Abstract
Copy number variations at chromosome 16p11.2 contribute to neurodevelopmental disorders, including autism spectrum disorder (ASD). This study seeks to improve our understanding of the biological basis of behavioral phenotypes common in ASD, in particular the prominent and prevalent disruption of spoken language seen in children with the 16p11.2 BP4–BP5 deletion. We examined the auditory and language white matter pathways with diffusion MRI in a cohort of 36 pediatric deletion carriers and 45 age-matched controls. Diffusion MR tractography of the auditory radiations and the arcuate fasciculus was performed to generate tract specific measures of white matter microstructure. In both tracts, deletion carriers exhibited significantly higher diffusivity than that of controls. Cross-sectional diffusion parameters in these tracts changed with age with no group difference in the rate of maturation. Within deletion carriers, the left-hemisphere arcuate fasciculus mean and radial diffusivities were significantly negatively correlated with clinical language ability, but not non-verbal cognitive ability. Diffusion metrics in the right-hemisphere arcuate fasciculus were not predictive of language ability. These results provide insight into the link between the 16p11.2 deletion, abnormal auditory and language pathway structures, and the specific behavioral deficits that may contribute to neurodevelopmental disorders such as ASD.
Abbreviations: AD, axial diffusivity; ASD, autism spectrum disorder; CELF, clinical evaluation of language fundamentals; CNV, copy number variation; DTI, diffusion tensor imaging; FA, fractional anisotropy; GFA, generalized fractional anisotropy; HARDI, high angular resolution diffusion imaging; MD, mean diffusivity; RD, radial diffusivity
Keywords: 16p11.2 deletion, Diffusion MR, Auditory system, Arcuate fasciculus, Language, Autism
Highlights
-
•
We examined auditory and language white matter tracts in children with the 16p11.2 BP4–BP5 deletion.
-
•
Diffusivity was enhanced in auditory radiation and arcuate fasciculus.
-
•
Arcuate fasciculus microstructure was correlated with language ability in deletion carriers.
-
•
There are correlations in the brain structure and behavioral phenotype in the 16p11.2 deletion carriers.
1. Introduction
Most studies of autism spectrum disorder (ASD) and other neuropsychiatric disorders are performed with a population defined by a diagnosis based on behavioral testing. However, ASD is a clinically and etiologically heterogeneous group of disorders, complicating analyses and leading at times to contradictory conclusions (Amaralet al., 2008). However, by pursuing a “genetics first” approach to the study design, we can more directly examine the underlying mechanisms influencing neurobehavioral phenotypes in a more etiologically-homogeneous population. This study examines the structure of auditory and language neural systems in children with a rare genetic copy number variation (CNV) that contributes to neurodevelopmental disorders, including ASD.
Deletion or duplication of the BP4–BP5 segment of chromosome 16p11.2 has been associated with developmental disorders including language impairments, mild to moderate intellectual disability, schizophrenia, altered body mass index, epilepsy and ASD (Weiss et al., 2008; Bochukova et al., 2010; Fernandez et al., 2010; Jacquemont et al., 2011; Rosenfeld et al., 2010; Hanson et al., 2010; Zufferey et al., 2012). The 16p11.2 CNV is approximately 600 kb and contains 29 genes. Human and mouse data demonstrate that the consequences of a 16p11.2 deletion are generally more severe than the reciprocal duplication (Horev et al., 2011; Stefansson et al., 2014). Concordantly, many of the deletions are acquired de novo, while many of the cases of duplication are inherited. ASD is present in approximately 15%–25% of individuals with a 16p11.2 deletion, and approximately 1% of individuals with an ASD diagnosis have a 16p11.2 CNV (Zufferey et al., 2012). A detailed analysis of the behavioral and cognitive phenotypes in 16p11.2 deletion carriers shows that disruption of language production is one of the most consistent features (Hanson et al., 2015). We therefore hypothesize that the 16p11.2 deletion results in anatomic abnormalities of the auditory and language systems, which in turn form the biological basis for behavioral dysfunctions.
We are also motivated to examine the auditory and language systems in these children given prior structural and functional findings in individuals with ASD. Magnetoencephalography (MEG) of cortical activity has shown delayed latency of the auditory evoked field in ASD (Roberts et al., 2010). Diffusion MR is sensitive to white matter architecture and has been used to detect white matter abnormalities in language and auditory pathways associated with ASD (Nagae et al., 2012; Lange et al., 2010). A prior report, using hypothesis-independent tract-based spatial statistics, detected changes in diffusion metrics globally in the brain in children with 16p11.2 deletions (Owen et al., 2014). In contrast, in this study, we have used diffusion MR fiber tractography in a hypothesis-driven approach to delineate and quantitatively assess two key white matter tracts known to be implicated in language processing: the auditory radiation and arcuate fasciculus. Diffusion tractography enables an entire functional white matter tract to be spatially selected for measurement. The arcuate fasciculus is integral to language function and interconnects Broca's and Wernicke's areas to cortices throughout the temporal, parietal and frontal lobes (Catani and Mesulam, 2008). The auditory radiation is a primary sensory tract which carries auditory input from the medial geniculate nucleus of the thalamus to the primary auditory cortex. The auditory radiation intersects other white matter tracts, that renders it difficult to reconstruct using standard DTI fiber tracking methods, necessitating the use of high angular resolution diffusion imaging (HARDI) fiber tracking (Berman et al., 2013).
