Abstract
We report a patient with Cryptococcus (C.) neoformans infection, who developed a case of sarcoid-like reaction (SLR). There have been reports of SLRs associated with malignancies. Although differentiating sarcoidosis from SLR is difficult, the patient was diagnosed as SLR because propionibacterium acnes bacterial (PAB) antibody staining of biopsy specimens was negative and the chest radiological findings improved after antifungal treatment. To our knowledge, this is the first report of SLR occurring during cryptococcal infection, and we believe that cryptococcal infection should be considered as a potential cause of SLR.
Background
We believe that cryptococcal infection should be considered as a potential cause of sarcoid-like reaction (SLR).
Case presentation
A 59-year-old woman with abnormal lung shadows on chest radiography was seen at our hospital in June 2010. She had a history of thyroid cancer and underwent partial thyroidectomy 2 years previously. She was not a smoker, and did not have a history of allergy or risk factors for immunodeficiency. A chest radiograph demonstrated bilateral hilar lymphadenopathy and multiple nodular shadows (figure 1A). Chest CT revealed multiple 2–15 mm nodules that were distributed along the pulmonary vasculature (figure 1B), and marked paratracheal, hilar and subcarinal lymphadenopathy (figure 1C). Bronchoalveolar lavage (BAL) from right B8 was performed. The cell count was 2.0×105/ml with 79% macrophages, 19% lymphocytes, 2% eosinophils and no neutrophils. The BAL fluid (BALF) CD4/CD8 ratio was 4.4. The recovery of BALF was low 36%.
Figure 1.
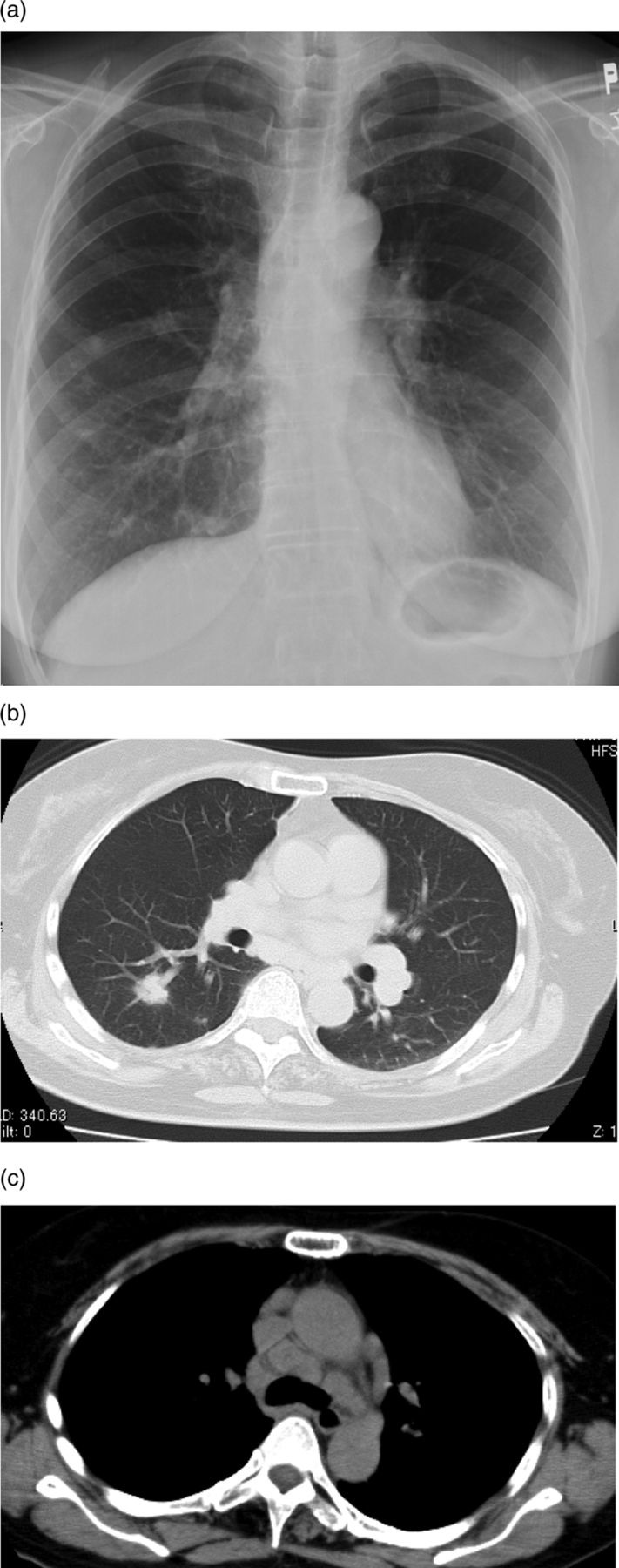
(A) Plain chest radiograph showing bilateral hilar lymphadenopathy and multiple pulmonary nodular shadows. (B) Chest CT showing multiple 2–15 mm nodules distributed along the pulmonary vasculature. (C) Marked paratracheal, parahilar and subcarinal lymphadenopathy is seen.
