Abstract
Background
Millions of children are born to parents affected by major psychoses. Cognitive dysfunctions seen in patients are already detectable in these children. In parallel, childhood maltreatment increases the risk of adult psychoses through unknown mechanisms. We investigated whether high-risk offspring exposed to abuse/neglect displayed more cognitive precursors of adult psychoses in childhood and adolescence than nonexposed offspring.
Methods
We used a stepwise selection strategy from a 25-year follow-up of 48 densely affected kindreds including 1500 adults (405 patients with schizophrenia or bipolar disorder) to select high-risk offspring aged 6–22 years for inclusion in our study. All offspring were assessed for childhood trauma from direct interviews with the offspring, parents and relatives and from the review of lifetime medical records of parents and children and administered a neuropsychological battery including IQ and 4 of the most impaired neuropsychological domains in psychoses.
Results
Our study included 66 high-risk offspring. Those who were exposed to abuse/neglect had significantly lower IQ (effect size [ES] = 0.61) than nonexposed offspring and displayed poorer cognitive performance in visual episodic memory (ES = 0.67) and in executive functions of initiation (ES = 1.01). Moreover, exposed offspring presented more combinations of cognitive deficits that were associated with lower Global Assessment of Functioning scores.
Limitations
Exposure to abuse/neglect was not assessed in the control group, thus the study could not test whether the effect of childhood maltreatment occured only in a high-risk setting and not in the general population.
Conclusion
In high-risk youths, maltreatment in childhood/adolescence may negatively impact cognitive domains known to be impaired in adults with psychoses, suggesting an early mediating effect in the association between abuse/neglect and adult psychoses. This finding provides a target for future developmental and preventive research.
Introduction
Exposure to abuse and neglect in childhood increases the risk of later occurrence of schizophrenia1–4 and bipolar disorder (BD).5–7 Child abuse may antedate psychotic experience in youths,3 and at least 40% of patients with psychosis retrospectively report personal exposure to abuse or neglect in childhood.2,5,8 Nevertheless, the mechanisms underlying this association are not well understood.1,2,9
Cognitive dysfunctions are central to schizophrenia and BD,10–13 and recent data suggest that the cognitive decline begins in childhood.14,15 The cognitive impairments that are typically shared by patients with schizophrenia and BD12,16,17 have a genetic basis,18,19 which does not preclude environmental influences from further impacting the developmental trajectory. Abuse and neglect are known to have a negative influence on cognitive functioning in community samples of healthy adults20,21 and children/adolescents22,23 as well as in patients with psychosis.8,24,25
Congruent with other studies,26–28 we have reported that children from densely affected multigenerational families who had a parent affected by schizophrenia or BD had full-scale IQ impairments as well as deficits in specific cognitive domains, such as visual and verbal episodic memory, working memory and executive functions of initiation.12,16 These domains are among the most impaired in adult patients and are consistently found to be associated with schizophrenia and BD.19,29,30 Based on 25 years of findings, we recently reported that phenotype, endophenotypes and genetic findings in these families were very similar to results in sporadic samples,15 supporting the findings of others that the familial and nonfamilial forms of illness share mechanisms.31,32
We hypothesized that children and adolescents at high risk for major psychoses who were exposed to childhood maltreatment would have greater cognitive dysfunctions that are known to be later associated with psychoses than nonexposed offspring. We focused on IQ functioning and 4 of the most impaired cognitive domains in psychoses: visual and verbal episodic memory, executive functions of initiation and working memory.12 A negative impact of childhood maltreatment on cognitive precursors of psychoses would inform on developmental mechanisms of the disease and could translate into prevention research.
Methods
Stepwise sampling strategy of offspring
The ascertainment of the sample of multigenerational families is described in detail in earlier reports15 and in Appendix 1, available at jpn.ca. We targeted all the multigenerational families densely affected by schizophrenia or BD in the catchment area of eastern Quebec, Canada. For the present study, we selected participants among 48 multigenerational families15 comprising an average number of 6 members affected by schizophrenia or BD per family. The inclusion criteria were having a parent with a definite DSM-IV diagnosis of schizophrenia or BD and having had a neuropsychological evaluation before the age of 23 years. The exclusion criteria were a diagnosis of a DSM-IV psychotic disorder, BD or recurrent major depression, and brain or metabolic disorders known to cause neuropsychological impairments.