This study is one component of the multi-site Simons Variation in Individuals Project (Simons VIP) which uses a “genetics first” approach to the study of ASD (The Simons VIP Consortium, 2012). Recent studies from the Simons VIP have observed global changes in brain volume and white matter microstructure associated with the 16p11.2 deletion and duplication (Owen et al., 2014; Qureshi et al., 2014). Here, we focus on the auditory and language systems to test the overall Simons VIP hypothesis that brain structure can be correlated with behavior when studying a genetically defined group. The first analysis in this study tests for microstructural and developmental changes to the auditory radiation and arcuate fasciculus in children with the 16p11.2 deletion compared to typically developing controls. The second analysis in this study correlates structural abnormalities of the arcuate fasciculus with clinically assessed language ability. By studying a group of subjects with a defined genetic etiology, we further our understanding of the link between the 16p11.2 deletion, altered language and auditory white matter, and impaired language ability.
2. Methods
2.1. Participants
Deletion carriers were recruited through the Simons VIP Connect website (The Simons VIP Consortium, 2012). The 16p11.2 deletion participants were identified with clinical chromosome microarrays and included individuals with the same recurrent ~600 kb deletion (chr16:29, 652,990–30,199,351; hg19) and did not have other known genetic diagnoses or pathogenic CNVs. Age-matched neurotypical participants had a chromosome microarray to rule out abnormal CNVs at the 16p11.2 locus or elsewhere in the genome. The exclusion criteria included a psychiatric or neurologic diagnosis in a control participant, inability to speak English fluently, drug use, or significant structural abnormalities on MRI.
This study included a total of 81 children imaged at either the Children's Hospital of Philadelphia (N = 29) or the University of California (N = 52). Informed consent was provided and the study was approved by the institutional review board. A total of 36 pediatric deletion carriers and 45 controls were studied. The mean age of the deletion carriers was 11.6 years (standard deviation: 2.3 years; range: 8–16 years) with 10 left-handed, 26 right-handed, 21 males, and 15 females. The mean age of control children was 12.2 years (standard deviation: 2.9 years; range: 7–17 years) with 8 left-handed, 37 right-handed, 24 males, and 21 females. There was no significant difference in chronological age between groups (p > 0.1). There was no significant difference in the ratio of handedness or gender between groups (p > 0.2 and p > 0.8, respectively; Fisher's exact test).
Participants were administered cognitive measures by experienced and licensed child psychologists. Standardization of measurements across sites included mandatory training to research reliability on all measures through in-person training sessions and webinars for all psychologists, as well as cross-site reliability and maintenance through monthly conference calls and periodic videotape review. Cognitive and language measures utilized for the current analyses included the Clinical Evaluation of Language Fundamentals, Fourth Edition (CELF-4), as a measure of basic language functioning, and the Differential Ability Scales, 2nd Edition, Special Nonverbal Composite (DAS-II), as a measure of nonverbal intellectual functioning (Semel et al., 2003; Elliott, 2007).
2.2. Imaging
Imaging was performed with a 3 T Tim Trio scanner (Siemens Medical Solutions) with a 32 channel RF head coil. The two imaging sites each have the same scanner with identical software, pulse sequences, and head coils. Inter-site equivalency of image data quality was confirmed by scanning 5 subjects at both sites (Owen et al., 2013). MR acquisition included diffusion tensor imaging (DTI), high angular resolution diffusion imaging (HARDI), and high-resolution structural imaging.
The whole-brain DTI acquisition used 30 diffusion gradient directions at b = 1000 s/mm2, one b = 0 s/mm2 volume, TR/TE = 10 s/80 ms, voxel size 2 × 2 × 2 mm, and 128 × 128 matrix. The whole-brain HARDI acquisition included 64 gradient directions at b = 3000 s/mm2, TR/TE = 13.9 s/119 ms, voxel size = 2×2 × 2 mm, and 128 × 128 matrix. DTI acquisition time was 6 min and HARDI acquisition time was 18.2 min. Two b = 0 s/mm2 volumes are included in the HARDI acquisition. Both HARDI and DTI acquisitions utilized the Siemens 511C works in progress (WIP) sequence with a Stejskal–Tanner monopolar, spin-echo echo planar sequence, parallel acceleration factor of 2 with GRAPPA, and axial slices. The DTI data were used for tractography and measurement of the arcuate fasciculus. The HARDI data were used for tractography of the auditory radiation and DTI parameters were measured. The b = 0 s/mm2 volume from the HARDI dataset was registered to the b = 0 s/mm2 volume from the DTI dataset using FMRIB's Linear Image Registration Tool (Jenkinson, and Smith, 2001). Structural imaging was performed with a T1-weighted multi-echo 3D magnetization-prepared rapid acquisition gradient echo (ME-MPRAGE) and TE = 1.64 ms, TR = 2530 ms, TI = 1200 ms, flip angle = 7°, and 1 mm isotropic resolution (van der Kouwe et al., 2008).
2.3. Tractography and measurements
The left and right arcuate fasciculi were defined with deterministic DTI tractography using coronal starting and target regions of interest as previously described (Nagaeet al., 2012; Mori et al., 1999). Prior studies have indicated that a majority of left-handed individuals have left-hemispheric language dominance (Szaflarski et al., 2002; Pujol et al., 1999). Thus, left-hemispheric language dominance was assumed in all subjects. Probabilistic tractography using the solid angle q-ball HARDI reconstruction was used to delineate the auditory radiation from the thalamus to the auditory cortex (Berman et al., 2008). The starting and target regions of interest were generated with the white matter parcellation provided by Freesurfer applied to the structural imaging (Fischl et al., 1999). As previously shown, a HARDI tractography approach is necessary for traversing the crossing fibers within the auditory radiation (Berman et al., 2013). DTI (b = 1000 s/mm2) measures of fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) were made within the 3D region encompassed by the arcuate fasciculus and auditory radiation (Fig. 1). Radial diffusivity is the mean of the second and third eigenvalues of the diffusion tensor. In addition, the HARDI derived generalized fractional anisotropy (GFA) was measured within the auditory radiation.