A right partial lobectomy (S6) and mediastinal lymphadenectomy of #2 were performed by video-assisted thoracoscopy ∼2 months after she was first seen. Histopathology of the S6 specimen showed non-caseating granulomas consisting of many epithelioid and Langhans’ giant cells and fibrosis (figure 2A). Grocott staining of the specimen revealed many small round cryptococci (figure 2B). A serum Cryptococcus neoformans antigen test was positive. Sarcoid-like granulomas without cryptococci were also seen in the #2 lymph node specimen; however, neither the S6 nor the lymph node specimens stained positive of propionibacterium acnes bacterial (PAB) antibody.
Figure 2.
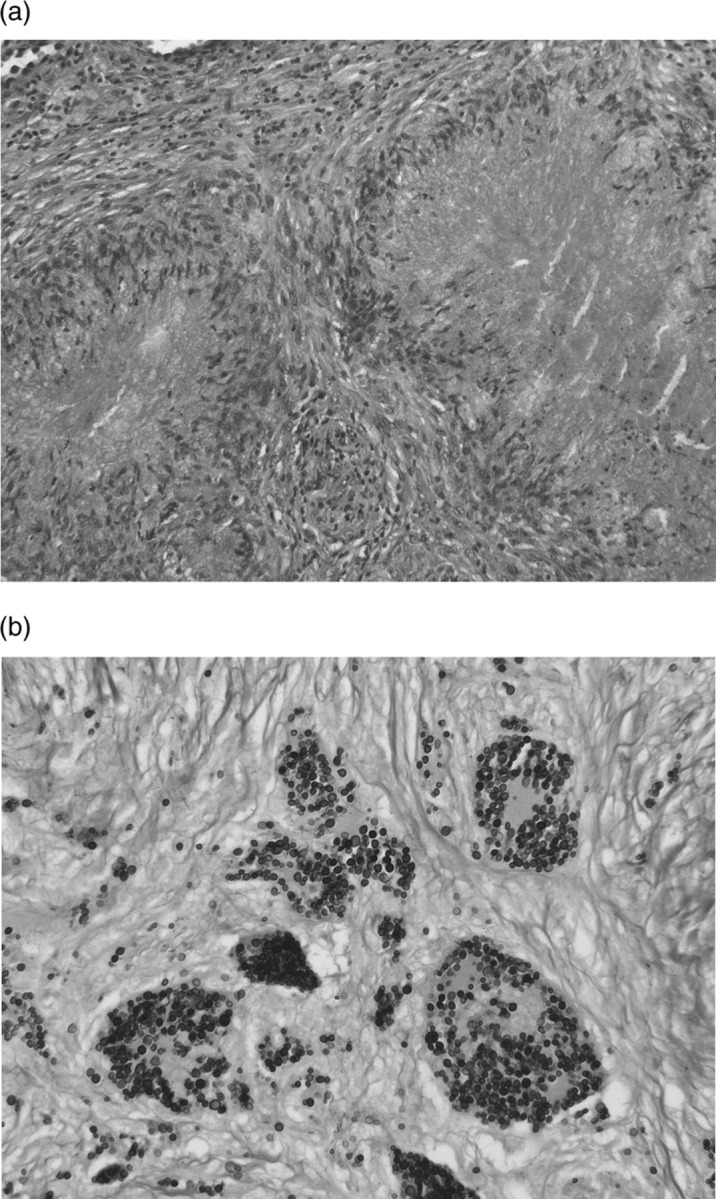
(A) Histopathology of the S6 specimen showing non-caseating granulomas consisting of many epithelioid and Langhans’ giant cells and fibrosis. (B) Grocott staining of the specimen revealing many small round cryptococci.
