Abstract
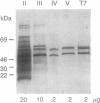
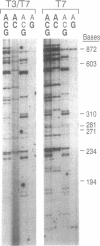
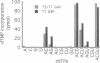
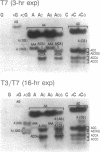
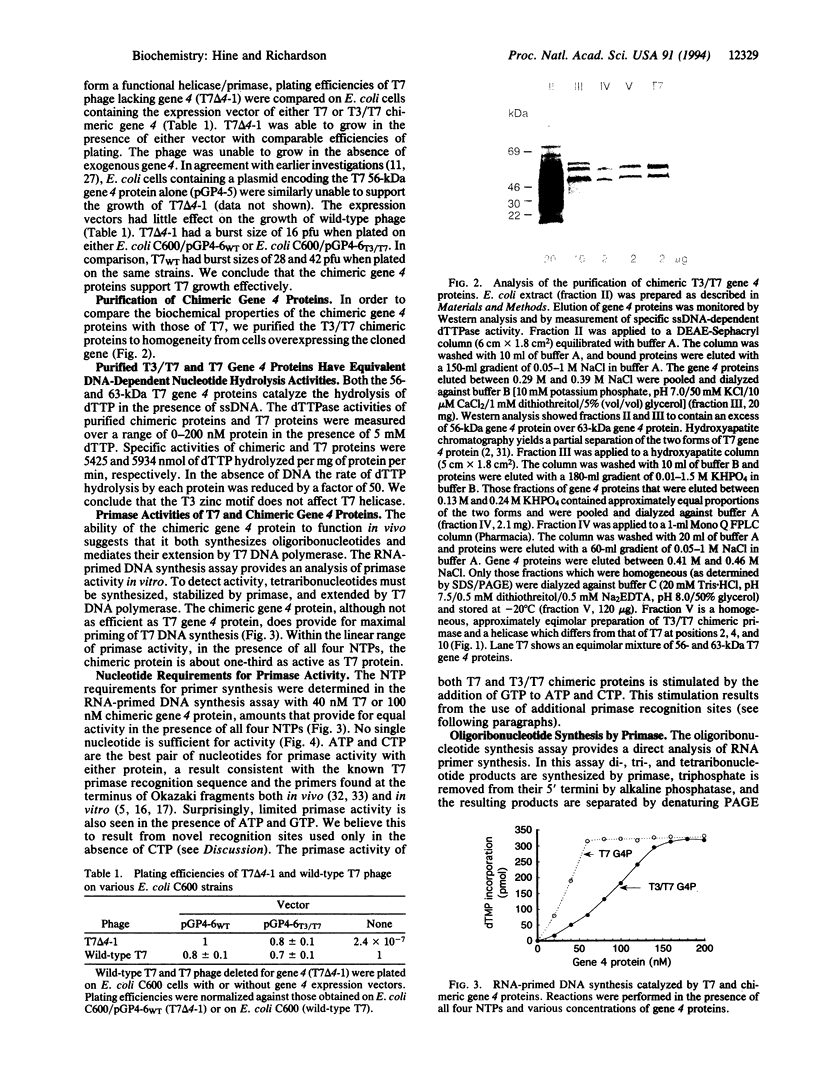
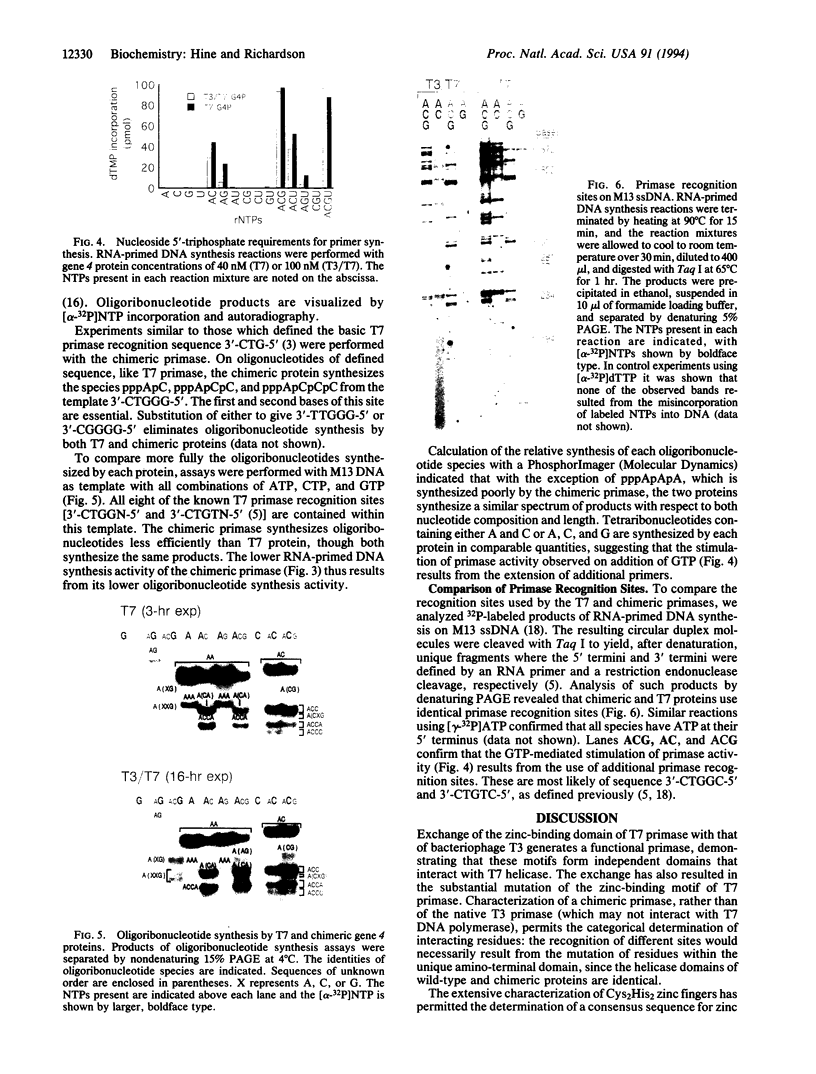
Two colinear bacteriophage T7 gene 4 proteins provide helicase and primase functions in vivo. T7 primase differs from T7 helicase by an additional 63 residues at the amino terminus. This terminal domain contains a zinc-binding motif which mediates an interaction with the basic primase recognition sequence 3'-CTG-5'. We have generated a chimeric primase in which the 81 amino-terminal residues are derived from the primase of phage T3 and the 484 carboxyl-terminal residues are those of phage T7 helicase. The amino-terminal domain of T3 primase is 50% homologous with that of T7 primase. The resulting T3/T7 chimeric protein is a functional primase in vivo. While the primase activity of the purified protein is about one-third that of T7 primase, the recognition sites used and the oligoribonucleotides synthesized from these sites are identical. We conclude that the residues responsible for the interaction with the sequence 3'-CTG-5' are conserved between the chimeric and T7 proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck P. J., Gonzalez S., Ward C. L., Molineux I. J. Sequence of bacteriophage T3 DNA from gene 2.5 through gene 9. J Mol Biol. 1989 Dec 20;210(4):687–701. doi: 10.1016/0022-2836(89)90102-2. [DOI] [PubMed] [Google Scholar]
- Bernstein J. A., Richardson C. C. A 7-kDa region of the bacteriophage T7 gene 4 protein is required for primase but not for helicase activity. Proc Natl Acad Sci U S A. 1988 Jan;85(2):396–400. doi: 10.1073/pnas.85.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein J. A., Richardson C. C. Characterization of the helicase and primase activities of the 63-kDa component of the bacteriophage T7 gene 4 protein. J Biol Chem. 1989 Aug 5;264(22):13066–13073. [PubMed] [Google Scholar]
- Bernstein J. A., Richardson C. C. Purification of the 56-kDa component of the bacteriophage T7 primase/helicase and characterization of its nucleoside 5'-triphosphatase activity. J Biol Chem. 1988 Oct 15;263(29):14891–14899. [PubMed] [Google Scholar]
- Cha T. A., Alberts B. M. Studies of the DNA helicase-RNA primase unit from bacteriophage T4. A trinucleotide sequence on the DNA template starts RNA primer synthesis. J Biol Chem. 1986 May 25;261(15):7001–7010. [PubMed] [Google Scholar]
