Abstract
Background
The neural substrate for clinical symptoms and neuropsychological performance in individuals with attention-deficit/hyperactivity disorder (ADHD) has rarely been studied and has yielded inconsistent results. We sought to compare the microstructural property of fibre tracts associated with the prefrontal cortex and its association with ADHD symptoms and a wide range of attention performance in youth with ADHD and healthy controls.
Methods
We assessed youths with ADHD and age-, sex-, handedness-, coil- and intelligence-matched controls using the Conners’ Continuous Performance Test (CCPT) for attention performance and MRI. The 10 target tracts, including the bilateral frontostriatal tracts (caudate to dorsolateral prefrontal cortex, ventrolateral prefrontal cortex and orbitofrontal cortex), superior longitudinal fasciculus (SLF) and cingulum bundle were reconstructed using diffusion spectrum imaging tractography. We computed generalized fractional anisotropy (GFA) values to indicate tract-specific microstructural property.
Results
We included 50 youths with ADHD and 50 healthy controls in our study. Youths with ADHD had lower GFA in the left frontostriatal tracts, bilateral SLF and right cingulum bundle and performed worse in the CCPT than controls. Furthermore, alteration of the right SLF GFA was most significantly associated with the clinical symptom of inattention in youths with ADHD. Finally, youths with ADHD had differential association patterns of the 10 fibre tract GFA values with attention performance compared with controls.
Limitations
Ten of the youths with ADHD were treated with methylphenidate, which may have long-term effects on microstructural property.
Conclusion
Our study highlights the importance of the SLF, cingulum bundle and frontostriatal tracts for clinical symptoms and attention performance in youths with ADHD and demonstrates the involvement of different fibre tracts in attention performance in these individuals.
Introduction
Attention deficit is one of the core symptoms of attention-deficit/hyperactivity disorder (ADHD) that lasts throughout adulthood.1 Children with ADHD tend to have deficits in focused attention, cognitive and behavioural inhibition, sustained attention (the ability to maintain focus on a task over time) and vigilance (the attentional capacity to remain alert even when less stimulated, such as through the stimuli of nontargets).2 To differentiate individuals with or without ADHD,3 the Continuous Performance Test (CPT) has been widely used to measure attention performance.2
Some neuropsychological functions have been proposed as potential endophenotypes for ADHD as they are more closely linked to the neurobiological substrate of ADHD than clinical symptoms4,5 and are associated with gene susceptibility for ADHD.4 Using the CPT and derived tasks, high reaction time (RT) variability has been associated with an absence of the 7-repeat allele in the dopamine receptor 4 gene (DRD4).6–8 Higher commission errors, impulsive responses and high RT variability were also correlated to those homozygous for the 10-repeat allele in the dopamine transporter gene.9,10 In Korean children with ADHD, associations have been discovered between fewer commission errors and homozygosity of the 4-repeat allele at DRD4.11 On the other hand, more commission errors were reported for those with the G1287A genotype of the norepinephrine transporter gene.12 Therefore, the CPT may provide information more closely related to the underlying neuropathology of ADHD than the clinical symptoms do.
Increasingly, studies have adopted imaging techniques to explore the neuropathology of ADHD. Structural and functional neuroimaging findings have highlighted that prefrontal cortex abnormality may contribute to dysfunction in attention regulation, resulting in the behavioural manifestation of ADHD.13 Among the subdivisions of the prefrontal cortex, the dorsolateral prefrontal cortex (DLPFC) plays a specific role in attention and executive functions,14 the ventrolateral prefrontal cortex (VLPFC) in behavioural inhibition,15,16 the orbitofrontal cortex (OFC) in motivation and reward, and the anterior cingulate cortex (ACC) in reward-based decision making.17 Volumetric and functional MRI studies have also consistently reported reduced cortical thickness18 and activation in these prefrontal areas in children and adults with ADHD, respectively, during the CPT.19,20 Although alteration in the frontostriatal tracts was the first to be proposed as a possible neuropathology of ADHD,13,16,21,22 its association with clinical symptoms and neuropsychological performance has rarely been studied and produces inconsistent results. For example, alteration in the frontostriatal tracts may be correlated not only with symptoms of inattention, but also with intraindividual variability of RT and executive functions in individuals with ADHD as well as typically developing controls.23,24 However, attention performance was noted only in controls — not in children21,25 or adults26 with ADHD.
The parietal cortex, especially the superior parietal lobule and inferior parietal lobule, has also been linked to visual attention in both shifting attention and sustained attention.27 The superior parietal lobule participates in the transmission of spatial information for body parts, the caudal part of the inferior parietal lobule is responsible for visual attention, and the inferior parietal lobule plays a role in working memory.28 The superior longitudinal fasciculus (SLF) as well as the interconnecting frontal, parietal and temporal cortices are involved in attention performance29 and working memory30 in control children. Alteration in the SLF has been demonstrated in children31–33 and adults34,35 with ADHD. All in all, positive correlations between attention performance and the microstructural property of the right SLF have been found in adults with ADHD34 and in control children,29 but not in healthy adults.36
The cingulum bundle is determined by fibre tracts extending from the ACC to the posterior cingulate cortex (PCC) and connects the frontal lobe with the precuneus, PCC, hippocampus and parahippocampus.37 The anterior portion of the cingulum bundle allows for communication between regions of the limbic system and has been related to the evaluation of rewards.38 On the other hand, the posterior part of the cingulum bundle interconnects the cognitive functions.38 The microstructural property of the right cingulum bundle is related to sustained attention and working memory in healthy adults.36 However, alteration in the cingulum bundle that was noted in children31 and adults34,35 with ADHD was not supported by some recent studies in children23,32 and adults26 with ADHD. While some studies suggest the involvement of the cingulum bundle in intraindividual variability of RT in children with ADHD,23 attention processing in patients with Parkinson disease39 and elderly adults without dementia,40 other studies have failed to demonstrate such an association in adults with ADHD.26,34
Diffusion spectrum imaging (DSI) is one of the high angular resolution diffusion imaging techniques that aims to address the limitations of diffusion tensor imaging (DTI) in order to resolve crossing fibres.41 Compared with DTI, which acquires diffusion-weighted images of varying directions at fixed diffusion sensitivity (b value, typically 1000 s/mm2), DSI acquires diffusion-weighted images of varying directions and different b values (typically from zero to a few thousand seconds/mm2).42 Whereas DTI reconstructs water molecular diffusion through a diffusion tensor based on the Gaussian model of translational displacement,43 DSI reconstructs water molecular diffusion as an average propagator based on a Fourier relation between the diffusion signal and average propagator.41,42 The advantage of DSI lies in the fact that the directions of the maxima of the average propagator correspond to the underlying crossing fibre directions,44 whereas the principal direction of the DTI points to a direction that is somewhere between the crossing fibres.