Treatment with oral voriconazole (VRCZ) (400 mg/day) was begun in September 2010. One month later, the serum C neoformans antigen test was turned to negative and an improvement was seen on the chest radiograph (figure 3A) and CT improved after 6 months treatment (figure 3B,C). Oral VRCZ was administered for 6 months and then changed to itraconazole (ITCZ) (200 mg/day) by the patient's request for a further 6 months.
Figure 3.
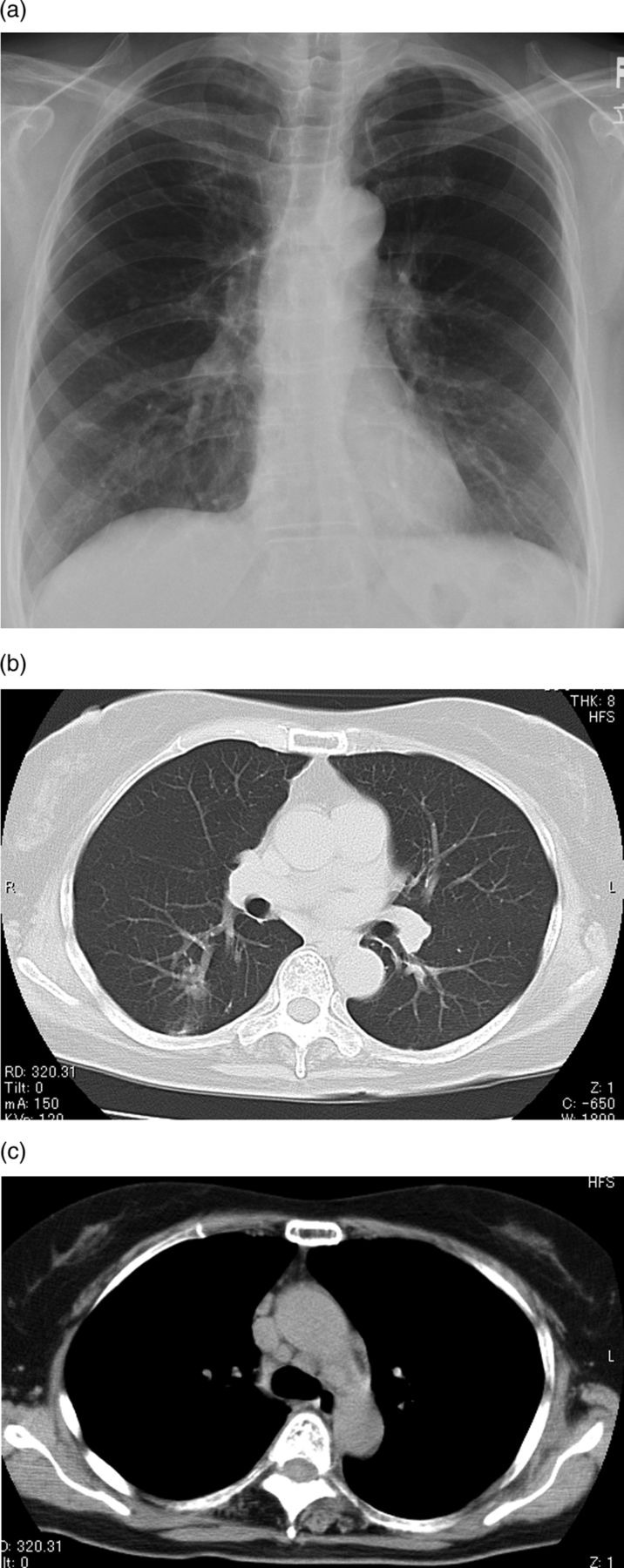
(A) Plain chest radiograph showing improvements of bilateral hilar lymphadenopathy and nodular shadows. (B) Chest CT showing decreases of the size of small nodules. (c) Hilar and mediastinal lymphadenopathy showing decreases in size.
Investigations
Laboratory findings indicated the following: white cell count, 3400 cells/mm3, with 70% neutrophils, 24% lymphocytes, 4% monocytes, 2% eosinophils; and serum C-reactive protein, 0.02 mg/dl. Her liver and renal functions, serum fasting glucose, calcium, lysozyme, ACE and soluble interleukin 2 (IL-2) receptor levels were normal. As the differential counts of white blood cells and the levels of immunoglobulin and serum CD4/CD8 ratio were normal, the patient was regarded as immunocompetent. A sputum culture grew normal flora, and a tuberculin skin test was negative.