- Desjarlais J. R., Berg J. M. Toward rules relating zinc finger protein sequences and DNA binding site preferences. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7345–7349. doi: 10.1073/pnas.89.16.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Fairall L., Schwabe J. W., Chapman L., Finch J. T., Rhodes D. The crystal structure of a two zinc-finger peptide reveals an extension to the rules for zinc-finger/DNA recognition. Nature. 1993 Dec 2;366(6454):483–487. doi: 10.1038/366483a0. [DOI] [PubMed] [Google Scholar]
- Fujiyama A., Kohara Y., Okazaki T. Initiation sites for discontinuous DNA synthesis of bacteriophage T7. Proc Natl Acad Sci U S A. 1981 Feb;78(2):903–907. doi: 10.1073/pnas.78.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiasa H., Sakai H., Tanaka K., Honda Y., Komano T., Godson G. N. Mutational analysis of the primer RNA template region in the replication origin (oric) of bacteriophage G4: priming signal recognition by Escherichia coli primase. Gene. 1989 Dec 7;84(1):9–16. doi: 10.1016/0378-1119(89)90133-9. [DOI] [PubMed] [Google Scholar]
- Hinton D. M., Nossal N. G. Bacteriophage T4 DNA primase-helicase. Characterization of oligomer synthesis by T4 61 protein alone and in conjunction with T4 41 protein. J Biol Chem. 1987 Aug 5;262(22):10873–10878. [PubMed] [Google Scholar]
- Ilyina T. V., Gorbalenya A. E., Koonin E. V. Organization and evolution of bacterial and bacteriophage primase-helicase systems. J Mol Evol. 1992 Apr;34(4):351–357. doi: 10.1007/BF00160243. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Richardson C. C. Replication of duplex DNA by bacteriophage T7 DNA polymerase and gene 4 protein is accompanied by hydrolysis of nucleoside 5'-triphosphates. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1525–1529. doi: 10.1073/pnas.74.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger D. H., Schroeder C. Bacteriophage T3 and bacteriophage T7 virus-host cell interactions. Microbiol Rev. 1981 Mar;45(1):9–51. doi: 10.1128/mr.45.1.9-51.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi B. F., Xu W. X., Otwinowski Z., Freedman L. P., Yamamoto K. R., Sigler P. B. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991 Aug 8;352(6335):497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- Marmorstein R., Carey M., Ptashne M., Harrison S. C. DNA recognition by GAL4: structure of a protein-DNA complex. Nature. 1992 Apr 2;356(6368):408–414. doi: 10.1038/356408a0. [DOI] [PubMed] [Google Scholar]
- Matson S. W., Richardson C. C. DNA-dependent nucleoside 5'-triphosphatase activity of the gene 4 protein of bacteriophage T7. J Biol Chem. 1983 Nov 25;258(22):14009–14016. [PubMed] [Google Scholar]