Generalized fractional anisotropy (GFA) is a DSI analogue of the fractional anisotropy (FA) in DTI.45 Whereas FA is defined as the standard deviation (SD) of the eigenvalues of the diffusion tensor normalized by the norm of the eigenvalues,46 GFA is defined as the SD of the orientation distribution function (ODF) values divided by their norm.45 Here, the ODF value in a particular direction can be calculated as the second moment of the average propagator, indicating the displacement probability in that particular direction.42 Similar to FA, GFA is a nonspecific index often used to indicate the microstructural property of white matter as determined by the diameter, packing density, directional coherence and myelination of the axons.
The inconsistency in the literature can be explained by small sample sizes, different neuropsychological tasks and varying methods to define the region of interest (ROI), resulting in little overlap of ROIs across studies. Among the constructed fibre tracts, the frontostriatal tracts, SLF and cingulum bundle have all been reported to be altered in individuals with ADHD. However, their associations with attention performance remain unclear. Therefore, the present study compared the white matter microstructural property associated with the prefrontal cortex and its association with ADHD symptoms as well as a wide range of attention performance between youths with ADHD and individually matched controls. We chose 10 white matter tracts, including the bilateral frontostriatal tracts (caudate–DLPFC, caudate–VLPFC and caudate–OFC; Fig. 1); the SLF (Fig. 2) and the cingulum bundle (Fig. 2). We also used DSI, instead of DTI, to reconstruct tractography and measure the microstructural property of selected fibre tracts for a better resolution of the crossing fibres.41 We hypothesized that the microstructural property of these target tracts could differ between youths with ADHD and controls and that altered diffusion anisotropy of these target tracts could correlate with the severity of ADHD core symptoms and impaired attention performance.
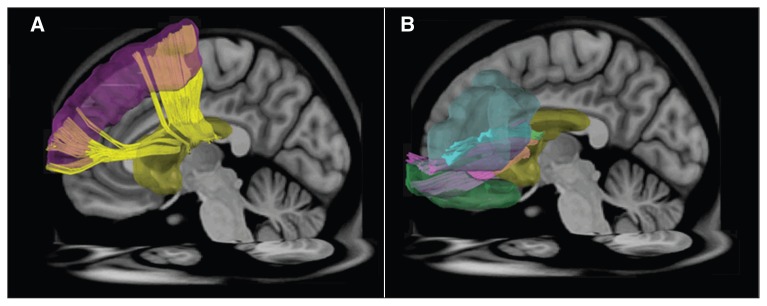
Fig. 1.
Regions of interest (ROIs) and reconstructed targeted tracts in the left hemisphere. (A) The ROIs at the dorsolateral prefrontal cortex (purple), caudate nucleus (yellow) and the dorsolateral prefrontal–caudate tract (yellow). (B) The ROIs at the ventrolateral prefrontal cortex (light blue), orbitofrontal cortex (green), caudate nucleus (yellow), ventrolateral prefrontal–caudate tract (light blue) and orbitofrontal–caudate tract (pink).
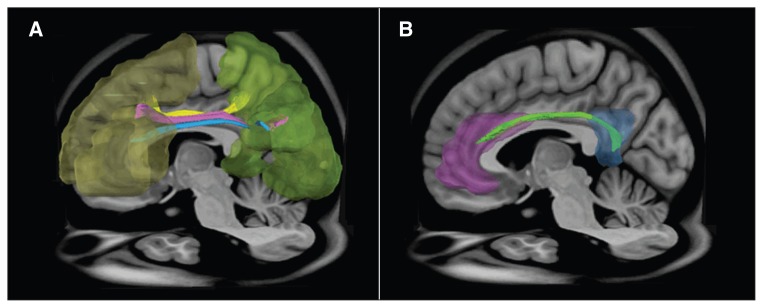
Fig. 2.
Regions of interest (ROIs) and reconstructed targeted tracts in the left hemisphere. (A)The ROIs in the frontal lobe (yellow), parietal lobes (green) and superior longitudinal fasciculus I (yellow), II (pink) and III (blue). (B) The ROIs at the anterior cingulate cortex (purple), posterior cingulate cortex (blue) and cingulum bundle (light green).
Methods
Participants
We recruited youths with ADHD from the Department of Psychiatry, National Taiwan University Hospital; ADHD was clinically diagnosed according to DSM-IV criteria, and 1 of us (S.S.-F.G.) further confirmed the diagnosis through psychiatric interviews using the Chinese Kiddie epidemiologic version of the Schedule for Affective Disorders and Schizophrenia (K-SADS-E).47 We recruited sex-, age-, handedness-, IQ- and coil-matched control youths from the same school districts as the ADHD group; the control youths were referred by school principals and teachers. Controls and their parents also underwent the Chinese K-SADS-E interview to confirm that they did not have a lifetime or current diagnosis of ADHD or other psychiatric disorders.48,49 Youths with psychosis, mood disorders, learning disabilities, substance use, autism-spectrum disorders, neurologic disorders or a full-scale IQ score lower than 80 were excluded from the study.
The Research Ethics Committee of National Taiwan University Hospital approved our study protocol. Written informed consent was obtained from both participants and parents after clearly explaining the purpose and detailed experimental procedures of this study. All participants received the same psychiatric, neuropsychological and MRI assessments.
The Conners’ Continuous Performance Test
The Conners’ Continuous Performance Test (CCPT) is a 14-minute computerized task for individuals aged 6 years and older.50 It requires participants to tap on the spacebar when any character except “X” is shown on the screen. There are 6 blocks in the CCPT, including 3 sub-blocks that each contain 20 letter presentations, for a total of 360 trials. The sub-blocks differ in interstimulus intervals (ISI) of 1, 2, and 4 s, and the sequence of ISI conditions is presented randomly. There are 12 indexes covering different domains of CCPT performance: omission errors (the number of times not responding to a target), commission errors (the number of times responding to a nontarget), RT (the period of time between the presentation of the stimulus and the response), variability (intraindividual variability in RT), perseveration (RT < 100 ms), detectability (the ability to discriminate between targets and nontargets), hit RT standard errors (consistency of RT), response style (a function of the ratio of hit target [hit rate] to hit non-target [false alarm rate] stimuli), Hit RT changed by blocks (the slope of change in RT over 6 blocks as the test progresses), standard errors of hit RT changed by blocks, hit RT changed by ISIs (whether performance decreases with longer ISIs), and standard errors of hit ISI change (whether performance becomes more variable with longer ISIs).
Based on the factor analysis by Egeland and Kovalik-Gran,51 indexes of CCPT can be grouped into 4 dimensions: focused attention (RT variability and hit RT standard errors, detectability and omission errors), hyperactivity/impulsivity (commission errors, RT, response style and perseverations), sustained attention (hit RT changed by blocks and standard errors of hit RT changed by blocks) and vigilance (hit RT ISI change and standard errors of hit ISI change).
