Hizentra, CSL Behring GmbH Marburg, Germany at the same dose) were administered once a week at 0.2 g/kg with a programmable pump (Crono Super PID, Canè, Italy). The first three infusions were carried out in the hospital and thereafter continued at home when the patient, after being trained, felt to be secure with the treatment. The patient was even informed about the possible problems linked to the infusion and their management and she was provided with an emergency telephone number.
Outcome and follow-up
After a 3-year treatment period, her neurological picture was stable, further exacerbation did not occurred and no new lesions were detected at MRI. The mean serum IgG trough level during SCIg treatment was higher than it was during previous IVIg treatment(748 vs 640 mg/dl). Anti-Ro antibodies serum levels were negative in yearly controls.
We also documented an improvement of the quality of life as measured using the validated Italian version of the Medical Outcome Study Short Form 36 (SF-36) questionnaire, when compared with baseline levels (from 37 to 52 as for global indexes). Patient's satisfaction was related to the easier self-management of the treatment with increased autonomy in organising daily-life activities. The patient is now continuing SCIg infusions without prednisone. No major side effects were documented except for mild and self-resolving local reactions (redness on the site of SCIg administration).
Discussion
CVID is the most common symptomatic primary immunodeficiency disease occurring in adult life. Besides bacterial, viral and fungal infections and/or granulomatous diseases, neurological involvement is rarely described in CVID.1 Ziegner et al5 reported 14 patients with CVID and encephalomyelitis possibly secondary to viral infections, autoimmune disease and vitamin deficiency. Some of the patients had features known to be associated with vitamin E deficiency, and thus it is possible that this could be the real cause, at least in some cases.1 Indeed, two CVID patients who developed neurological disease because of vitamin E deficiency due to an associated enteropathy have been described.6 Patients presented with sensory loss, ataxia, tremor and retinitis pigmentosa. Clinicians must consider checking for vitamin E deficiency in all CVID patients with evidence of an enteropathy, as early detection and appropriate treatment may avoid arrest or reverse the neurological degenerative disease.6 In CVID, myelitis has been reported only in a few cases.5 7 Kumar et al7 recently described a patient in whom the neurological involvement was supposed to be linked to granulomatous disease. She was treated with infliximab that resolved systemic symptoms (fever and night swears) and reduced the thoracic lymphadenopathy. However, the neurological deficits were not affected by infliximab therapy.
To our knowledge, this is the only report of a patient with recurrent myelitis and CVID successfully treated with SCIg. The clinical and instrumental pictures did not confirm the hypothesis of multiple sclerosis. We decided to treat her with SCIg at doses higher than usually employed in replacement therapy in CVID. High-dose IVIg is a recognised treatment in Guillain-Barré syndrome and in some immune-mediated neuropathies with a chronic course, such as chronic inflammatory demyelinating polyneuropathy and multifocal motor neuropathy (MMN).8 In severe steroid-resistant postinfectious encephalomyelitis IVIg can be useful.9 More recently, even SCIg has been tested in patients with MMN, with initial beneficial effects.10
Several mechanisms have been implicated in the anti-inflammatory properties of IVIg.11 Recently, a novel mechanism of action involving the decreased cytokine production by a particular pro-inflammatory subset of monocytes has been proposed.12 The study is related to patient with CVID, it is thus possible that higher serum IgG concentrations could reduce the incidence or the severity of inflammatory complications in these patients.12 Since it is not known which of the multiple effects exerted by IVIg are necessary and/or responsible for its efficacy in different immune-mediated diseases,11 we cannot exclude that even SCIg, despite the different kinetics, could act as immunomodulatory agent. Our hypothesis is that, at doses higher than those usually employed in primary immunodeficiency, SCIg could act on T regulatory cells that have been implicated in the development of autoimmune disease.13
The presence of anti-Ro/SSA antibodies in our patient is particularly intriguing. Hummers et al,14 in a case–control study, demonstrated anti-Ro/SSA antibodies in 77% (10/13) of cases with recurrent transverse myelitis, compared with 33% (4/12) of control subjects. It is thus possible that anti-Ro/SSA antibodies have a role in the pathogenesis of recurrent myelitis. As with other autoimmune markers, anti-Ro/SSA antibodies may be directly pathogenic or may represent an epiphenomenon of the key pathogenic event that finally leads to the specific disease. The diagnostic role of anti-Ro/SSA antibodies in rheumatic diseases is controversial, although a significant association with Sjogren's syndrome, systemic lupus erythematosus, myositis and to a lesser extent with systemic sclerosis has been reported. Anti-Ro/SSA antibodies are even present in drug-induced subacute cutaneous lupus erythematosus. However, our patient was not on any medication implicated in this phenomenon. No data are available for anti-Ro/SSA antibodies and CVID. In our patient, interestingly, after Ig therapy, anti-Ro/SSA antibodies became undetectable. Thus, this could be another mechanism by which the Ig could reduce the risk of recurrence of myelitis.