- Matson S. W., Richardson C. C. Nucleotide-dependent binding of the gene 4 protein of bacteriophage T7 to single-stranded DNA. J Biol Chem. 1985 Feb 25;260(4):2281–2287. [PubMed] [Google Scholar]
- Matson S. W., Tabor S., Richardson C. C. The gene 4 protein of bacteriophage T7. Characterization of helicase activity. J Biol Chem. 1983 Nov 25;258(22):14017–14024. [PubMed] [Google Scholar]
- Mendelman L. V., Beauchamp B. B., Richardson C. C. Requirement for a zinc motif for template recognition by the bacteriophage T7 primase. EMBO J. 1994 Aug 15;13(16):3909–3916. doi: 10.1002/j.1460-2075.1994.tb06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelman L. V., Notarnicola S. M., Richardson C. C. Evidence for distinct primase and helicase domains in the 63-kDa gene 4 protein of bacteriophage T7. Characterization of nucleotide binding site mutant. J Biol Chem. 1993 Dec 25;268(36):27208–27213. [PubMed] [Google Scholar]
- Mendelman L. V., Notarnicola S. M., Richardson C. C. Roles of bacteriophage T7 gene 4 proteins in providing primase and helicase functions in vivo. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10638–10642. doi: 10.1073/pnas.89.22.10638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelman L. V., Richardson C. C. Requirements for primer synthesis by bacteriophage T7 63-kDa gene 4 protein. Roles of template sequence and T7 56-kDa gene 4 protein. J Biol Chem. 1991 Dec 5;266(34):23240–23250. [PubMed] [Google Scholar]
- Nakai H., Richardson C. C. Dissection of RNA-primed DNA synthesis catalyzed by gene 4 protein and DNA polymerase of bacteriophage T7. Coupling of RNA primer and DNA synthesis. J Biol Chem. 1986 Nov 15;261(32):15217–15224. [PubMed] [Google Scholar]
- Nakai H., Richardson C. C. Leading and lagging strand synthesis at the replication fork of bacteriophage T7. Distinct properties of T7 gene 4 protein as a helicase and primase. J Biol Chem. 1988 Jul 15;263(20):9818–9830. [PubMed] [Google Scholar]
- Nardelli J., Gibson T. J., Vesque C., Charnay P. Base sequence discrimination by zinc-finger DNA-binding domains. Nature. 1991 Jan 10;349(6305):175–178. doi: 10.1038/349175a0. [DOI] [PubMed] [Google Scholar]
- Notarnicola S. M., Richardson C. C. The nucleotide binding site of the helicase/primase of bacteriophage T7. Interaction of mutant and wild-type proteins. J Biol Chem. 1993 Dec 25;268(36):27198–27207. [PubMed] [Google Scholar]