MRI assessments
We obtained MRIs using a Siemens Trio 3 T MRI system with a 32-channel phased array head coil, except 3 dyads with a 16-channel phased array head coil. All participants were asked to lie still on the table, and head movement was restricted with expandable foam cushions. We acquired transaxial sections covering the whole brain, with the imaging planes parallel to the line that connects the anterior commissure and the posterior commissure on the sagittal localizer.
We acquired T1-weighted images of the whole head using a 3-dimensional magnetization-prepared rapid gradient echo (MPRAGE) sequence with the following parameters: repetition time (TR) 2530 ms, echo time (TE) 3.4 ms, slice thickness 1.0 mm, matrix size 256 × 256, field of view (FOV) 256 × 256 mm. Diffusion-weighted images (DWIs) were acquired using a pulsed-gradient spin-echo echo-planar imaging sequence with a twice-refocused balanced echo.52 The DWI volumes were acquired by applying diffusion-sensitive gradients with different strengths and directions to fill isotropic grid points in the diffusion-encoding space (q-space).42 A total of 102 DWI volumes corresponding to the grid points within a half sphere of the q-space (DSI102) were acquired with the maximum diffusion sensitivity value (bmax) set to 4000 s/mm2. The acquisition scheme was a modified version (a half sphere rather than a full sphere) of the optimal q-space sampling method, DSI 203, as previously described.53 Other acquisition parameters were as follows: TR 9600 ms, TE 130 ms, matrix size 80 × 80, FOV 200 × 200 mm2, and slice thickness 2.5 mm without gap. The scan time for each DSI acquisition was approximately 16.5 min.
DSI reconstruction and template-based tract-specific analysis
Having acquired DSI data, we obtained the diffusion probability density function (PDF) on the basis of the Fourier association between the PDF and q-space signal.54 Within each voxel, there were 102 samples at the grid points within a half sphere of the q-space. These 102 samples were first projected around the origin to fill the other half sphere based on the fact that the q-space data were symmetric around the origin. The 8 corners outside of the sphere were filled with zeros, resulting in a 7 × 7 × 7 data grid in the q-space. A Hanning filter with a width of 17 units was applied to the q-space data and followed by a 3-dimensional Fourier transformation of the q-space signal to obtain the PDF. The ODF (Ψ(u)) was computed by obtaining the second moment of the PDF along each of the 362 radial directions (6-fold tessellated icosahedron). At each voxel, GFA was quantified in terms of SD(Ψ)/RMS(Ψ), where SD is the standard deviation and RMS is the root mean square. The GFA indicates the directionality of the ODF and ranges from zero (when the diffusion is completely isotropic) to 1 (when the diffusion is restricted to only 1 direction).45 To determine the local tract directions in each voxel, we used an iterative approach to decompose the ODF into several constituent Gaussian ODFs.55 The resulting local tract direction field was obtained for tractography.
To avoid subjective variation arising from manual tractography, we used a template-based approach. First, a study-specific DSI template (SSDT) was constructed from all the recruited participants using a coregistration method under the framework of large deformation diffeomorphic metric mapping.56 Two of us (W.-Y.I.T., Y.-C.L., who has more than 7 years’ experience in DSI tractography) reconstructed 10 target tracts, namely the bilateral frontostriatal tracts (i.e., caudate-VLPFC, caudate-DLPFC, caudate-OFC, Fig. 1), SLF (Fig. 2) and cingulum bundles (Fig. 2), under consensus. They placed appropriate ROIs on the SSDT to segment the target tracts. The appropriate ROIs were defined in the automated anatomic labelling (AAL) system57 using the WFU PickAtlas version 3.0.4. The coordinates of these ROIs in AAL were transformed to SSDT after performing the coregistration between SSDT and the International Consortium for Brain Mapping 152 template using a Dartel toolbox in SPM12. We performed a streamline-based fibre tracking algorithm with whole brain seeding using DSI studio (http://dsi-studio.labsolver.org) based on the local tract directions. Having obtained tractography of each target tract on SSDT, the coordinates of streamlines were aligned along the proceeding direction of each tract bundle and were saved as the sampling coordinates of GFA. The sampling coordinates of target tracts were then transformed from SSDT to individual DSI data set according to the established transformation between SSDT and native DSI space. Finally, the GFA values of each target tract were sampled on the corresponding sampling coordinates in the native DSI space.
Statistical analyses
We conducted statistical analyses using SAS version 9.2 software (SAS Institute). The α value was preselected at the level of p < 0.05. The descriptive results are displayed as the mean ± SD for continuous variables. Because of the matched case–control study design, we used the paired t test to compare the mean scores of IQ and ADHD symptom counts, CCPT performance, and GFA values of the white matter tracts between the ADHD and control groups. We computed the Cohen d to indicate small (0.2–0.5), medium (0.5–0.8) and large (≥ 0.8) effect sizes.
To control for the inflation of type I error in computing multiple bivariate correlations, we used multiple linear regression models with the backward elimination procedure to determine the association between the attention measures (i.e., ADHD symptoms and CCPT indexes) as dependent variables and the GFA values of the 10 target tracts as independent variables. We used the backward elimination procedure to identify the fitted model containing variables from these 10 tracts, which maintained significant effects on each of the attention measures. We calculated R2 values to present the proportion of the variance of each attention measure that can be explained by the microstructural property, presented by the GFA values, of some of the fibre tracts in the final fitted model.
Results
Participants
The sample consisted of 50 youths with ADHD and 50 controls. The demographic and clinical characteristics of participants are shown in Table 1. Among the 50 youths with ADHD, 26 (52%) had the combined type and 24 (48%) had the predominantly inattentive type. Seventeen of the youths with ADHD had comorbid oppositional defiant disorder (ODD), 2 youths had comorbid conduct disorder, and 3 youths had both comorbid ODD and conduct disorder. Two of the control youths had ODD. Both the ADHD and control youths were well-matched at the individual level, with no significant group differences in the distribution of sex, handedness, age, full-scale IQ or type of coil used during MRI assessment. Despite no group difference in performance IQ, youths with ADHD had lower verbal IQ than controls (Table 1).
Table 1.