Histopathology of the S6 specimen showed non-caseating granulomas consisting of many epithelioid and Langhans’ giant cells and fibrosis. Grocott staining of the specimen revealed many small round cryptococci. A serum C neoformans antigen test was positive. Sarcoid-like granulomas were also seen in the #2 lymph node specimen; however, neither the S6 nor the lymph node specimens stained positive of PAB antibody. Brain CT scan and examination of cerebrospinal fluid smear were negative ruling out central nervous system (CNS) disease.
Differential diagnosis
Although we initially thought that the patient's lung lesions were thyroid cancer metastase, there was no evidence of tumour recurrence in the thyroid. We then suspected malignant lymphoma or sarcoidosis, based on the marked mediastinal and hilar lymphadenopathy. BAL from right B8 was performed. The BALF analysis was not completely reliable, because the recovery of BALF was low. Although the results were not conclusively for diagnosis, we suspected sarcoidosis or SLR from the BALF findings. Cryptococcosis was not suggested as the BALF did not have any neutrophils. The patient's serum ACE and soluble IL-2 receptor levels were within the normal range; we suspected lung cancer metastases too and performed a segmentectomy and mediastinal lymphadenectomy by video-assisted thoracoscopy. The lung and lymph node specimens had only non-caseating granulomas, and no malignant cells were seen. Although many small round cryptococci were seen in the right lung specimens, they were not detected in mediastinal lymph node specimen. Differentiating sarcoidosis from SLR based on the presence of cryptococci is difficult because sarcoidosis can precede cryptococcal infection, whereas cryptococcal infection may lead to SLR.
Cryptococcal infections commonly occur in individuals with impaired T cell-mediated immune responses, such as AIDS patients and patients receiving immunosuppressive agents. Sarcoidosis patients have also been thought to be increased risk for Cryptococcus infection.1–4
Recent work proposes that SLR in sarcoidosis may be caused by Propionibacterium (P.) acnes infection. Abe et al5 have isolated P. acnes from 31 of 40 lymph nodes biopsies from 40 Japanese patients with sarcoidosis. Eishi et al6 immunised mice with a crude bacterial extract of P. acnes and recovered a hybridoma clone that produced an antibody (PAB antibody) that had similar reactivity by ELISA and immunohistochemistry as a monoclonal antibody (SG5) developed from a crude homogenate of lymph node affected by sarcoidosis. Eishi7 reported that genomes of P. acnes were found in 81–100% of sarcoidosis patient's lymph node samples. The PAB antibody was used to evaluate specimens from the patient, and the results were negative. Large (10–40 mm diameter) pulmonary nodules due to sarcoidosis can occur but are unusual.8 In this case, the largest nodule of 15 mm in size may show the possibility of SLR. And the radiological findings improved only by antifugal treatment. Therefore, we believe that the patient's lymphadenopathy resulted from an SLR to cryptococcocal infection.
SLR has been reported in patients with malignancy, including metastatic breast cancer and invasive adenocarcinoma of the colon,9–11 and is thought to be secondary to immune response to tumour cells. Granulomas are typically found adjacent to the tumour or a lymphatic drainage route. SLR has also been reported in fungal or tuberculous infections.1 12 To our knowledge, this is the first case of SLR to cryptococcosis in an immunocompetent woman. We believe that cryptococcal infection should be considered a potential cause of SLR.
Treatment
Oral VRCZ (400 mg/day) was administered for 6 months and then changed to ITCZ (200 mg/day) for a further 6 months by the patient's request. VRCZ is an effective and well-tolerated treatment for refractory or less-common invasive fungal infections. VRCZ was associated with satisfactory global responses in 50% of the overall cohort; specially, successful outcomes were observed in 47% of patients whose infections failed to respond to previous antifungal therapy. In this case, the lesion widely spread in the lung field and lymph nodes, and was treated as refractory cryptococcosis.13
Outcome and follow-up
I treated this patient using VRCZ for 6 months and then changed to ITCZ for a further 6 months without progression.