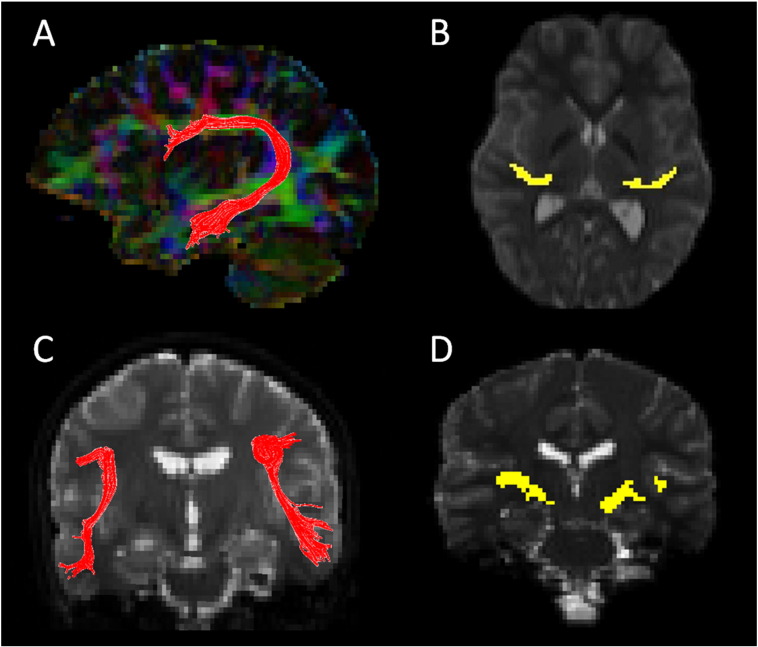
Fig. 1.
Fiber tractography of the arcuate fasciculus from a 16p11.2 deletion carrier (panels A and C) and the auditory radiation from a control subject (panels B and D) are shown. The arcuate fasciculus images are sagittal and coronal projections of a 3D rendering. The auditory radiation images are axial and coronal slices through the delineated pathway.
2.4. Statistical methods
Statistics were computed using the JMP 11.0 (SAS) software platform. To examine group differences, a linear mixed model was used to predict diffusion metrics with group, age, site of MR scan, and hemisphere as effects and subject as the random variable. The regression coefficient for hemisphere is reported as hemispheric asymmetry and represents the difference between the left and right hemisphere measures. Positive hemispheric asymmetry indicates a greater measure in the left hemisphere. Group differences were further examined for each hemisphere separately with a linear regression including group, age, and site of MR scan as effects.
To examine the relationship between CELF-4 core language index and diffusion in the left and right arcuate fasciculi, a linear regression was used with chronological age, non-verbal IQ, site of MR scan, and CELF-4 score as effects. Non-verbal IQ is included as a covariate to isolate the relationship between language score and diffusion metrics. The analysis of CELF-4 core language index was performed separately in the deletion and controls groups because non-verbal IQ is significantly different between groups by approximately one standard deviation and thus controlling for non-verbal IQ across both populations simultaneously is not possible (Miller and Chapman, 2001).
3. Results
3.1. Population
A total of eight deletion carriers met the diagnostic criteria for ASD. Among the deletion carriers, other diagnoses included attention deficit disorder (n = 8), anxiety disorder (n = 5), articulation disorder (n = 23), behavior disorder (n = 4), coordination disorder (n = 14), enuresis (n = 8), language disorder (n = 13), learning disorder (n = 3), mood disorder (n = 2), and intellectual disability (n = 2).
The entire cohort of 36 deletion carriers and 45 typically developing children had DTI of sufficient quality for the group comparison of SLF measures. A subset of 34 deletion carriers (including 7 with an ASD diagnosis) and 45 typically developing children had both DTI and HARDI acquisitions of sufficient quality to be included in the auditory radiation analysis. In this subset, there was no significant group difference in age (p > 0.05).
Not all subjects successfully completed the entire neuropsychiatric testing battery. A subset of 31 children with deletions and 44 typically developing children completed the neuropsychiatric testing and MR imaging necessary for examining correlation with language function. The mean CELF-4 core language index was significantly higher in the control group than the deletion group (mean ± SD for controls: 104.8 ± 13.3, deletions: 75.1 ± 20.0, p < 0.001). The deletion carriers with and without ASD diagnosis did not have significantly different NVIQ (p > 0.7) or CELF-4 (p > 0.3). The non-verbal IQ of the control group was significantly higher than the non-verbal IQ of the deletion group (mean ± SD for controls: 106.5 ± 12.2, deletions: 91.1 ± 14.3, p < 0.001). The 25% percentile for the control group non-verbal IQ was 98 and the 75% percentile for the deletion group was 99, indicating a low degree of overlap between the distributions.
3.2. Group differences
Tables 1 and 2 summarize the group differences in diffusion metrics for the arcuate fasciculus. The deletion carriers exhibited significantly higher arcuate fasciculus mean diffusivity (p < 0.005, Table 1). The increase in mean diffusivity was driven by an increase in radial diffusivity (p < 0.02) and also a trend towards increased axial diffusivity (p = 0.052). Group comparison by hemisphere indicates that mean diffusivity and radial diffusivity were elevated in both the left and right deletion carrier hemispheres (Table 2 for p-values). Deletion carrier axial diffusivity was significantly elevated in the right hemisphere (p < 0.05) but not the left hemisphere.
Table 1.