Demographic and clinical characteristics of study participants
| Group, mean ± SD* | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | ADHD (n = 50) | Control (n = 50) | t | p value |
| Male sex, no. (%) | 38 (76) | 38 (76) | — | — |
| Handedness, right:left | 49:1 | 49:1 | — | — |
| Coil, 32:16 | 47:3 | 47:3 | — | — |
| Age, yr† | 11.26 ± 2.93 | 11.22 ± 2.79 | 0.40 | 0.69 |
| Full-scale IQ | 110.28 ± 11.52 | 111.78 ± 11.02 | −0.89 | 0.38 |
| Performance IQ | 109.38 ± 13.54 | 108.54 ± 13.19 | 0.40 | 0.69 |
| Verbal IQ | 109.62 ± 10.44 | 113.44 ± 9.88 | −2.66 | 0.011 |
| DSM-IV symptom count‡ | ||||
| Inattention (0–9) | 7.74 ± 1.34 | 0.38 ± 0.94 | 31.78 | < 0.001 |
| Hyperactivity (0–6) | 3.18 ± 1.96 | 0.15 ± 0.44 | 10.88 | < 0.001 |
| Impulsivity (0–3) | 1.71 ± 1.11 | 0 ± 0 | 10.88 | < 0.001 |
ADHD = attention-deficit/hyperactivity disorder; SADS-E = Schedule for Affective Disorders, epidemiologic version; SD = standard deviation.
Unless otherwise indicated.
Range 7–18 yr.
Based on psychiatric interview using the Kiddie-SADS-E interviews of current symptoms.
Microstructural property
Youths with ADHD had significantly lower mean GFA values than control youths in the 3 left frontostriatal tracts (i.e., caudate–VLPFC, caudate–DLPFC, caudate–OFC tracts), the bilateral SLF and the right cingulum bundle (Table 2). The effect sizes were higher than 0.3 in these 6 tracts. We further analyzed the group differences in mean GFA values after removing the 6 participants using a different type of coil or the 2 left-handed participants. All findings remained significant, even after comparing the 47 youths with ADHD to the 47 control youths who used a 32-channel phased array head coil (See the Appendix, Table S1, available at jpn.ca). We found that the results were generally consistent when comparing the 49 right-handed youths with ADHD and the 49 right-handed control youths, with the exception that the effect size of the group difference in the mean GFA value of the left caudate-OFC tract decreased (p = 0.06, Cohen d −0.268; Table S3).
Table 2.
Comparisons of the generalized fractional anisotropy values of 10 fibre tracts between youths with ADHD and controls
| Group, mean ± SD | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Target tracts | Side | ADHD (n = 50) | Control (n = 50) | t | p value | Cohen d |
| Caudate–ventrolateral prefrontal cortex | Left | 0.236 ± 0.016 | 0.241 ± 0.015 | −2.22 | 0.031 | −0.32 |
| Right | 0.235 ± 0.019 | 0.238 ± 0.017 | −1.15 | 0.25 | −0.17 | |
| Caudate–dorsolateral prefrontal cortex | Left | 0.221 ± 0.017 | 0.227 ± 0.017 | −2.12 | 0.039 | −0.35 |
| Right | 0.227 ± 0.022 | 0.232 ± 0.019 | −1.42 | 0.16 | −0.24 | |
| Caudate–orbitofrontal cortex | Left | 0.213 ± 0.012 | 0.217 ± 0.011 | −2.06 | 0.044 | −0.35 |
| Right | 0.212 ± 0.015 | 0.216 ± 0.015 | −1.44 | 0.16 | −0.27 | |
| Superior longitudinal fasciculus | Left | 0.198 ± 0.023 | 0.206 ± 0.022 | −2.38 | 0.021 | −0.36 |
| Right | 0.223 ± 0.025 | 0.231 ± 0.021 | −2.08 | 0.040 | −0.35 | |
| Cingulum | Left | 0.301 ± 0.027 | 0.307 ± 0.023 | −1.35 | 0.19 | −0.24 |
| Right | 0.279 ± 0.029 | 0.288 ± 0.019 | −2.39 | 0.021 | −0.37 | |
ADHD = attention-deficit/hyperactivity disorder; SD = standard deviation.
Attention performance
Youths with ADHD generally performed worse than control youths across the 4 dimensions of attention performance assessed using the CCPT (Table 3), with effect sizes higher than 0.5 except in the indexes of response style and standard error of RT ISI change. Compared with control youths, youths with ADHD had more omission errors, higher standard errors of hit RT and variability and lower detectability (impaired focused attention); youths with ADHD also had more commission errors and a higher rate of perseverations (impulsivity), higher scores with standard errors of hit RT changed by blocks (impaired sustained attention) and longer hit RT with higher standard errors (p = 0.06) and increased ISIs (declined vigilance) than controls.
Table 3.
Comparisons of performance factors on the Conner’s Continuous Performance Test between youths with ADHD and controls
| Group, mean ± SD | |||||
|---|---|---|---|---|---|
|
|
|||||
| Factor | ADHD (n = 50) | Control (n = 50) | t | p value | Cohen d |
| Focused attention | |||||
| Omission | 11.12 ± 11.97 | 4.02 ± 4.71 | 3.9 | < 0.001 | 0.78 |
| RT SE | 12.20 ± 7.08 | 6.72 ± 3.27 | 5.21 | < 0.001 | 0.99 |
| Variability | 26.24 ± 19.54 | 11.16 ± 9.84 | 4.99 | < 0.001 | 0.98 |
| Detectability | 0.338 ± 0.364 | 0.52 ± 0.356 | −3.03 | 0.004 | −0.51 |
| Impulsivity | |||||
| Commission | 23.04 ± 7.75 | 18.62 ± 8.02 | 3.5 | 0.001 | 0.56 |
| Reaction time | 408.92 ± 90.25 | 375.79 ± 56.71 | 2.33 | 0.024 | 0.44 |
| Response style | 0.622 ± 0.377 | 0.778 ± 2.389 | −0.47 | 0.64 | −0.09 |
| Perseveration | 14.98 ± 20.26 | 3.22 ± 6.42 | 4.02 | < 0.001 | 0.78 |
| Sustained attention | |||||
| RT block change | 0.023 ± 0.036 | 0.007 ± 0.025 | 2.62 | 0.012 | 0.52 |
| RT SE block change | 0.107 ± 0.095 | 0.046 ± 0.065 | 3.77 | < 0.001 | 0.75 |
| Vigilance | |||||
| RT ISI change | 0.098 ± 0.048 | 0.064 ± 0.031 | 4.23 | < 0.001 | 0.84 |
| RT SE ISI change | 0.121 ± 0.188 | 0.059 ± 0.126 | 1.94 | 0.06 | 0.39 |
ADHD = attention-deficit/hyperactivity disorder; ISI = interstimulus interval; RT = reaction time; SD = standard deviation; SE = standard error.
Correlations of fibre tract property with clinical symptoms and CCPT performance
The GFA values of the right caudate-VLPFC, bilateral caudate–DLPFC, SLF and cingulum bundle were negatively correlated with inattention symptoms in youths with ADHD (Appendix, Table S2), but the significant effects remained only for the right SLF in the final model (regression coefficient estimate β = −26.33, p < 0.001, R2 = 0.23).