Healthy control sample
Control participants were recruited among the same population through advertisements. The exclusion criteria were the same as those for the offspring, with the addition of any personal lifetime DSM Axis I diagnosis or a positive family history of schizophrenia- or BD-spectrum disorders. We obtained written consent from all participants aged 14–22 years as well as from the parents of participants younger than 18 years. The ethics committee of our university-affiliated mental health institute and the Ethics Committee on Health Research of Laval University approved our study.
We did not assess exposure to abuse and neglect in the control group because the present study did not aim to test a gene × environment interaction (i.e., we did not intend to test whether the effect of childhood maltreatment would occur only in a high-risk setting and not in the general population).
Measurements
Adverse life events
Our goal was to focus on interpersonal trauma (abuse and neglect), considering its relevance in the development of psychosis.33,34 We also gathered information on other childhood stressful events. We compiled from known instruments35,36 a list of 30 previously tested items assessing trauma and other life events to create the childhood adverse life events chart (CALEC; Appendix 1, Tables S1 and S2). Items 1–9 determined the presence of traumatic events (abuse and neglect), and items 10–30 determined the presence of other stressful events. Information on the CALEC and on the comparison of items with other instruments is provided in the Appendix. Categories of abuse and neglect were comparable to those reported in a meta-analysis on childhood adversities1 and in established instruments.35,36
In contrast to retrospective reporting of childhood trauma in adulthood, the CALEC involved an expert rating of exposure to trauma based on all available lifetime information collected throughout the longitudinal follow-up (further details are provided in Appendix 1). The chart was rated blind by a clinical PhD psychologist specialized in childhood trauma (N.B.). Two other researchers independently reviewed the lifetime information. The interrater agreement was satisfactory, with 93% agreement on presence or absence of childhood maltreatment. Raters also obtained satisfactory agreement for the different types of abuse and neglect, with κ scores ranging from 0.58 (moderate) to 1.0 (perfect), with a median of 0.83.
Neuropsychological assessments
We selected the cognitive domains that have been previously reported as impaired (p < 0.05) in high-risk offspring compared with healthy controls12 (i.e., visual episodic memory, verbal episodic memory, working memory, executive functions of initiation) in addition to full-scale IQ. Measures are detailed in Appendix 1. Assessments were made blind by a certified psychologist or by PhD students supervised by a senior neuropsychologist (E.G.).
Psychiatric and clinical ascertainment
A best-estimate lifetime diagnostic procedure based on multiple sources of information was administered among the offspring, their parents and adult relatives.37 This procedure involved reviewing all available medical records, family interviews and a semistructured interview. We administered the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS)38 to the parents of children younger than 18 years in the presence of the child or the Structured Clinical Interview for DSM Disorders (SCID)39 to participants aged 18 years or older. Based on the available lifetime information, we rated global functioning using the Global Assessment of Functioning (GAF).40 Lifetime substance abuse or dependence was also coded using all available lifetime information.
Statistical analysis
Data were analyzed using SPSS version 17.0. We used a multivariate analysis of covariance (MANCOVA) comparing cognitive z scores to evaluate whether the exposed and non-exposed offspring differed on global IQ and on the set of the 4 cognitive domains retained (visual and verbal episodic memory, working memory, executive functions of initiation). To verify whether socioeconomic status and level of other stressful events had an effect on the association between childhood maltreatment and cognitive performance, we performed a MANCOVA with these independent variables as covariates. Subsequent ANCOVAs were performed to evaluate group differences on each cognitive domain separately. All analyses were controlled for age and sex. Given the 4 cognitive domains, we corrected for multiple analyses using Bonferroni correction by dividing the significance level of 0.05 by 4. The significance level was then fixed at p = 0.0125.
Given the presence of siblings (n = 19 sibships), we accounted for the nonindependence of observations within the same sibship by means of a multilevel regression analysis with the MIXED procedure of SAS version 9.3 (SAS Institute Inc.). The hierarchical structure of the data are modelled according to a random effect. We obtained degrees of freedom using the Kenward–Roger method,41 available with the option DDFM = KR in the MODEL statement of the MIXED procedure.