Comparisons of arcuate fasciculus diffusion metrics in typically developing (TD) control and deletion carriers groups are shown. Slope values were computed from the model across both groups. Group comparison marginal means are reported which factor out age, site, and hemisphere. Developmental trajectory and hemispheric asymmetry values are for both groups. Bold p-values are < 0.05.
| Group comparison |
Developmental trajectory |
Hemispheric asymmetry |
|||
|---|---|---|---|---|---|
| TD mean (±SE) |
Deletion mean (±SE) |
p-Value | Slope vs age (years) (p-value) |
Left–right (p-value) |
|
| Mean diffusivity 10−4 mm2/s |
7.34 (±0.031) |
7.47 (±0.034) |
<0.005 | −0.064 (<0.0001) |
−0.038 (<0.05) |
| Fractional anisotropy | 0.535 (±0.0039) |
0.529 (±0.0042) |
0.3 | 0.0034 (<0.002) |
0.019 (<0.001) |
| Axial diffusivity 10−4 mm2/s |
12.1 (±0.048) |
12.2 (±0.051) |
0.052 | −0.047 (<0.001) |
0.16 (<0.001) |
| Radial diffusivity 10−4 mm2/s |
4.94 (±0.040) |
5.08 (±0.043) |
<0.02 | −0.062 (<0.001) |
−0.12 (<0.001) |
Table 2.
Comparisons of arcuate fasciculus diffusion metrics by hemisphere in typically developing (TD) control and deletion carrier groups are shown. Bold p-values are < 0.05.
| Left hemisphere |
Right hemisphere |
|||||
|---|---|---|---|---|---|---|
| TD mean (±SE) |
Deletion mean (±SE) |
p-Value | TD mean (±SE) |
Deletion mean (±SE) |
p-Value | |
| Mean diffusivity 10−4 mm2/s |
7.32 (±0.035) |
7.45 (±0.037) |
<0.02 | 7.35 (±0.034) |
7.49 (±0.036) |
<0.01 |
| Fractional anisotropy |
0.545 (±0.0044) |
0.535 (±0.0047) |
0.15 | 0.525 (±0.0042) |
0.522 (±0.0045) |
0.6 |
| Axial diffusivity 10−4 mm2/s |
12.2 (±0.055) |
12.3 (±0.059) |
0.2 | 12.0 (±0.058) |
12.2 (±0.062) |
<0.05 |
| Radial diffusivity 10−4 mm2/s |
4.88 (±0.043) |
5.03 (±0.047) |
<0.02 | 5.00 (±0.042) |
5.14 (±0.045) |
<0.05 |
Table 3 summarizes the group differences in diffusion metrics for the auditory radiations. The deletion carriers exhibited significantly elevated mean diffusivity (p < 0.05) which was primarily driven by elevated axial diffusivity (p < 0.002). Measures of fractional anisotropy and the HARDI derived generalized FA did not significantly differ between groups.
Table 3.
Comparisons of auditory radiation diffusion metrics in typically developing (TD) control and deletion carrier groups are shown. Slope values were computed from the model across both groups. Group comparison marginal means are reported which factor out age, site, and hemisphere. Developmental trajectory slopes are for both groups. Bold p-values are < 0.05.
| Group comparison |
Developmental trajectory |
|||
|---|---|---|---|---|
| TD | Deletion | p-Value | Slope vs age (years) (p-value) |
|
| Mean diffusivity 10−4 mm2/s |
7.51 (±0.034) |
7.62 (±0.038) |
<0.05 | −0.045 (<0.0001) |
| Fractional anisotropy | 0.481 (±0.0056) |
0.492 (±0.0063) |
0.25 | 0.0051 (<0.002) |
| Generalized FA | 0.484 (±0.0047) |
0.494 (±0.0052) | 0.14 | 0.0037 (<0.01) |
| Axial diffusivity 10−4 mm2/s |
11.8 (±0.062) |
12.1 (±0.069) |
<0.002 | −0.01 (0.5) |
| Radial diffusivity 10−4 mm2/s |
5.38 (±0.051) |
5.40 (±0.057) |
0.8 | −0.064 (<0.0001) |
3.3. Developmental variation
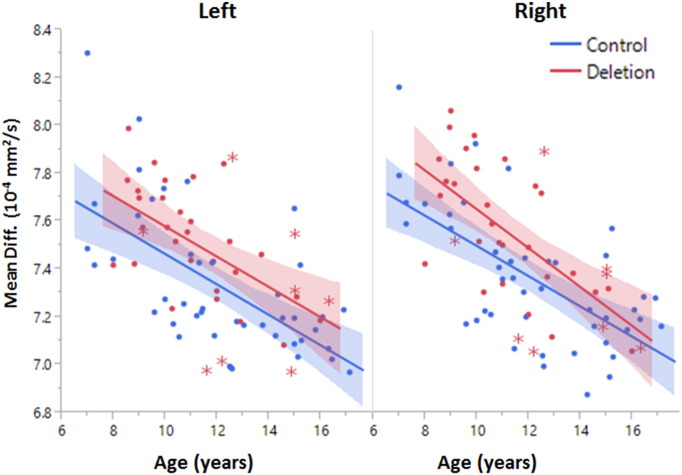
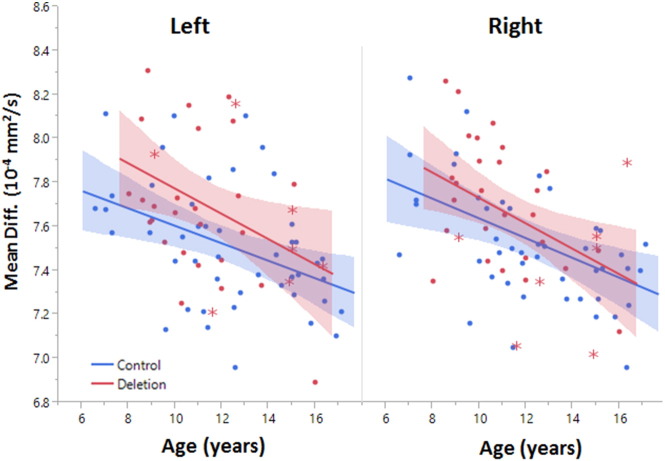
Significant correlation of arcuate fasciculus and auditory radiation diffusion metrics with age were observed in the cross-sectional data and are summarized in Tables 1 and 3. Mean diffusivity and radial diffusivity significantly decreased and measures of anisotropy significantly increased during maturation for both control and deletion carrier groups. In both the auditory radiation and arcuate fasciculus, no significant interaction between age and group was observed for any diffusion metric, indicating no differences in the rates of maturation. Figs 2 and 3 show the developmental variation of both white matter tracts.