Discussion
SLR has been reported in patients with malignancy, including metastatic breast cancer and invasive adenocarcinoma of the colon, and is thought to be secondary to immune response to tumour cells. Granulomas are typically found adjacent to the tumour or a lymphatic drainage route. SLR has also been reported in patients with fungal or tuberculous infections. Because AIDS patients in Japanese female over 50 years are extremely rare, we did not examine HIV test in this case. To our knowledge, this is the first case of SLR to cryptococcosis in an immunocompetent woman. We believe that cryptococcal infection should be considered as a potential cause of SLR.
Although we initially thought that the patient's lung lesions were thyroid cancer metastase, there was no evidence of tumour recurrence in the thyroid. We then suspected malignant lymphoma or sarcoidosis, based on the marked mediastinal and hilar lymphadenopathy. However, because the patient's serum ACE and soluble IL-2 receptor levels were within the normal range, we suspected lung cancer metastases too and performed a segmentectomy and mediastinal lymphadenectomy by video-assisted thoracoscopy. The lung and lymph node specimens had only non-caseating granulomas, and no malignant cells were seen. Although many small round cryptococci were seen in the right lung specimens, they were not detected in the mediastinal lymph node specimen. Differentiating sarcoidosis from SLR based on the presence of cryptococci is difficult because sarcoidosis can precede cryptococcal infection, whereas cryptococcal infection may lead to SLR. The therapies are very different depending on the cause, sarcoidosis or SLR. If the lesion results from sarcoidosis, steroid therapy may be necessary, and if the lesion results from SLR, the primary cause of SLR should be treated. Therefore, the diagnosis from the surgical specimen was very important. As cryptoccocosis was the primary cause in this case, antifungal therapy was begun and both the lung and lymph node lesions improved.
Learning points.
Differentiating sarcoidosis from sarcoid-like reaction (SLR) based on the presence of cryptococci is difficult because sarcoidosis can precede cryptococcal infection, whereas cryptococcal infection may lead to SLR.
SLR has also been reported in patients of fungal or tuberculous infections.
We believe that cryptococcal infection should be considered as a potential cause of SLR.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Nottebart HC, McGehee RF, Utz JP. Cryptococcosis complicating sarcoidosis. Am Rev Respir Dis 1973;107:1060–3. [DOI] [PubMed] [Google Scholar]
- 2.Allen KS, Glickstein M, Arger PH, et al. Cryptococcosis associated with sarcoidosis: CT and MR findings. J Comput Assist Tomogr 1988;12:420–2. [DOI] [PubMed] [Google Scholar]
- 3.Giner V, Casademont J, Cardellach F. Cryptoccoccal meningoencepalitis and sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:229–30. [PubMed] [Google Scholar]
- 4.Ross JJ, Katz JD. Cryptococcal meningitis and sarcoidosis. Scand J Infect Dis 2002;34:937–9. [DOI] [PubMed] [Google Scholar]
- 5.Abe C, Iwai K, Mikami R, et al. Frequent isolation of Propionibacterium acnes from sarcoidosis lymph nodes. Zentbl Bakteriol Mikrobiol Hyg 1984; 25:541–7. [DOI] [PubMed] [Google Scholar]
- 6.Eishi Y. Seeking a causative agent of sarcoidosis. Nihon Rinsho 1994;52:78–83. [PubMed] [Google Scholar]
- 7.Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol 2002;40:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michael GR, Steve DG, Marvin IS. Lung nodules in a woman with a history of breast cancer. Chest 2007;132:1697–701. [DOI] [PubMed] [Google Scholar]
- 9.McNeill M, Zanders TB, Morris MJ. A 49-year-old man with concurrent diagnosis of lung cancer, sarcoidosis, and multiple regions of adenopathy on positron emission tomography. Chest 2009;135:546–9. [DOI] [PubMed] [Google Scholar]
- 10.Risbano MG, Groshong SD, Schwalz MI. Lung nodules in a woman with a history of breast cancer. Chest 2007;132:1697–701. [DOI] [PubMed] [Google Scholar]
- 11.Malani AK, Gupta C, Singh J, et al. A 63-year old woman with colon cancer and mediastinal lymphadenopathy. Chest 2007;131:1970–3. [DOI] [PubMed] [Google Scholar]
- 12.Bretza J, Mayfield JD. Mycobacterium intracellulare presenting as a sarcoid-like illness. South Med J 1978;71:872–4. [DOI] [PubMed] [Google Scholar]
- 13.John RP, Kieren A, Thomas JW, et al. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin Infect Dis 2003;36:1122–31. [DOI] [PubMed] [Google Scholar]