Table 4 presents different association patterns of the microstructural property of fibre tracts, as indicated by the GFA values and CCPT indexes in the final fitted model, using the backward elimination method in control youths and youths with ADHD separately. Generally speaking, the CCPT indexes were significantly associated with all tracts of interest in the control group, whereas no CCPT indexes were significantly related to the frontostriatal tracts to connect to the VLPFC in youths with ADHD. The GFA values of the right caudate–VLPFC, left caudate-DLPFC and left caudate–OFC explained the dimension of focused attention in control youths, while only significant associations in the left caudate–DLPFC and right caudate-OFC tracts were attributed to ADHD. The severity of the impulsivity dimension was related to the GFA values of all 3 frontostriatal tracts, right SLF and right cingulum bundle in control youths, but only to those of the left caudate–OFC tract, right SLF and left cingulum bundle in youths with ADHD. Sustained attention was related to the GFA values of the left caudate–VLPFC, right caudate-–DLPFC tracts, left caudate–OFC and left cingulum bundle in control youths, and to those of the bilateral caudate–DLPFC tracts, right SLF and right cingulum bundle in youths with ADHD. The GFA values of the left caudate–DLPFC tract and right cingulum bundle correlated with vigilance in control youths, while the microstructural property of the left caudate–DLPFC tract and left cingulum bundle accounts for vigilance in youths with ADHD.
Table 4.
Final model of significant correlates from white matter fibre tracts for the attention dimensions as assessed by the CCPT
| CCPT dimension, β (p value) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Focused attention | Impulsivity | Sustained attention | Vigilance | |||||||||
|
|
|
|
|
|||||||||
| Group, fibre tract | Omission | RT SE | Variability | Detectability | Commission | RT | Response style | Perseveration | RT BC | RT SE BC | RT ISI C | RT SE ISI BC |
| Control | ||||||||||||
| L caudate–VLPFC | — | — | — | — | −1840.31 (0.031) | — | — | — | 0.770 (0.07) | — | — | — |
| R caudate–VLPFC | −126.0 (0.025) | — | — | — | — | — | — | −391.8 (0.002) | — | — | — | — |
| L caudate–DLPFC | — | — | — | −151.6 (0.005) | 1284.68 (0.09) | — | 10.79 (0.07) | −304.0 (0.026) | — | — | — | −2.546 (0.041) |
| R caudate–DLPFC | — | — | — | — | — | 64.74 (0.019) | −17.76 (0.005) | 339.43 (0.007) | 0.599 (0.035) | 1.13 (0.05) | — | — |
| L caudate–OFC | 160.38 (0.08) | — | — | — | — | −107.90 (0.031) | −21.80 (0.028) | 459.12 (0.038) | −1.47 (0.035) | — | — | — |
| R caudate–OFC | — | — | — | — | — | — | 23.66 (0.003) | — | — | — | — | — |
| R SLF | — | — | — | — | — | — | −8.86 (0.010) | 122.67 (0.09) | — | — | — | — |
| L cingulum | — | — | — | — | — | — | — | — | — | −0.890 (0.06) | — | — |
| R cingulum | — | — | — | — | — | — | 11.95 (0.002) | −148.19 (0.07) | — | — | — | 1.91 (0.08) |
| F (p value) | F2,47 = 2.70 (0.08) | — | — | F1,48 = 8.89 (0.005) | F2,47 = 2.47 (0.10) | F2,47 = 3.09 (0.06) | F6,43 = 3.24 (0.010) | F6,43 = 3.05 (0.014) | F3,46 = 3.27 (0.029) | F2,47 = 2.42 (0.010) | — | F2,47 = 2.56 (0.09) |
| R2 | 0.10 | — | — | 0.16 | 0.10 | 0.12 | 0.31 | 0.30 | 0.18 | 0.09 | — | 0.10 |
| ADHD | ||||||||||||
| L caudate–DLPFC | — | — | −407.0 (0.013) | — | — | — | — | — | −0.855 (0.010) | — | — | −3.82 (0.015) |
| R caudate–DLPFC | — | — | — | — | — | — | — | — | — | −1.598 (0.032) | — | — |
| L caudate–OFC | — | — | — | — | — | — | 16.26 (< 0.001) | −345.32 (< 0.001) | — | — | — | — |
| R caudate–OFC | −334.72 (0.002) | −179.81 (0.005) | — | −400.65 (0.033) | — | — | — | — | — | — | — | — |
| R SLF | — | — | — | — | −1388.5 (0.007) | — | — | — | −0.693 (0.036) | — | — | — |
| L cingulum | — | — | — | — | — | −4.43 (0.023) | — | — | — | — | −0.569 (0.022) | — |
| R cingulum | — | — | — | — | — | — | — | — | 0.627 (0.037) | 1.00 (0.08) | — | — |
| F (p value) | F1,48 = 10.73 (0.002) | F1,48 = 8.53 (0.005) | F1,48 = 6.69 (0.013) | F1,48 = 4.83 (0.033) | F1,48 = 8.02 (0.007) | F1,48 = 5.55 (0.023) | F1,48 = 17.96 (0.001) | F1,48 = 17.88 (0.001) | F3,46 = 3.85 (0.015) | F2,47 = 2.62 (0.08) | F1,48 = 5.59 (0.022) | F1,48 = 6.33 (0.015) |
| R2 | 0.18 | 0.15 | 0.12 | 0.09 | 0.14 | 0.10 | 0.27 | 0.27 | 0.20 | 0.10 | 0.10 | 0.12 |
ADHD = attention-deficit/hyperactivity disorder; BC = changed by blocks; CCPT = Conner’s Continuous Performance Test; DLPFC = dorsolateral prefrontal cortex; ISI = interstimulus interval; L = left; OFC = orbitofrontal cortex; RT = reaction time; R = right; SE = standard errors; SLF = superior longitudinal fasciculus; VLPFC = ventrolateral prefrontal cortex.
Discussion
Our findings provided the first data on the correlations between the microstructural property of the right SLF and multiple dimensions of attention in youths with ADHD by taking advantage of the strengths from using DSI tractography analysis, comprehensively investigating several major fibre tracts associated with ADHD, assessing multiple dimensions of attention through clinical interviews and CCPT, using a relatively larger sample size than previous investigations and maintaining a strictly matched case–control study design. We also found that youths with ADHD had lower GFA in the left frontostriatal tracts, bilateral SLF and right cingulum bundle; performed worse in focused attention, sustained attention, inhibition and vigilance; and had altered right SLF microstructural property (which was most significantly associated with inattention symptoms of ADHD) than controls. Moreover, the microstructural property of the target tracts generally was either associated with or had differential patterns of associations with attention performance in youths with ADHD than controls in terms of the fewer attention components involved or the lack of association with any of the attention performance, as detailed in the sections that follow.