There are several advantages linked to the use of SCIg.15 The administration eludes the high peaks of immunoglobulin levels experienced after intravenous large boluses administration, thus lowering the occurrence of related adverse events. Few systemic adverse reactions are documented after the SCIg administration route that may be appropriate for patients with previous adverse reactions to IVIg.15 A local reaction in the injection site is the most common event that is rarely severe and generally well tolerated by most patients. Finally, there is no need for venous access and even the requirement for premedication with corticosteroids and antihistamines is decreased. Indeed, during the treatment period, our patient neither developed recurrent infections nor other episodes of myelitis and MRI did not detect subclinical new spinal cord lesions.
The treatment was safe and well tolerated and the patient reported us her satisfaction. She also felt confident in the management of the therapy because of the training period and of the good and useful information obtained from health professionals.
Results from new studies indicate that SCIg can be used even in the treatment of autoimmune diseases, linked or not to CVID.10 16 Our case suggests the effectiveness and safety of SCIg in maintaining remission in a woman with recurrent severe inflammatory myelitis associated with CVID.
Learning points.
Subcutaneous immunoglobulin (SCIg) permitted to maintain a long-stable remission in a patient with recurrent myelitis associated with common variable immunodeficiency.
SCIg can play an anti-inflammatory action interfering at different levels in the immune response.
SCIg permitted to reduce the risk of systemic adverse reactions and improved the quality of life in a patient with antibody deficiency.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Wood P, Stanworth S, Burton J, et al. Recognition, clinical diagnosis and management of patients with primary antibody deficiencies: a systematic review. Clin Exp Immunol 2007;149:410–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinti I, Soresina A, Spadaro G, et al. Long-term follow-up and outcome of a large cohort of patients with Common Variable Immunodeficiency. J Clin Immunol 2007;27:308–16. [DOI] [PubMed] [Google Scholar]
- 3.Borchers AT, Gershwin ME. Transverse myelitis. Autoimmunity Rev 2012;11:231–48. [DOI] [PubMed] [Google Scholar]
- 4.Sa’ MJ. Acute transverse myelitis: a practical reappraisal. Autoimmunity Rev 2009;9:128–31. [DOI] [PubMed] [Google Scholar]
- 5.Ziegner UHM, Kobayashi RH, Cunningham-Rundles C, et al. Progressive neurodegeneration in patients with primary immunodeficiency diseases on IVIG treatment. Clin Immunol 2002;102:19–24. [DOI] [PubMed] [Google Scholar]
- 6.Aslam A, Misbah SA, Talbot K, et al. Vitamin E deficiency induced neurological disease in common variable immunodeficiency: two cases and a review of the literature of vitamin E deficiency. Clin Immunol 2004;112:24–9. [DOI] [PubMed] [Google Scholar]
- 7.Kumar N, Hagan JB, Abraham RS, et al. Common variable immunodeficiency-associated myelitis. Report of treatment with infliximab. J Neurol 2008;255:1821–4. [DOI] [PubMed] [Google Scholar]
- 8.Kivity S, Katz U, Daniel N, et al. Evidence for the use of intravenous immunoglobulins—a review of the literature. Clin Rev Allergy Immunol 2010;38:201–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravaglia S, Piccolo G, Ceroni M, et al. Severe steroid-resistant post-infectious encephalomyelitis: general features and effects of IVIg. J Neurol 2007;254:1518–23. [DOI] [PubMed] [Google Scholar]
- 10.Misbah SA, Baumann A, Fazio R, et al. A smooth transition protocol for patients with multifocal motor neuropathy going from intravenous to subcutaneous immunoglobulin therapy: an open-label proof-of-concept study. J Peripher Nerv Syst 2011;16:92–7. [DOI] [PubMed] [Google Scholar]
- 11.Seite JF, Shoenfeld Y, Youinou P, et al. What is the contents of the magic draft IVIg? Autoimmun Rev 2008;7:435–9. [DOI] [PubMed] [Google Scholar]
- 12.Siedlar M, Strach M, Bukowska-Strakova K, et al. Preparations of intravenous immunoglobulin diminish the number and pro-inflammatory response of CD14+CD16++ monocytes in common variable immunodeficiency (CVID) patients. Clin Immunol 2011;139:122–32. [DOI] [PubMed] [Google Scholar]
- 13.Kessel A, Ammuri H, Peri R, et al. Intravenous immunoglobulin therapy affects T regulatory cells by increasing their suppressive function. J Immunol 2007;179:5571–5. [DOI] [PubMed] [Google Scholar]
- 14.Hummers LK, Krishnan C, Casciola-Rosen L, et al. Recurrent transverse myelitis associates with anti-Ro (SSA) autoantibodies. Neurology 2004;62:147–9. [DOI] [PubMed] [Google Scholar]
- 15.Berger M, Rojavin M, Kiessling P, et al. Pharmacokinetics of subcutaneous immunoglobulin and their use in dosing of replacement therapy in patients with primary immunodeficiencies. Clin Immunol 2011;139:133–41. [DOI] [PubMed] [Google Scholar]
- 16.Danieli MG, Pettinari L, Moretti R, et al. Subcutaneous immunoglobulin in polymyositis and dermatomyositis: a novel application. Autoimmunity Rev 2011;10:144–9. [DOI] [PubMed] [Google Scholar]