We calculated effect sizes (ES) using the difference of adjusted means (LSMeans) between offspring exposed to trauma and nonexposed offspring standardized by a pooled standard deviation (SD). The pooled SD was obtained by dividing the standard error of the difference of LSMeans by the square root of the following equation:
In complement to the analyses on continuous cognitive variables, we also compared exposed and nonexposed offspring using a categorical analysis in which a cognitive deficit was defined as a score below the 16th percentile, a cut-off often used in clinical neuropsychology.42,43 We used a χ2 analysis to make the comparison.
Results
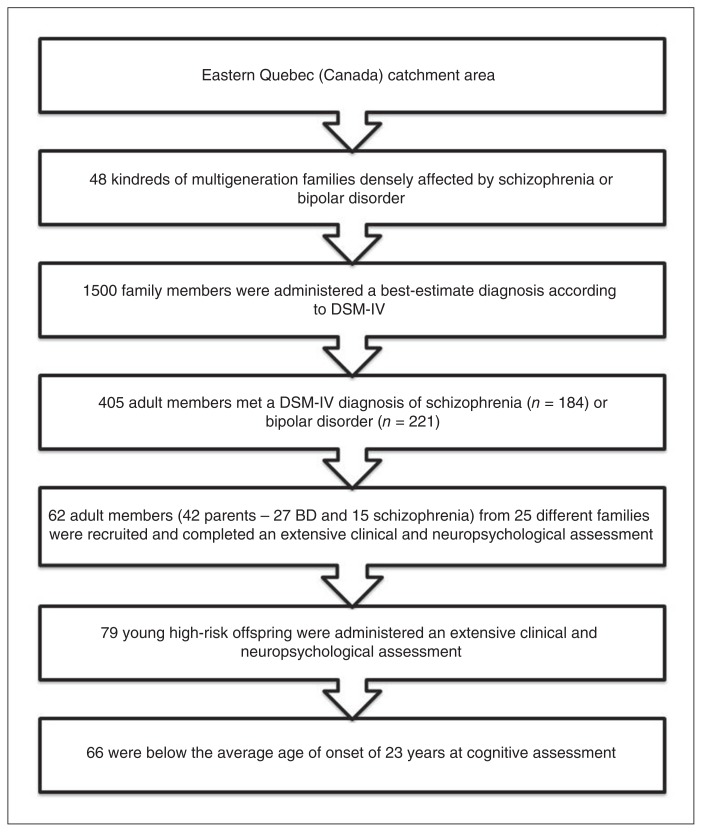
Our stepwise selection strategy yielded a high-risk sample of 66 offspring of a parent with schizophrenia (n = 23) or BD (n = 43; Fig. 1). The sample comprised 23 singletons and 19 sibships (14 sibships of 2 siblings and 5 of 3 siblings). The mean age of participants at cognitive assessment was 17.2 ± 4.10 years, and 49% were male. The sample of healthy controls whose cognitive performance we used to convert the neuropsychological test scores of high-risk offspring to z scores comprised 170 individuals balanced for age and sex (mean age 16.1 ± 4.51 yr, 49% male). Thirty of the 66 offspring (46%) were exposed to childhood maltreatment, as indexed by the CALEC, with an average of 2.03 ± 1.00 different types of abuse or neglect. Detailed frequencies are provided in Appendix 1, Table S2. Exposed offspring did not differ from nonexposed offspring in sex and age at the time of cognitive assessment except that the nonexposed offspring were from families with higher socioeconomic status (Blishen index, see Appendix 1, Table S3).
Fig. 1.
Stepwise sampling approach to narrow down the early disease mechanisms in this high-risk sample. Starting with a 20-year follow-up of 48 densely affected multigenerational families (1500 clinically characterized adult members), we identified 405 members affected by a DSM-IV schizophrenia or bipolar disorder (BD). We included 66 high-risk offspring aged 7–22 years who met our inclusion criteria.