Alteration of white matter microstruactual property
Most previous studies investigating white matter tract property in individuals with ADHD focused mainly on the frontostriatal tracts.21,24,25 Our finding of decreased diffusion anisotropy in the left frontostriatal tracts in youths with ADHD is consistent with those of some previous studies using DTI22,31 and DSI,21,24 but inconsistent with those of other studies showing increased diffusion anisotropy in children with ADHD.58,59 Most of the previous studies did not particularly emphasize the difference between the left and right sides of frontostriatal tracts.22,31 In addition, a previous study from our group reported decreased diffusion anisotropy in the bilateral frontostriatal tracts.21 However, the reduced cortical thickness,60 grey matter volume and white matter volume61 in the left prefrontal cortex of individuals with ADHD provided collateral evidence to support the altered frontostriatal tracts on the left side in those with ADHD, as discovered in our study.
Our findings of decreased diffusion anisotropy of the SLF in youths with ADHD lends evidence to support previous studies.31–33 Despite the lack of change of the cingulum bundle property in individuals with ADHD reported in our prior investigation23 and in another study32 with a much larger independent sample and a different DSI tractography approach from our earlier investigation, current studies support decreased diffusion anisotropy of the cingulum bundle in youths with ADHD.31
Correlations between the SLF and ADHD symptoms and attention performance
Because fibre tracts other than the frontostriatal tracts have been less studied, the novelty of the present study is to demonstrate that the right SLF, rather than the frontostriatal tracts, was mostly associated with inattention symptoms in youths with ADHD. The SLF connects areas in the dorsal attention network, parietal lobe and frontal eye field.62 This explains why the function of the SLF has been widely accepted for spatial attention, although only a few human studies on the structural connectivity of the SLF with regards to attention1,29 and working memory63 have been conducted in control youths. Until now, only 1 previous study of ADHD reported a correlation between attention performance and the right SLF in adults with ADHD.34
To the best of our knowledge, this is the first study to demonstrate the association between the SLF and the inattention symptom/attention performance in youths with ADHD, and such a positive association has been reported only in adults with ADHD34 and control children.29 Our findings of different association patterns led us to conclude that the SLF microstructural property was associated with cognitive and behavioural inhibition and sustained attention in youths with ADHD, but with focused attention in controls. Such a finding lends evidence to support the hypothesis that those with ADHD may engage in adaptive processing strategies to compensate for impairments in other brain regions.64
Furthermore, our results are in line with those of studies in healthy participants that demonstrate the importance of the SLF, especially the right side, in attention performance29 and working memory,30 which are considered to be major neuropsychological deficits in individuals with ADHD.65 In addition, our findings raised a noteworthy issue: most studies reported correlations between the frontostriatal tracts and ADHD symptoms without considering the effects of other related white matter tracts.21,24 Fibre tracts with different microstructural property and fibre tracts involved in neuropsychological impairment in patients with ADHD are important candidates to examine in order to determine how these altered pathways contribute to the etiology of ADHD.
Correlations between frontostriatal tracts and attention performance in youths with ADHD
Our finding that the frontostriatal tracts property was associated with the performance of attention tasks in both youths with ADHD and controls is consistent with those of some previous studies.23,24 Although the associations of the caudate–VLPFC tract with all attention dimensions were noted in controls, no such associations were found in youths with ADHD. Our results support other recent evidence of such an association noted only in control children and not in those with ADHD.21,25 One of the explanations for the different association patterns between the ADHD and control groups is that children with ADHD may compensate through the use of other brain regions and tracts when performing attention tasks.64 For instance, our findings demonstrated that the performance of focused attention relied on the frontostriatal tracts connecting the VLPFC, DLPFC and OFC in control youths. However, youths with ADHD mainly relied on the right caudate–OFC. Consequently, we hypothesized that the tracts responsible for the performance of focused attention shifted to the right caudate–OFC owing to alteration in the left frontostriatal tracts in youths with ADHD.
Correlations between the cingulum bundle and attention performance in youths with ADHD
The ACC is a critical region for executive attention, cognitive control and self-regulation,66 while the cingulum bundle is involved in sustained attention36 and executive functions38 in healthy individuals. However, only 1 previous study from our group focused on this issue in patients with ADHD and demonstrated the correlation between the cingulum bundle and intraindividual variability of RT in youths with ADHD.23 The present study provided further evidence of the association between cingulum bundle integrity and a wider range of attention performance, such as inhibition, sustained attention and vigilance, in both controls and youths with ADHD.
Limitations
Our findings should be interpreted in the context of some limitations. First, owing to a cross-sectional study design, we could not determine whether the different diffusion anisotropy of white matter tracts between youths with ADHD and controls reflects the underlying neuropathology of ADHD or the consequences of a compensatory neurodevelopmental process. Second, although 10 youths with ADHD had been treated with methylphenidate before scanning and their last dose of methylphenidate was at least 1 week before the neuropsychological and MRI assessment, long-term effects of medication on microstructural property may still exist. Third, our sensitivity analysis produced almost consistent results after removing the 6 participants in whom a different type of coil was used or after removing the 2 left-handed participants. Therefore, the significant group difference in the mean GFA for some tracts is unlikely to be a chance variation. Finally, we did not correct for multiple comparisons in our analyses given the scarce literature on this topic.67 We presented original statistical results to allow readers to evaluate their importance and significance.7 Research in an independent sample would be necessary to replicate and validate the present findings.
Conclusion
This study provides strong evidence to support altered white matter tract property in the left frontostriatal tracts, bilateral SLF and right cingulum bundle in youths with ADHD. Besides the frontostriatal tracts, our data suggest that the SLF and cingulum bundle are also involved in the core neuropathological underpinnings of ADHD since the property of these tracts was associated with several attention components. In addition, such differential association patterns between youths with ADHD and controls implied the possibility of a compensatory mechanism. We suggest further studies to examine other fibre tracts theoretically associated with ADHD neuropathology and that such studies use different tasks to assess more dimensions of neuropsychological functions, conduct subgroup analyses if the sample size is large enough and collect longitudinal data.
Acknowledgements
This work was supported by the National Science Council (NSC99-2627-B-002-015, NSC100-2627-B-002-014, NSC101-2627-B-002-002, NSC101-2321-B-002-079) and the National Taiwan University Hospital (NTUH101-S1910), Taiwan. The manuscript preparation was supported by the National Health Research Institute (NHRI-EX102-0008PI) and Ministry of Economic Affairs (101-EC-17-A-19-S1-175), Taiwan.
Footnotes
Competing interests: None declared.
Contributors: H.-L. Chiang and S.S.-F. Gau designed the study. W.-Y.I. Tseng and S.S.-F. Gau acquired the data, which H.-L. Chiang, Y.-J. Chen, Y.-C. Lo and S.S.-F. Gau analyzed. H.-L. Chiang, Y.-J. Chen and S.S.F. Gau wrote the article, which all authors reviewed and approved for publication.