- Omichinski J. G., Clore G. M., Schaad O., Felsenfeld G., Trainor C., Appella E., Stahl S. J., Gronenborn A. M. NMR structure of a specific DNA complex of Zn-containing DNA binding domain of GATA-1. Science. 1993 Jul 23;261(5120):438–446. doi: 10.1126/science.8332909. [DOI] [PubMed] [Google Scholar]
- Patel S. S., Hingorani M. M., Ng W. M. The K318A mutant of bacteriophage T7 DNA primase-helicase protein is deficient in helicase but not primase activity and inhibits primase-helicase protein wild-type activities by heterooligomer formation. Biochemistry. 1994 Jun 28;33(25):7857–7868. doi: 10.1021/bi00191a013. [DOI] [PubMed] [Google Scholar]
- Qian X., Gozani S. N., Yoon H., Jeon C. J., Agarwal K., Weiss M. A. Novel zinc finger motif in the basal transcriptional machinery: three-dimensional NMR studies of the nucleic acid binding domain of transcriptional elongation factor TFIIS. Biochemistry. 1993 Sep 28;32(38):9944–9959. doi: 10.1021/bi00089a010. [DOI] [PubMed] [Google Scholar]
- Rebar E. J., Pabo C. O. Zinc finger phage: affinity selection of fingers with new DNA-binding specificities. Science. 1994 Feb 4;263(5147):671–673. doi: 10.1126/science.8303274. [DOI] [PubMed] [Google Scholar]
- Romano L. J., Richardson C. C. Characterization of the ribonucleic acid primers and the deoxyribonucleic acid product synthesized by the DNA polymerase and gene 4 protein of bacteriophage T7. J Biol Chem. 1979 Oct 25;254(20):10483–10489. [PubMed] [Google Scholar]
- Rosenberg A. H., Patel S. S., Johnson K. A., Studier F. W. Cloning and expression of gene 4 of bacteriophage T7 and creation and analysis of T7 mutants lacking the 4A primase/helicase or the 4B helicase. J Biol Chem. 1992 Jul 25;267(21):15005–15012. [PubMed] [Google Scholar]
- Scherzinger E., Lanka E., Hillenbrand G. Role of bacteriophage T7 DNA primase in the initiation of DNA strand synthesis. Nucleic Acids Res. 1977 Dec;4(12):4151–4163. doi: 10.1093/nar/4.12.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T., Okazaki T. RNA-linked nascent DNA pieces in phage T7-infected Escherchia coli. II. Primary structure of the RNA portion. Nucleic Acids Res. 1979 Nov 24;7(6):1603–1619. doi: 10.1093/nar/7.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. Template recognition sequence for RNA primer synthesis by gene 4 protein of bacteriophage T7. Proc Natl Acad Sci U S A. 1981 Jan;78(1):205–209. doi: 10.1073/pnas.78.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda K., Okazaki T. Specificity of recognition sequence for Escherichia coli primase. Mol Gen Genet. 1991 May;227(1):1–8. doi: 10.1007/BF00260698. [DOI] [PubMed] [Google Scholar]