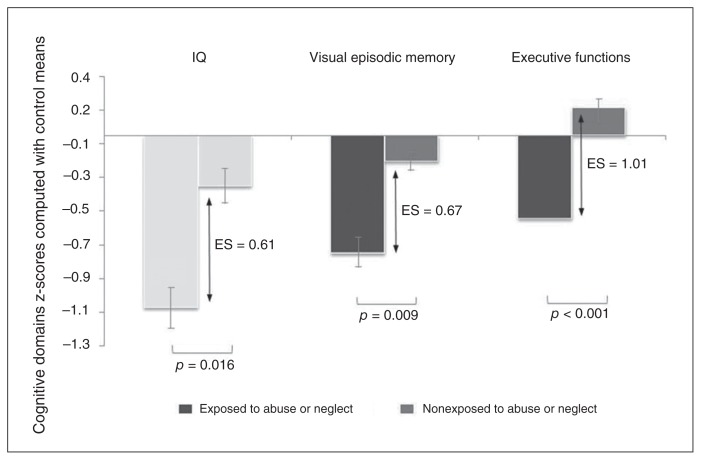
Offspring exposed to abuse/neglect had lower cognitive performance than nonexposed offspring (MANCOVA; Wilks’ λ = 0.63, F5,57 = 6.74, p < 0.001) even when controlling for socioeconomic status (Wilks’ λ = 0.66, F5,56 = 5.91, p < 0.001) or other stressful events (Wilks’ λ = 0.71, F5,56 = 4.70, p = 0.001; Table 1). Post hoc ANCOVAs (Table 1 and Fig. 2) revealed a lower global IQ (p = 0.016) in exposed than in nonexposed offspring (mean 93.17 ± 11.24 v. 101.28 ± 13.70). With regards to the specific cognitive domains, exposed offspring had lower performance than nonexposed offspring in visual episodic memory (p = 0.009) and executive functions of initiation (p < 0.001), but not in verbal memory or working memory. This result was congruent with the categorical analysis showing that 67% of exposed offspring presented visual episodic memory impairments, whereas only 28% of nonexposed offspring had such impairments (odds ratio [OR] 5.2; Appendix 1, Table S4). The categorical analysis with executive functions yielded an OR of 4.67 (Appendix 1, Table S4). Note that offspring exposed to abuse or neglect were similar to nonexposed offspring in terms of sociodemographic and clinical characteristics, including substance abuse or dependence and nonpsychotic DSM diagnoses (Appendix, Table S3). Group differences remained when controlling for confounding factors: when IQ was entered as a covariate instead of a dependent variable, the effect of trauma on visual episodic memory (F1,59.3 = 4.31, p = 0.042) and executive functions (F1,33 = 9.08, p = 0.005) remained. When socioeconomic status was entered as a covariate, the effect of trauma on visual episodic memory (F1,60.7 = 6.71, p = 0.012), executive functions (F1,30.7 = 14.62, p < 0.001) and IQ (F1,60.3 = 4.59, p = 0.036) remained significant. When substance abuse or dependence was entered as a covariate, the effect of trauma on visual episodic memory (F1,23 = 6.56, p = 0.018), executive functions (F1,32.6 = 15.87, p < 0.001) and IQ (F1,57.7 = 6.64, p = 0.013 remained significant. When nonpsychotic DSM diagnoses were entered as covariates, the effect of trauma on visual episodic memory (F1,61 = 7.94, p = 0.007), executive functions (F1,59.6 = 17.50, p < 0.001) and IQ (F1,33.6 = 4.94, p = 0.033) remained significant.
Table 1.
ANCOVAs comparing the cognitive functioning of offspring exposed and nonexposed to abuse or neglect*
| Group, adjusted mean ± SE† | ANCOVA‡ | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Domain | Nonexposed, n = 36 | Exposed, n = 30 | Statistic | p value | ES |
| IQ | −0.301 ± 0.198 | −1.021 ± 0.224 | F1,60.3 = 6.15 | 0.016 | 0.61 |
| Visual episodic memory | −0.153 ± 0.142 | −0.702 ± 0.161 | F1,61.9 = 7.26 | 0.009 | 0.67 |
| Verbal episodic memory | −0.533 ± 0.190 | −0.964 ± 0.209 | F1,62.0 = 2.30 | 0.14 | 0.37 |
| Executive functions (initiation) | 0.164 ± 0.108 | −0.494 ± 0.123 | F1,34.4 = 16.70 | < 0.001 | 1.01 |
| Working memory | −0.014 ± 0.107 | −0.235 ± 0.121 | F1,45.8 = 1.86 | 0.180 | 0.34 |
ANCOVA = analysis of covariance; ES = effect size; MANCOVA = multivariate analysis of covariance; SE = standard error.
Offspring exposed to abuse/neglect had lower cognitive performance than nonexposed offspring (MANCOVA; Wilks’ λ = 0.63, F5,57 = 6.74, p < 0.001).
The offsprings’ raw scores on each neuropsychological test were converted to z scores based on the cognitive performance of 170 young healthy controls. Means were adjusted for age and sex.