Clinical trial registration: This work was registered before study implementation (ClinicalTrials.gov number, NCT01677793, NCT00916851). The authors assert that all procedures contributing to this work comply with the ethical standards of relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975 as revised in 2008.
References
- 1.Ge H, Yin X, Xu J, et al. Fiber pathways of attention subnetworks revealed with tract-based spatial statistics (TBSS) and probabilistic tractography. PLoS ONE. 2013;8:e78831. doi: 10.1371/journal.pone.0078831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egeland J, Kovalik-Gran I. Measuring several aspects of attention in one test: the factor structure of conners’s continuous performance test. J Atten Disord. 2010;13:339–46. doi: 10.1177/1087054708323019. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JN, Erkanli A, Conners CK, et al. Relations between Continuous Performance Test performance measures and ADHD behaviors. J Abnorm Child Psychol. 2003;31:543–54. doi: 10.1023/a:1025405216339. [DOI] [PubMed] [Google Scholar]
- 4.Shang CY, Gau SS. Association between the DAT1 gene and spatial working memory in attention deficit hyperactivity disorder. Int J Neuropsychopharmacol. 2014;17:9–21. doi: 10.1017/S1461145713000783. [DOI] [PubMed] [Google Scholar]
- 5.Shang CY, Gau SS. Visual memory as a potential cognitive endophenotype of attention deficit hyperactivity disorder. Psychol Med. 2011;41:2603–14. doi: 10.1017/S0033291711000857. [DOI] [PubMed] [Google Scholar]
- 6.Manor I, Tyano S, Eisenberg J, et al. The short DRD4 repeats confer risk to attention deficit hyperactivity disorder in a family-based design and impair performance on a continuous performance test (TOVA) Mol Psychiatry. 2002;7:790–4. doi: 10.1038/sj.mp.4001078. [DOI] [PubMed] [Google Scholar]
- 7.Bellgrove MA, Hawi Z, Lowe N, et al. DRD4 gene variants and sustained attention in attention deficit hyperactivity disorder (ADHD): effects of associated alleles at the VNTR and −521 SNP. Am J Med Genet B Neuropsychiatr Genet. 2005;136B:81–6. doi: 10.1002/ajmg.b.30193. [DOI] [PubMed] [Google Scholar]
- 8.Johnson KA, Kelly SP, Robertson IH, et al. Absence of the 7-repeat variant of the DRD4 VNTR is associated with drifting sustained attention in children with ADHD but not in controls. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:927–37. doi: 10.1002/ajmg.b.30718. [DOI] [PubMed] [Google Scholar]
- 9.Loo SK, Specter E, Smolen A, et al. Functional effects of the DAT1 polymorphism on EEG measures in ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42:986–93. doi: 10.1097/01.CHI.0000046890.27264.88. [DOI] [PubMed] [Google Scholar]
- 10.Bellgrove MA, Hawi Z, Kirley A, et al. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005;43:1847–57. doi: 10.1016/j.neuropsychologia.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Kim B, Koo MS, Jun JY, et al. Association between dopamine D4 receptor gene polymorphism and scores on a continuous performance test in Korean children with attention deficit hyperactivity disorder. Psychiatry Investig. 2009;6:216–21. doi: 10.4306/pi.2009.6.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song DH, Jhung K, Song J, et al. The 1287 G/A polymorphism of the norepinephrine transporter gene (NET) is involved in commission errors in Korean children with attention deficit hyperactivity disorder. Behav Brain Funct. 2011;7:12. doi: 10.1186/1744-9081-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. 2010;35:278–300. doi: 10.1038/npp.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durston S, Davidson MC, Thomas KM, et al. Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. Neuroimage. 2003;20:2135–41. doi: 10.1016/j.neuroimage.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1285–92. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Casey BJ, Epstein JN, Buhle J, et al. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. Am J Psychiatry. 2007;164:1729–36. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- 17.Williams ZM, Bush G, Rauch SL, et al. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci. 2004;7:1370–5. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- 18.Shaw P, Lerch J, Greenstein D, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:540–9. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 19.Rubia K, Smith AB, Halari R, et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am J Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- 20.Cubillo A, Halari R, Smith A, et al. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with attention deficit hyperactivity disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012;48:194–215. doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Wu YH, Gau SS, Lo YC, et al. White matter tract integrity of frontostriatal circuit in attention deficit hyperactivity disorder: association with attention performance and symptoms. Hum Brain Mapp. 2014;35:199–212. doi: 10.1002/hbm.22169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamm L, Barnea-Goraly N, Reiss AL. Diffusion tensor imaging reveals white matter abnormalities in attention-deficit/hyperactivity disorder. Psychiatry Res. 2012;202:150–4. doi: 10.1016/j.pscychresns.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin HY, Gau SS, Huang-Gu SL, et al. Neural substrates of behavioral variability in attention deficit hyperactivity disorder: based on ex-Gaussian reaction time distribution and diffusion spectrum imaging tractography. Psychol Med. 2013;44:1751–64. doi: 10.1017/S0033291713001955. [DOI] [PubMed] [Google Scholar]
- 24.Shang CY, Wu YH, Gau SS, et al. Disturbed microstructural integrity of the frontostriatal fiber pathways and executive dysfunction in children with attention deficit hyperactivity disorder. Psychol Med. 2013;43:1093–107. doi: 10.1017/S0033291712001869. [DOI] [PubMed] [Google Scholar]
- 25.De Zeeuw P, Mandl RC, Hulshoff Pol HE, et al. Decreased frontostriatal microstructural organization in attention deficit/hyperactivity disorder. Hum Brain Mapp. 2012;33:1941–51. doi: 10.1002/hbm.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konrad A, Dielentheis TF, El Masri D, et al. White matter abnormalities and their impact on attentional performance in adult attention-deficit/hyperactivity disorder. Eur Arch Psychiatry Clin Neurosci. 2012;262:351–60. doi: 10.1007/s00406-011-0251-1. [DOI] [PubMed] [Google Scholar]
- 27.Thakral PP, Slotnick SD. The role of parietal cortex during sustained visual spatial attention. Brain Res. 2009;1302:157–66. doi: 10.1016/j.brainres.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 28.Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–69. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- 29.Klarborg B, Skak Madsen K, Vestergaard M, et al. Sustained attention is associated with right superior longitudinal fasciculus and superior parietal white matter microstructure in children. Hum Brain Mapp. 2013;34:3216–32. doi: 10.1002/hbm.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters BD, Szeszko PR, Radua J, et al. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr Bull. 2012;38:1308–17. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavuluri MN, Yang S, Kamineni K, et al. Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65:586–93. doi: 10.1016/j.biopsych.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamilton LS, Levitt JG, O’Neill J, et al. Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuroreport. 2008;19:1705–8. doi: 10.1097/WNR.0b013e3283174415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawrence KE, Levitt JG, Loo SK, et al. White matter microstructure in subjects with attention-deficit/hyperactivity disorder and their siblings. J Am Acad Child Adolesc Psychiatry. 2013;52:431–40 e434. doi: 10.1016/j.jaac.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konrad A, Dielentheis TF, El Masri D, et al. Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. Eur J Neurosci. 2010;31:912–9. doi: 10.1111/j.1460-9568.2010.07110.x. [DOI] [PubMed] [Google Scholar]
