Abstract
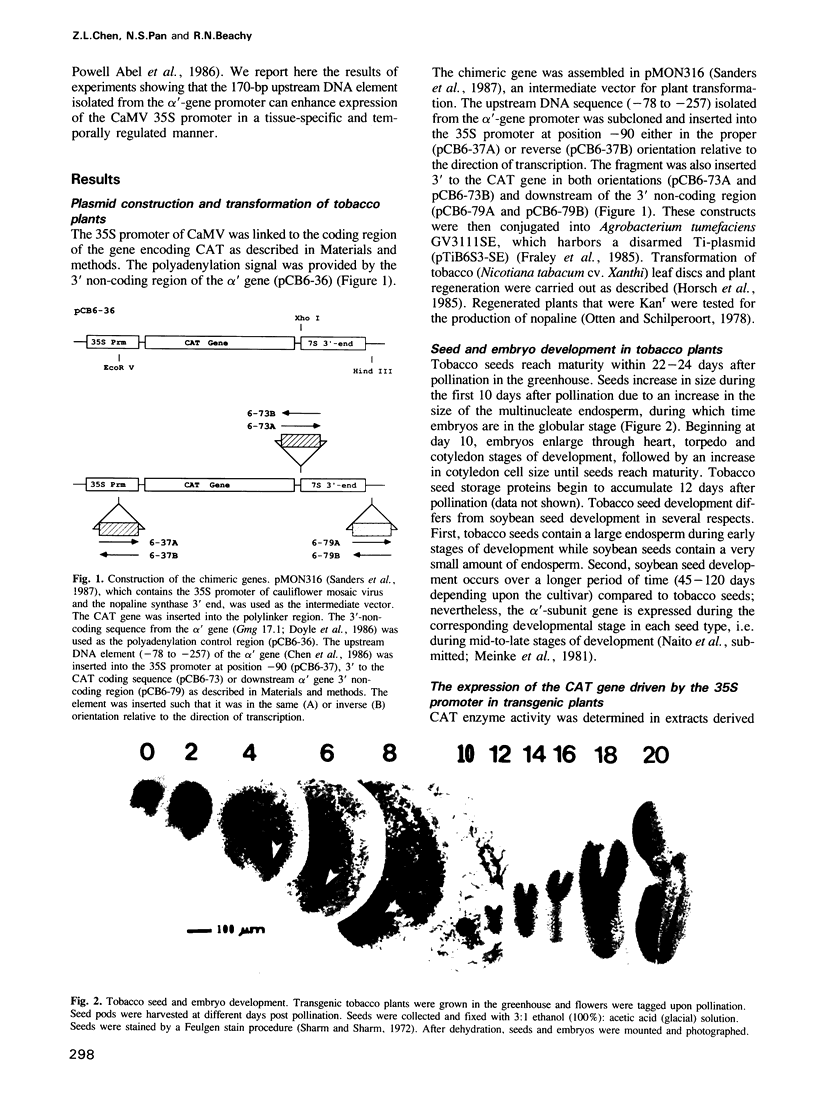
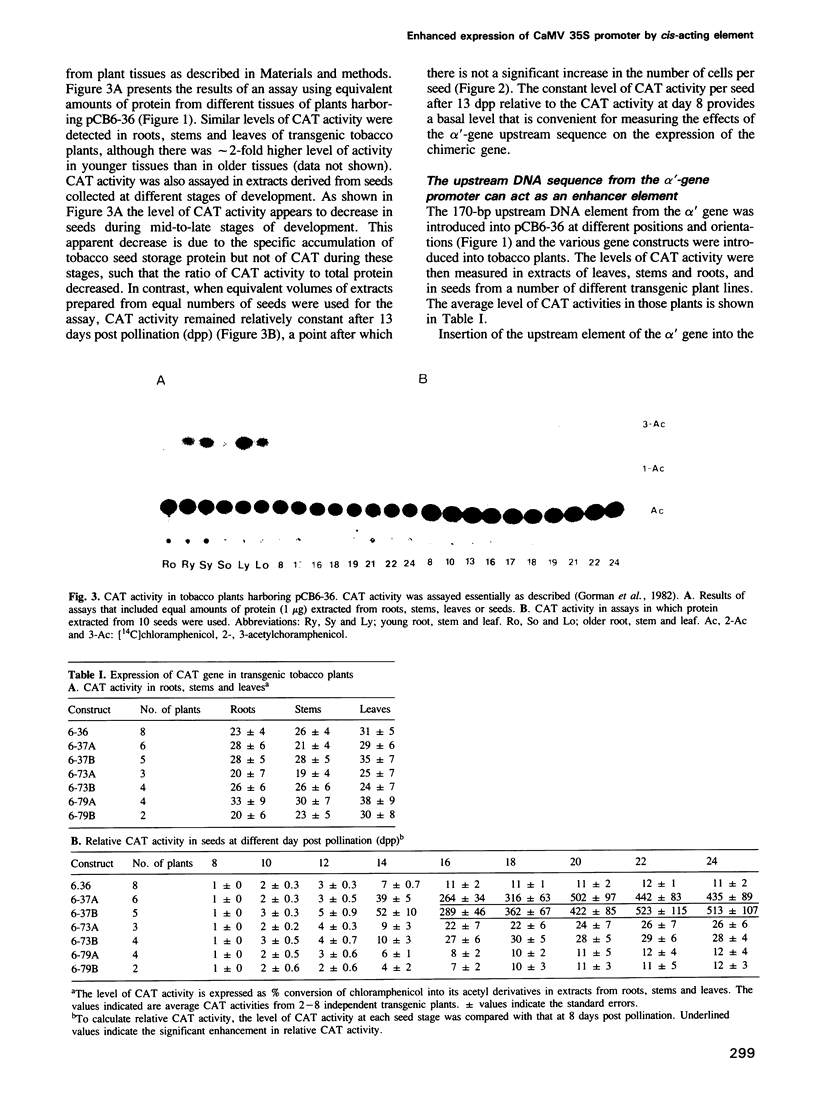
Genes encoding β-conglycinin, a soybean seed storage protein, are expressed only in seeds during mid-to-late stages in embryogeny. It was previously determined that a DNA sequence ˜200 nucleotides upstream of the transcriptional start site of the gene encoding the α'-subunit of β-conglycinin is essential for regulated gene expression in transgenic plants. The regulatory effect of this DNA element was tested by inserting the element in different positions and different orientations within a chimeric constitutively expressed reporter gene. The reporter gene was comprised of the 35S promoter from cauliflower mosaic virus (CaMV), a gene encoding chloramphenicol acetyltransferase (CAT) and the polyadenylation signal from the α'-subunit gene. The element had no significant effect on the expression of the CAT gene in roots, stems, or leaves, regardless of the position of its insertion (i.e. 5 or 3 of the gene). However, there was 25- to 40-fold enhancement of CAT gene expression in seeds during mid-to-late stages of embryo development when the element was placed in either orientation within the 35S promoter. There was 2- to 4-fold enhancement of CAT activity when the element was placed 3' of the CAT coding sequence. No enhancement was detected when the element was placed downstream of the 3' non-coding region. This is, to our knowledge, the first identification of a cis-acting element that enhances gene expression in a tissue-specific and temporally regulated manner during embryo development in plants.