To account for possible correlation among participants within the same sibship, a multilevel model was carried out using the MIXED procedure of SAS version 9.1.3 (SAS Institute Inc.). Sibships nested in the group were used as the second level and were modelled according to a random effect. Degrees of freedom were obtained using the Kenward–Roger method.41
Fig. 2.
Effect of childhood maltreatment on cognitive functioning in childhood/adolescence. Cognitive performance on full-scale IQ (Wechsler Intelligence Scale for Children-III/Wechsler Adult Intelligence Scale-III), visual episodic memory (Rey Complex Figure Test) and executive functions of initiation (Verbal Fluency Test) in the exposed (n = 30) and nonexposed (n = 36) offspring. Measures are detailed in Appendix 1, available at jpn.ca. The analyses of covariance compared the cognitive z scores between the exposed and nonexposed offspring (Table 1). The z scores were calculated based on the cognitive performance of 170 young healthy controls balanced for age and sex. Error bars: standard errors. Analyses controlled for age and sex. Effect sizes (ES) were calculated using the difference of adjusted means between exposed and nonexposed offspring, standardized by a pooled standard deviation.
Notably, the offspring of parents with schizophrenia and BD had a similar overall rate of exposure to abuse/neglect (p = 0.19, Appendix 1, Table S5), and exposure had a similar effect on cognition in both groups (Appendix 1, Table S6).
Visual episodic memory and executive functions of initiation were correlated to a degree (r = 0.46, p < 0.001), but exposure had a separate effect on each function. We ran multiple regressions showing that abuse/neglect remained associated with visual memory (p = 0.034, Appendix 1, Table S7) when executive functions were included in the model and that, conversely, the association remained with executive functions when visual memory was entered in the model (p < 0.001, Appendix 1, Table S8).
We next reanalyzed our data in girls and in boys separately; results of the association between childhood maltreatment and poorer cognitive performance remained consistent in all analyses (Appendix 1, Tables S9 and S10).
We finally analyzed the distribution of cognitive deficits (absence, 1 deficit, ≥ 2 deficits) in the exposed and nonexposed offspring; our findings suggested a higher occurrence of combined deficits in the exposed offspring (p < 0.001, Appendix 1, Table S11 and S12). In a second step, we looked at differences in the GAF functional severity scores among the 3 groups of offspring (nonexposed to maltreatment, exposed to maltreatment with ≤ 1 cognitive deficit, exposed to maltreatment with a combination of deficits) using an ANCOVA (F5,56 = 5.91, p < 0.001). Post hoc analyses showed that the exposed offspring with combined deficits differed from nonexposed offspring (GAF of 56.7 v. 72; Appendix 1, Table S13).
Discussion
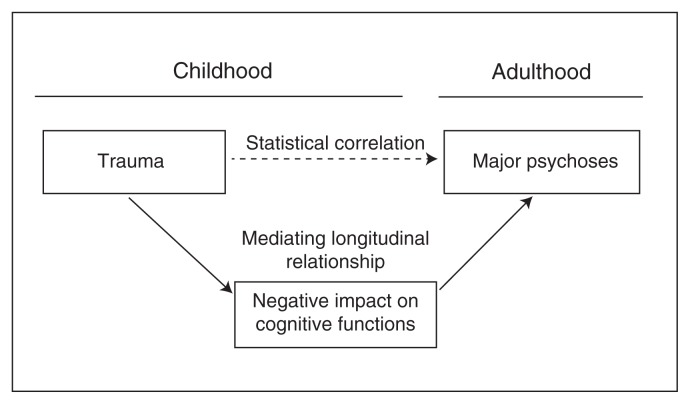
To our knowledge, our study is the first to investigate the effect of childhood abuse and neglect on IQ and on cognitive precursors of adult psychoses in children and adolescents at high genetic risk for psychoses. Exposed offspring displayed poorer cognitive performance than nonexposed offspring in terms of full-scale IQ, visual episodic memory and executive functions of initiation. Offspring exposed to childhood maltreatment also expressed an aggregation of cognitive deficits that was associated with early impairments in social functioning. Our findings add to the emerging evidence that psychoses may be the adult end-point of a declining cognitive trajectory starting in childhood.9,14 Our results suggest a mediating developmental mechanism for the established association between childhood maltreatment and later psychoses.1,25 They may also relate to recent findings that the offspring of parents with affective psychosis who transitioned to psychosis were more likely to have been exposed to abuse than those who did not transition to psychosis.7 In the present sample of high-risk offspring, abuse and neglect in childhood would impact the cognitive functions that could henceforth entail a progressive deviating trajectory toward later disease onset (Fig. 3). This has clinical implications, as every year 6 million children in the United States alone are involved in reports to child protective services owing to abuse and neglect.44 Our data raise the public health challenge of identifying among exposed children with a family history of psychosis those who would transition to nonaffective or affective psychosis. A recent meta-analysis has suggested that psychosis incidence would be reduced by up to 33% by eliminating childhood adversity and trauma.1
Fig. 3.