Fig. 2.
Developmental trajectory of the arcuate fasciculus. Mean diffusivity decreases with age in both hemispheres. Deletion carriers exhibit higher mean diffusivity than controls (p < 0.005). The shaded regions represent the 95th percentile confidence interval of the lines. The slopes of the lines were not significantly different between groups. Deletion carriers with an ASD diagnosis are indicated with asterisk markers.
Fig. 3.
Developmental trajectory of the auditory radiation. Mean diffusivity significantly decreases with age in both hemispheres. Deletion carriers exhibit higher mean diffusivity than controls (p < 0.05). The shaded regions represent the 95th percentile confidence interval of the lines. The rate of maturation was not significantly different between groups. Deletion carriers with an ASD diagnosis are indicated with asterisk markers.
3.4. Language ability
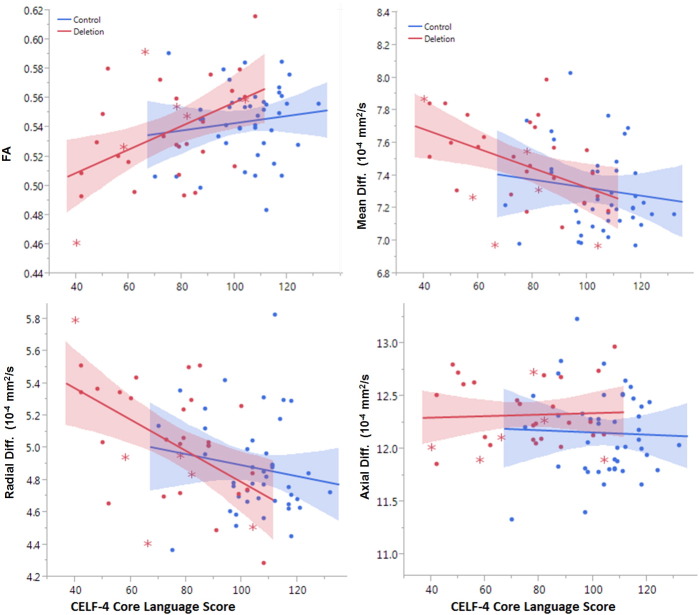
In the deletion group, left-hemisphere arcuate fasciculus mean diffusivity (p < 0.05, R2 = 0.55) and radial diffusivity (p = 0.02, R2 = 0.60) exhibited a significant negative relationship with CELF-4 core language index, but not non-verbal cognitive ability (Table 4). Left hemisphere arcuate fasciculus FA showed a trend level relationship with language in the deletion group (p = 0.07, R2 = 0.45). In controls, left-hemisphere arcuate fasciculus axial diffusivity was correlated with CELF-4 core language index and non-verbal IQ (p = 0.02 each, R2 = 0.28). Fig. 4 shows the relationship of DTI parameters with CELF-4 in both the deletion carrier and control groups. Auditory radiation mean diffusivity in the control group was predictive of language ability (p < 0.02, R2 = 0.31, Table 5).
Table 4.
Correlations of diffusion metrics with language ability as measured with the CELF-4 are shown for the arcuate fasciculus. Regression coefficients and coefficient standard errors for MD, AD, and RD are scaled ×10−7 and FA is scaled ×10−4. The p-values are reported to one significant figure and p-values under 0.05 are marked with an asterisk and in bold. MD: Mean diffusivity; FA: fractional anisotropy; AD: axial diffusivity; RD: radial diffusivity.
| Deletion carriers |
Controls |
|||||||
|---|---|---|---|---|---|---|---|---|
| Left |
Right |
Left |
Right |
|||||
| Regress. coef. (standard err.) p-value |
CELF core language index |
Non-verbal IQ |
CELF core language index |
Non-verbal IQ |
CELF core language index |
Non-verbal IQ |
CELF core language index |
Non-verbal IQ |
| MD |
−5.5 (2.6) p < 0.05* |
1.5 (3.6) p = 0.7 |
−3.4 (2.5) p = 0.2 |
−0.6 (3.4) p = 0.9 |
7.6 (4.0) p = 0.065 |
7.8 (4.5) p = 0.09 |
2.8 (4.2) p = 0.5 |
−2.4 (4.7) p = 0.6 |
| FA | 7.0 (3.8) p = 0.07 |
−1.3 (5.1) p = 0.8 |
4.9 (4.4) p = 0.3 |
−1.2 (6.0) p = 0.8 |
0.9 (4.4) p = 0.8 |
−1.4 (5.0) p = 0.8 |
6.1 (4.3) p = 0.2 |
−6.3 (4.7) p = 0.2 |
| AD | −1.1 (4.5) p = 0.8 |
3.5 (6.1) p = 0.6 |
−0.5 (6.3) p = 0.9 |
0.1 (8.5) p = 0.9 |
14 (6.0) p = 0.02* |
−16 (7.0) p = 0.02* |
6.7 (6.1) p = 0.3 |
−9.9 (6.7) p = 0.2 |
| RD |
−8.5 (3.5) p = 0.02* |
1.5 (4.7) p = 0.8 |
−6.3 (3.5) p = 0.08 |
1.1 (4.7) p = 0.8 |
3.0 (4.7) p = 0.5 |
−2.9 (5.2) p = 0.6 |
−3.7 (4.7) p = 0.4 |
03.0 (5.2) p = 0.6 |
Fig. 4.