- 35.Makris N, Buka SL, Biederman J, et al. Attention and executive systems abnormalities in adults with childhood ADHD: A DT-MRI study of connections. Cereb Cortex. 2008;18:1210–20. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi M, Iwamoto K, Fukatsu H, et al. White matter microstructure of the cingulum and cerebellar peduncle is related to sustained attention and working memory: a diffusion tensor imaging study. Neurosci Lett. 2010;477:72–6. doi: 10.1016/j.neulet.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 37.Lawes IN, Barrick TR, Murugam V, et al. Atlas-based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. Neuroimage. 2008;39:62–79. doi: 10.1016/j.neuroimage.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 38.Peters BD, Ikuta T, Derosse P, et al. Age-related differences in white matter tract microstructure are associated with cognitive performance from childhood to adulthood. Biol Psychiatry. 2014;75:248–56. doi: 10.1016/j.biopsych.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng Z, Shemmassian S, Wijekoon C, et al. DTI correlates of distinct cognitive impairments in Parkinson’s disease. Hum Brain Mapp. 2014;44:1751–64. doi: 10.1002/hbm.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kantarci K, Senjem ML, Avula R, et al. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 2011;77:26–34. doi: 10.1212/WNL.0b013e31822313dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wedeen VJ, Wang RP, Schmahmann JD, et al. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41:1267–77. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 42.Wedeen VJ, Hagmann P, Tseng WY, et al. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med. 2005;54:1377–86. doi: 10.1002/mrm.20642. [DOI] [PubMed] [Google Scholar]
- 43.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–54. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 44.Lin CP, Wedeen VJ, Chen JH, et al. Validation of diffusion spectrum magnetic resonance imaging with manganese-enhanced rat optic tracts and ex vivo phantoms. Neuroimage. 2003;19:482–95. doi: 10.1016/s1053-8119(03)00154-x. [DOI] [PubMed] [Google Scholar]
- 45.Tuch DS. Q-ball imaging. Magn Reson Med. 2004;52:1358–72. doi: 10.1002/mrm.20279. [DOI] [PubMed] [Google Scholar]
- 46.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–19. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 47.Gau SS, Chong MY, Chen TH, et al. A 3-year panel study of mental disorders among adolescents in Taiwan. Am J Psychiatry. 2005;162:1344–50. doi: 10.1176/appi.ajp.162.7.1344. [DOI] [PubMed] [Google Scholar]
- 48.Gau SS, Chiang HL. Association between early attention-deficit/hyperactivity symptoms and current verbal and visuo-spatial short-term memory. Res Dev Disabil. 2013;34:710–20. doi: 10.1016/j.ridd.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Gau SS, Huang WL. Rapid visual information processing as a cognitive endophenotype of attention deficit hyperactivity disorder. Psychol Med. 2014;44:435–46. doi: 10.1017/S0033291713000640. [DOI] [PubMed] [Google Scholar]
- 50.Conners CK. Staff. M. Conners’ Continuous Performance Test II: computer program for Windows technical guide and software manual. North Tonwanda, NY: Mutli-Health Systems; 2000. [Google Scholar]
- 51.Egeland J, Kovalik-Gran I. Validity of the factor structure of Conners’ CPT. J Atten Disord. 2010;13:347–57. doi: 10.1177/1087054709332477. [DOI] [PubMed] [Google Scholar]
- 52.Reese TG, Heid O, Weisskoff RM, et al. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–82. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 53.Kuo LW, Chen JH, Wedeen VJ, et al. Optimization of diffusion spectrum imaging and q-ball imaging on clinical MRI system. Neuroimage. 2008;41:7–18. doi: 10.1016/j.neuroimage.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 54.Callaghan PT, Coy A, MacGowan D, et al. Diffraction-like effects in NMR diffusion studies of fluids in porous solids. [accessed 2015 Apr. 7];Nature. 1991 351:467–9. Available: www.nature.com/nature/journal/v351/n6326/abs/351467a0.html. [Google Scholar]
- 55.Yeh FC, Wedeen VJ, Tseng WY. Estimation of fiber orientation and spin density distribution by diffusion deconvolution. Neuroimage. 2011;55:1054–62. doi: 10.1016/j.neuroimage.2010.11.087. [DOI] [PubMed] [Google Scholar]
- 56.Hsu YC, Hsu CH, Tseng WY. A large deformation diffeomorphic metric mapping solution for diffusion spectrum imaging datasets. Neuroimage. 2012;63:818–34. doi: 10.1016/j.neuroimage.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 57.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 58.Davenport ND, Karatekin C, White T, et al. Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatry Res. 2010;181:193–8. doi: 10.1016/j.pscychresns.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silk TJ, Vance A, Rinehart N, et al. White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum Brain Mapp. 2009;30:2757–65. doi: 10.1002/hbm.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaw P, Lalonde F, Lepage C, et al. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2009;66:888–96. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mostofsky SH, Cooper KL, Kates WR, et al. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2002;52:785–94. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- 62.Luo L, Rodriguez E, Jerbi K, et al. Ten years of Nature Reviews Neuroscience: insights from the highly cited. Nat Rev Neurosci. 2010;11:718–26. doi: 10.1038/nrn2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vestergaard M, Madsen KS, Baare WF, et al. White matter microstructure in superior longitudinal fasciculus associated with spatial working memory performance in children. J Cogn Neurosci. 2011;23:2135–46. doi: 10.1162/jocn.2010.21592. [DOI] [PubMed] [Google Scholar]
- 64.Fassbender C, Schweitzer JB. Is there evidence for neural compensation in attention deficit hyperactivity disorder? A review of the functional neuroimaging literature. Clin Psychol Rev. 2006;26:445–65. doi: 10.1016/j.cpr.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willcutt EG, Doyle AE, Nigg JT, et al. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–46. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 66.Fjell AM, Walhovd KB, Brown TT, et al. Multimodal imaging of the self-regulating developing brain. Proc Natl Acad Sci U S A. 2012;109:19620–5. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Veazie PJ. When to combine hypotheses and adjust for multiple tests. Health Serv Res. 2006;41:804–18. doi: 10.1111/j.1475-6773.2006.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]