Putative risk model on the mediation effect of childhood maltreatment on cognitive deficits in children at risk of adult psychoses. In this model, childhood trauma would impact the developmental trajectory of visual episodic memory and executive functions, which would induce a deviation of the neurodevelopmental trajectory toward adult disease onset.
Our findings are also compatible with recent data suggesting that the phenotypic expression of psychopathology may be strongly influenced by exposure to maltreatment, that some neurobiological abnormalities identified in different psychopathologies may be limited to patients with a history of childhood maltreatment, and that the psychopathological effects of maltreatment may not appear immediately around the time of exposure but may manifest more subtly throughout development.45
Our observation that exposure to abuse and neglect may impact certain cognitive domains, such as visual memory and executive functions of initiation, in contrast to others is not unexpected for several reasons. First, our finding of the absence of association between trauma and verbal episodic memory and working memory appears congruent with those of previous reports in the general population.46,47 Second, it has been described previously that different cognitive domains may harbour distinct developmental trajectories.48 Different pathophysiological mechanisms may consequently underly deficits in different domains.49 Correspondingly, we previously reported a dynamic changing course of visual memory impairments from childhood until adulthood that contrasted with the more stable or static course of verbal memory in high-risk offspring.50
Our observation that exposure in the young offspring of parents with schizophrenia or BD would induce similar cognitive dysfunctions is consistent with observations that these 2 adult disorders share many genetic, phenotypic and endophenotypic characteristics15,51 and that the young offspring of parents with schizophrenia or BD display common cognitive impairments.9,16,27
The offspring of parents with schizophrenia or BD are likely to carry a genetic vulnerability expressed in greater biological sensitivity to stress,52 thus sensitizing them to abuse and neglect. They would pay a heavier developmental toll at 2 levels: they are likely to carry a genetic vulnerability and are also likely to be exposed to trauma, which are both risk factors for serious and recurring mental illness.52 We observed a rate of abuse and neglect of 46% in the affected families, whereas an approximate ratio of 30% has been reported in the general population.53,54 When we broke down the types of maltreatment, we noted that the offspring were not exposed to higher rates of sexual (11%) or physical abuse (20%) than the general population (5%–11% and 18%–19% respectively).53,54
We observed that some of the exposed offspring had a combination of cognitive deficits, whereas others had either average cognition or had only 1 deficit (Appendix 1, Table S8), suggesting interindividual differences in vulnerability among the children and adolescents born to affected parents. Regarding the neurobiological mechanisms, previous studies have suggested that childhood maltreatment may result in the overactivation of the hypothalamic–pituitary–adrenal (HPA) axis, which coordinates stress response, resulting in an increased sensitivity to stress.45 This HPA overreactivity might then affect brain structures such as the hippocampus at critical moments of brain development through glucocorticoid receptors that can diminish the hippocampus neurogenesis,45 which in turn would provoke the expression of the cognitive dysfunctions observed in our study.
Our observation that the exposed offspring with a combination of cognitive deficits were more likely to have a poorer global functioning deserves special attention. These youths born to affected parents and exposed to abuse/neglect should probably be prioritized for multimodal interventions to alleviate their difficulties of adaptation and possibly reduce the risk of a poorer adult outcome. For instance, cognitive remediation targeting the negative cognitive effect of abuse/neglect exposure warrants clinical research. Recent studies have suggested a positive effect of cognitive remediation in adolescents at risk for or affected by psychosis.55,56 Research should also consider whether psychotherapeutic interventions, which are specifically designed for abused and neglected children57 and which already address trauma consequences, such as dissociation, disorganized attachment and distorted attributions/mentalization, could normalize the cognitive risk trajectory.