Correlation of diffusion metrics and language ability in the left hemisphere arcuate fasciculus. Deletion carriers with an ASD diagnosis are indicated with asterisk markers. Scatter plots include variance from covariates which are controlled for in the multivariate regression.
Table 5.
Correlations of diffusion metrics with language ability as measured with the CELF-4 are shown for the auditory radiation. Regression coefficients and coefficient standard errors for MD, AD, and RD are scaled ×10−7 and FA is scaled ×10−4. The p-values are reported to one significant figure. The p-values <0.05 are bold and indicated with an asterisk. MD: Mean diffusivity; FA: fractional anisotropy; AD: axial diffusivity; RD: radial diffusivity.
| Deletion carriers |
Controls |
|||||||
|---|---|---|---|---|---|---|---|---|
| Left |
Right |
Left |
Right |
|||||
| Regress. coef. (standard err.) p-value |
CELF core language index |
Non-verbal IQ |
CELF core language index |
Non-verbal IQ |
CELF core language index |
Non-verbal IQ |
CELF core language index |
Non-verbal IQ |
| MD | −5.2 (3.5) p = 0.2 |
3.8 (4.7) p = 0.4 |
−5.4 (3.4) p = 0.1 |
5.7 (4.6) p = 0.2 |
11 (4.7) p = 0.02* |
−2.3 (4.4) p = 0.6 |
5.5 (5.4) p = 0.3 |
−1.1 (5.0) p = 0.8 |
| FA | 8.9 (7.5) p = 0.2 |
−2.8 (10.2) p = 0.8 |
2.7 (7.4) p = 0.7 |
−3.2 (10.0) p = 0.7 |
−0.8 (9.6) p = 0.9 |
−2.4 (8.9) p = 0.8 |
−6.5 (7.9) p = 0.4 |
11.5 (7.3) p = 0.1 |
| AD | 4.1 (8.1) p = 0.6 |
−0.2 (11) p = 0.9 |
−6.3 (7.3) p = 0.4 |
3.2 (9.9) p = 0.8 |
15 (9.9) p = 0.1 |
−7.5 (9.1) p = 0.4 |
−3.4 (9.7) p = 0.7 |
−14 (8.9) p = 0.1 |
| RD | −9.7 (6.3) p = 0.1 |
5.8 (8.6) p = 0.5 |
−4.9 (5.8) p = 0.4 |
4.1 (7.9) p = 0.4 |
9.3 (7.9) p = 0.2 |
0.3 (7.3) p = 0.9 |
1.0 (7.3) p = 0.2 |
5.2 (6.8) p = 0.5 |
4. Discussion
The results of this study demonstrate alterations to the microstructural organization of the auditory and language white matter tracts in children with 16p11.2 deletions. Among deletion carriers, variations in the microstructure of language pathways were found to be predictive of language ability, but not non-verbal cognition. As observed in the scatterplots, no apparent difference was observed between deletion carriers without an ASD diagnosis and the small number with an ASD diagnosis. These results provide insight into the links between 16p11.2 deletion, altered development of auditory and language pathways, and the specific behavioral deficits which may contribute to neurodevelopmental disorders such as ASD.
The increase in mean diffusivity among the 16p11.2 deletion children is indicative of greater water mobility between structures such as myelin, axonal membranes, and glial cells. Lower axonal density or decreased myelin content can contribute to increased mean diffusivity. A prior tract based spatial statistics (TBSS) study of this 16p11.2 population found increased AD throughout the brain and increased FA within the corpus callosum, superior corona radiata, and internal capsule (Owen et al., 2014). The current study similarly observed significantly elevated AD in the auditory radiation and right arcuate fasciculus. Elevated FA was not observed in either the auditory radiation or arcuate fasciculus which is consistent with the TBSS study which only observed FA changes in deep major white matter tracts. The TBSS study observed no changes in RD which survived multiple comparison correction. However we did observe significantly higher RD in the arcuate fasciculus. However, this apparent discrepancy between the current and the past study may be attributed to different study goals. The TBSS method used by Owen et al. is a whole brain voxelwise technique which requires multiple comparison correction. In this study, we used fiber tracking measures of entire white matter tracts to test specific hypotheses concerning the language and auditory systems. These large regions of interest increase measurement sensitivity and enable correlations with specific cognitive abilities such as language.
Many prior studies have demonstrated that the maturation of white matter results in decreasing mean diffusivity (Mukherjee et al., 2001). In this study, both the control and 16p11.2 groups demonstrated significant maturational trends as measured by decreasing mean diffusivity with increasing age. The rates of maturation were not different between groups. Thus, the child deletion group's mean diffusivity was elevated by a constant amount across ages compared to controls. This shift in diffusivity may be caused by a difference in the timing or rate of development during the prenatal or early childhood period. Alternatively, the difference in the 16p11.2 deletion group may represent permanent alterations to brain architecture that persist through adulthood.
Diffusion MR was acquired with a 2 mm isotropic resolution which introduces partial volume effects. The voxels may overlap two adjacent white matter tracts or contain fibers crossing on the microscopic level. Both the auditory radiation and arcuate fasciculus contain many white matter crossings. In regions of crossing fibers, mean diffusivity decreases but maintains its interpretation as a rotationally invariant measure of water mobility (Vos et al., 2012). FA, AD, and RD cannot, however, be interpreted as straightforward markers for a specific characteristic of microstructure in the presence of crossing white matter fibers (Wheeler-Kingshott and Cercignani, 2009). However, AD and RD may still maintain sensitivity to alterations in microstructure and may also indicate differences in fiber crossings, as evidenced by the observed group differences in this study. The crossing fibers also have implications for tractography, and a HARDI tractography method was necessary to delineate the auditory radiation (Berman et al., 2013). The arcuate fasciculus has extensive connectivity throughout the frontal, parietal, and temporal lobes. However, DTI tractography is sufficient to identify the core trunk of the arcuate fasciculus and produce measurements specific to the tract of interest.