Keywords: β-conglycinin, seed specific enhancer element, 35S promoter, transgenic plants
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Abel P. P., Nelson R. S., De B., Hoffmann N., Rogers S. G., Fraley R. T., Beachy R. N. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science. 1986 May 9;232(4751):738–743. doi: 10.1126/science.3457472. [DOI] [PubMed] [Google Scholar]
- Baumann G., Raschke E., Bevan M., Schöffl F. Functional analysis of sequences required for transcriptional activation of a soybean heat shock gene in transgenic tobacco plants. EMBO J. 1987 May;6(5):1161–1166. doi: 10.1002/j.1460-2075.1987.tb02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy R. N., Chen Z. L., Horsch R. B., Rogers S. G., Hoffmann N. J., Fraley R. T. Accumulation and assembly of soybean beta-conglycinin in seeds of transformed petunia plants. EMBO J. 1985 Dec 1;4(12):3047–3053. doi: 10.1002/j.1460-2075.1985.tb04044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. L., Schuler M. A., Beachy R. N. Functional analysis of regulatory elements in a plant embryo-specific gene. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8560–8564. doi: 10.1073/pnas.83.22.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J. J., Schuler M. A., Godette W. D., Zenger V., Beachy R. N., Slightom J. L. The glycosylated seed storage proteins of Glycine max and Phaseolus vulgaris. Structural homologies of genes and proteins. J Biol Chem. 1986 Jul 15;261(20):9228–9238. [PubMed] [Google Scholar]
- Ellis J. G., Llewellyn D. J., Dennis E. S., Peacock W. J. Maize Adh-1 promoter sequences control anaerobic regulation: addition of upstream promoter elements from constitutive genes is necessary for expression in tobacco. EMBO J. 1987 Jan;6(1):11–16. doi: 10.1002/j.1460-2075.1987.tb04711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Kuhlemeier C., Nagy F., Chua N. H. Organ-specific and light-induced expression of plant genes. Science. 1986 May 30;232(4754):1106–1112. doi: 10.1126/science.232.4754.1106. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. E., Breidenbach R. W. Proteins of Soybean Seeds: II. Accumulation of the Major Protein Components during Seed Development and Maturation. Plant Physiol. 1974 May;53(5):747–751. doi: 10.1104/pp.53.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulen Hildegard, Schell Jeff, Kreuzaler Fritz. Light-induced expression of the chimeric chalcone synthase-NPTII gene in tobacco cells. EMBO J. 1986 Jan;5(1):1–8. doi: 10.1002/j.1460-2075.1986.tb04169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlemeier C., Fluhr R., Green P. J., Chua N. H. Sequences in the pea rbcS-3A gene have homology to constitutive mammalian enhancers but function as negative regulatory elements. Genes Dev. 1987 May;1(3):247–255. doi: 10.1101/gad.1.3.247. [DOI] [PubMed] [Google Scholar]
- Otten L. A., Schilperoort R. A. A rapid micro scale method for the detection of lysopine and nopaline dehydrogenase activities. Biochim Biophys Acta. 1978 Dec 8;527(2):497–500. doi: 10.1016/0005-2744(78)90363-7. [DOI] [PubMed] [Google Scholar]
- Ow D. W., DE Wet J. R., Helinski D. R., Howell S. H., Wood K. V., Deluca M. Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science. 1986 Nov 14;234(4778):856–859. doi: 10.1126/science.234.4778.856. [DOI] [PubMed] [Google Scholar]
- Sanders P. R., Winter J. A., Barnason A. R., Rogers S. G., Fraley R. T. Comparison of cauliflower mosaic virus 35S and nopaline synthase promoters in transgenic plants. Nucleic Acids Res. 1987 Feb 25;15(4):1543–1558. doi: 10.1093/nar/15.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler M. A., Schmitt E. S., Beachy R. N. Closely related families of genes code for the alpha and alpha' subunits of the soybean 7S storage protein complex. Nucleic Acids Res. 1982 Dec 20;10(24):8225–8244. doi: 10.1093/nar/10.24.8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J., VAN Montagu M., Herrera-Estrella L. Photosynthesis-associated gene families: differences in response to tissue-specific and environmental factors. Science. 1986 Jul 4;233(4759):34–38. doi: 10.1126/science.233.4759.34. [DOI] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Timko M. P., Kausch A. P., Castresana C., Fassler J., Herrera-Estrella L., Van den Broeck G., Van Montagu M., Schell J., Cashmore A. R. Light regulation of plant gene expression by an upstream enhancer-like element. Nature. 1985 Dec 12;318(6046):579–582. doi: 10.1038/318579a0. [DOI] [PubMed] [Google Scholar]
- Walling L., Drews G. N., Goldberg R. B. Transcriptional and post-transcriptional regulation of soybean seed protein mRNA levels. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2123–2127. doi: 10.1073/pnas.83.7.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]