Microstructure of the left-hemisphere arcuate fasciculus correlated with language ability in both deletion carriers and controls. MD and RD of deletion subjects were correlated with language ability but not general cognitive ability. The abnormally high MD and RD observed in 16p11.2 deletion carriers may drive the relationship between microstructure and language ability. The correlation of left arcuate fasciculus radial diffusivity with language ability is consistent with past reports of increased MD and RD among subjects with specific language impairment (Roberts et al., 2014). These results establish a link between 16p11.2 deletion, abnormal development of the language pathways, and impaired language function. This relationship was not observed in the right-hemispheric arcuate fasciculus. As seen in Fig. 4, the control population demonstrated a plateauing of the relationship between CELF-4 and measures of FA, RD, and MD. This may indicate that the relationship is non-linear or that language ability (especially above “average” values) is not modulated by the small differences in tract RD and MD that exist among the control population. Factors other than tissue microstructure may primarily modulate language ability among the control population. Tissue microstructure should be interpreted in the context of language impairments, not the normal variations in language ability across the control population. However, AD of control subjects was correlated with language ability and inversely correlated with general cognitive ability. Axial diffusivity and radial diffusivity are sensitive to different properties of tissue microstructure and it is possible that different mechanisms modulate language ability in each group.
Language impairment is only one clinical component which may contribute to a diagnosis of ASD. It is unknown what combination of genetic, developmental, and/or environmental factors are necessary for an ASD diagnosis. Measures of brain phenotypes, such as diffusion MR, observe one stage in the cascade of mechanisms between genetics and neuropsychiatric disorder. Multiple factors may degrade white matter microstructure and result in behavioral dysfunctions. Indeed, increased mean diffusivity is commonly reported in many diseases and pathologies. Diffusion MR alone cannot specifically identify the mechanism by which the genes of 16p11.2 impact white matter development or how 16p11.2 deletion differs from other genetic etiologies of ASD or language disorder. For this reason, it is important for future studies to further integrate genetic data with behavioral phenotyping and examine the influence of genetics on white matter development.
The changes in brain microstructure observed with diffusion MR in the 16p11.2 deletion group are similar to changes previously observed in ASD. Elevated mean diffusivity has been observed in the arcuate fasciculus of individuals with ASD and has been correlated with language impairment (Fletcher et al., 2010; Nagae et al., 2012; Roberts et al., 2014). Abnormal superior temporal gyrus diffusion metrics have also been detected in ASD, indicating involvement of the auditory system (Lange et al., 2010). In this study, there was an insufficient number of deletion carriers with an ASD diagnosis to perform a full comparison of deletion carriers with and without ASD. However, the similarity between white matter abnormalities in the 16p11.2 deletion carriers and individuals with idiopathic ASD suggests a common link between brain structure and language function.
Acknowledgments
This work was supported by a grant from the Simons Foundation (SFARI # 216074 and # 251203) and is submitted on behalf of the Simons Variation in Individuals Project (Simons VIP) investigators. We are grateful to all of the families at the participating Simons Variation in Individuals Project (Simons VIP) sites, as well as the Simons VIP Consortium. We appreciate obtaining access to phenotypic data on the SFARI Base. Support from NIH K01MH096091 (PI: JIB) is also acknowledged.
Contributor Information
Jeffrey I. Berman, Email: bermanj@email.chop.edu.
Randall Buckner, Email: randy_buckner@harvard.edu.
Wendy K. Chung, Email: wkc15@columbia.edu.
John E. Spiro, Email: jspiro@simonsfoundation.org.
Elliott H. Sherr, Email: sherre@neuropeds.ucsf.edu.
References
- Amaral D.G., Schumann C.M., Nordahl C.W. Neuroanatomy of autism. Trends Neurosci. 2008;31(3):137–145. doi: 10.1016/j.tins.2007.12.005. 18258309 [DOI] [PubMed] [Google Scholar]
- Berman J.I., Chung S., Mukherjee P., Hess C.P., Han E.T., Henry R.G. Probabilistic streamline q-ball tractography using the residual bootstrap. NeuroImage. 2008;39(1):215–222. doi: 10.1016/j.neuroimage.2007.08.021. 17911030 [DOI] [PubMed] [Google Scholar]
- Berman J.I., Lanza M.R., Blaskey L., Edgar J.C., Roberts T.P. High angular resolution diffusion imaging probabilistic tractography of the auditory radiation. A.J.N.R. Am. j. neuroradiol. 2013;34(8):1573–1578. doi: 10.3174/ajnr.A3471. 23493892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochukova E.G., Huang N., Keogh J. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463(7281):666–670. doi: 10.1038/nature08689. 19966786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44(8):953–961. doi: 10.1016/j.cortex.2008.04.002. 18614162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott C.D. second edition. Pearson; San Antonio, TX: 2007. Differential Ability Scales. [Google Scholar]
- Fernandez B.A., Roberts W., Chung B. Phenotypic spectrum associated with de novo and inherited deletions and duplications at 16p11.2 in individuals ascertained for diagnosis of autism spectrum disorder. J. Med. Genet. 2010;47(3):195–203. doi: 10.1136/jmg.2009.069369. 19755429 [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Tootell R.B., Dale A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. 10619420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.T., Whitaker R.T., Tao R. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage. 2010;51(3):1117–1125. doi: 10.1016/j.neuroimage.2010.01.083. 20132894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson E., Bernier R., Porche K. The cognitive and behavioral phenotype of the 16p11.2 deletion in a clinically ascertained population. Biol. Psychiatry. 2015;77(9):785–793. doi: 10.1016/j.biopsych.2014.04.021. 25064419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson E., Nasir R.H., Fong A. Cognitive and behavioral characterization of 16p11.2 deletion syndrome. J. dev. behav. pediatr. JDBP. 2010;31(8):649–657. doi: 10.1097/DBP.0b013e3181ea50ed. 20613623 [DOI] [PubMed] [Google Scholar]
- Horev G., Ellegood J., Lerch J.P. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc. Natl. Acad. Sci. U. S. A. 2011;108(41):17076–17081. doi: 10.1073/pnas.1114042108. 21969575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S., Reymond A., Zufferey F. Mirror extreme BMI phenotypes associated with gene dosage at the chromosome 16p11.2 locus. Nature. 2011;478(7367):97–102. doi: 10.1038/nature10406. 21881559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. 11516708 [DOI] [PubMed] [Google Scholar]
- Lange N., DuBray M.B., Lee J.E. Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res. 2010;3(6):350–358. doi: 10.1002/aur.162. 21182212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.A., Chapman J.P. Misunderstanding analysis of covariance. J. abnorm. psychol. 2001;110(1):40–48. doi: 10.1037//0021-843x.110.1.40. 11261398 [DOI] [PubMed] [Google Scholar]
- Mori S., Crain B.J., Chacko V.P., van Zijl P.C. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. 9989633 [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Miller J.H., Shimony J.S. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221(2):349–358. doi: 10.1148/radiol.2212001702. 11687675 [DOI] [PubMed] [Google Scholar]
- Nagae L.M., Zarnow D.M., Blaskey L. Elevated mean diffusivity in the left hemisphere superior longitudinal fasciculus in autism spectrum disorders increases with more profound language impairment. A.J.N.R. Am. j. neuroradiol. 2012;33(9):1720–1725. doi: 10.3174/ajnr.A3037. 22492573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J.P., Chang Y.S., Pojman N.J. Aberrant white matter microstructure in children with 16p11.2 deletions. J. Neurosci. 2014;34(18):6214–6223. doi: 10.1523/JNEUROSCI.4495-13.2014. 24790192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J.P., Ziv E., Bukshpun P. Test–retest reliability of computational network measurements derived from the structural connectome of the human brain. Brain Connectivity. 2013;3(2):160–176. doi: 10.1089/brain.2012.0121. 23350832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J., Deus J., Losilla J.M., Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurol. 1999;52(5):1038–1043. doi: 10.1212/wnl.52.5.1038. 10102425 [DOI] [PubMed] [Google Scholar]
- Qureshi A.Y., Mueller S., Snyder A.Z. Opposing brain differences in 16p11.2 deletion and duplication carriers. J. Neurosci. 2014;34(34):11199–11211. doi: 10.1523/JNEUROSCI.1366-14.2014. 25143601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T.P.L., Heiken K., Zarnow D. Left hemisphere diffusivity of the arcuate fasciculus: influences of autism spectrum disorder and language impairment. A.J.N.R. Am. J. Neuroradiol. 2014;35(3):587–592. doi: 10.3174/ajnr.A3754. 24335547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T.P.L., Khan S.Y., Rey M. MEG detection of delayed auditory evoked responses in autism spectrum disorders: towards an imaging biomarker for autism. Autism Res. 2010;3(1):8–18. doi: 10.1002/aur.111. 20063319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld J.A., Coppinger J., Bejjani B.A. Speech delays and behavioral problems are the predominant features in individuals with developmental delays and 16p11.2 microdeletions and microduplications. J. neurodev. disord. 2010;2(1):26–38. doi: 10.1007/s11689-009-9037-4. 21731881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E.M., Wiig E.H., Secord W. the psychological corporation; San Antonio: 2003. Clinical Evaluation of Language Fundamentals (CELF-4) [Google Scholar]
- Stefansson H., Meyer-Lindenberg A., Steinberg S. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature. 2014;505(7483):361–366. doi: 10.1038/nature12818. 24352232 [DOI] [PubMed] [Google Scholar]
- Szaflarski J.P., Binder J.R., Possing E.T., McKiernan K.A., Ward B.D., Hammeke T.A. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurol. 2002;59(2):238–244. doi: 10.1212/wnl.59.2.238. 12136064 [DOI] [PubMed] [Google Scholar]
- The Simons VIP Consortium Simons variation in Individuals Project (Simons VIP): a genetics-first approach to studying autism spectrum and related neurodevelopmental disorders. Neuron. 2012;73(6):1063–1067. doi: 10.1016/j.neuron.2012.02.014. 22445335 [DOI] [PubMed] [Google Scholar]
- van der Kouwe A.J., Benner T., Salat D.H., Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage. 2008;40(2):559–569. doi: 10.1016/j.neuroimage.2007.12.025. 18242102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos S.B., Jones D.K., Jeurissen B., Viergever M.A., Leemans A. The influence of complex white matter architecture on the mean diffusivity in diffusion tensor MRI of the human brain. NeuroImage. 2012;59(3):2208–2216. doi: 10.1016/j.neuroimage.2011.09.086. 22005591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L.A., Shen Y., Korn J.M. Association between microdeletion and microduplication at 16p11.2 and autism. N. Engl. J. Med. 2008;358(7):667–675. doi: 10.1056/NEJMoa075974. 18184952 [DOI] [PubMed] [Google Scholar]
- Wheeler-Kingshott C.A., Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61(5):1255–1260. doi: 10.1002/mrm.21965. 19253405 [DOI] [PubMed] [Google Scholar]
- Zufferey F., Sherr E.H., Beckmann N.D. A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. J. Med. Genet. 2012;49(10):660–668. doi: 10.1136/jmedgenet-2012-101203. 23054248 [DOI] [PMC free article] [PubMed] [Google Scholar]