Limitations
Our study presents strengths and weaknesses. First, our goal was to compare in a large high-risk sample the offspring exposed to abuse and neglect with nonexposed high-risk offspring, and we found large effect differences between the 2 groups. A limitation, however, was that we did not aim to test a gene × environment interaction (i.e., we did not intend to test whether such an effect of maltreatment would be greater in a high-risk setting than in the general population). For testing such an interaction, we would have needed abuse/neglect measurements from a comparative sample of controls. Studies of gene × environment interactions would be a step further toward understanding developmental mechanisms implicated in psychoses. However, our results already bear significance for the millions of children born to affected parents, considering that 14%–24% of children have a parent with a mental illness58 and that about 3% of the population of Group of 7 countries (Canada, France, Germany, Italy, Japan, United Kingdom, United States) are affected by schizophrenia, BD and recurrent major depression (i.e., more than 20 million patients). Nevertheless, because abuse and neglect are known to have a negative influence on cognitive functioning in community samples of healthy adults20,21 and children/adolescents,22,23 our results may not be specific to the genetic high-risk population. Second, although our measure of abuse and neglect had the advantage of drawing information from multiple sources and from contemporary medical records instead of only retrospective self-reporting of childhood events by adults as in most previous studies, our measure was not prospective. Third, our instrument did not allow us to assess how much of the exposure to abuse or neglect was subjectively traumatic to the child — a factor known to be associated with outcome.59 Fourth, although our sample size of 66 participants was rather large in comparison to most prior studies of young high-risk offspring,50,60 a larger sample would be needed to eliminate type 2 errors and to clarify potential specific effects of different types of trauma. Fifth, our research highlighted an association between maltreatment in childhood/adolescence and performance in cognitive precursors of psychosis. Our hypothesis that childhood trauma would be responsible for the poor cognitive performance observed is supported by the literature, suggesting that childhood trauma leads to cognitive deficits.8,20–25 However, one might argue that cognitive deficits might increase the risk of maltreatment. Sixth, the present sample of 66 offspring was gathered from 25 of the 48 multigenerational families from the eastern Quebec catchment area; thus the representativeness of these 66 offspring with respect to those from the 23 other families would have to be confirmed. However, we recently reported that the phenotypic, endophenotypic and genetic findings in our multiaffected families15 strikingly resembled those previously reported in adult patients and relatives31,32 and in children born to affected parents61–63 from general or sporadic samples. Consequently, the present observations are likely to be generalizable to all offspring of parents affected by major psychosis.
Conclusion
To our knowledge, our study is the first to demonstrate a negative effect of childhood maltreatment on the cognitive precursors of adult psychoses in children and adolescents at high genetic risk for psychosis. Our study suggests that child abuse and neglect would be related to adult disease through a mediating mechanism occurring in childhood. This finding may influence future research on the neurobiological etiology and treatment of psychoses. Although completely preventing child abuse might be difficult, our study might orient the design of early intervention research aiming to alleviate the effect of maltreatment on brain microcircuitry and consequently reduce the risk of a poorer adult outcome.
Acknowledgments
We thank our research assistants, M.-C. Boisvert, L. Bélanger, J. Lavoie, L. René, V. Beaupré-Monfette, C. Poirier and V. Jomphe, and the families who participated in this study. This study was supported by a Canada Research Chair (#950-200810) in psychiatric genetics (M. Maziade) and by Canadian Institute of Health Research grants (#MOP-74430, #MOP-114988 and #MOP-119408). The funding organizations had no role in the writing of this manuscript.
Footnotes
Competing interests: None declared.
Contributors: N. Berthelot, T. Paccalet and M. Maziade designed the study. N. Berthelot, E. Gilbert, N. Gingras, N. Rouleau and M. Maziade acquired the data, which N. Berthelot, T. Paccalet, I. Moreau, C. Mérette and M. Maziade analyzed. N. Berthelot, T. Paccalet and M. Maziade wrote the article, which all authors reviewed and approved for publication.
References
- 1.Varese F, Smeets F, Drukker M, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38:661–71. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonoldi I, Simeone E, Rocchetti M, et al. Prevalence of self-reported childhood abuse in psychosis: a meta-analysis of retrospective studies. Psychiatry Res. 2013;210:8–15. doi: 10.1016/j.psychres.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Kelleher I, Keeley H, Corcoran P, et al. Childhood trauma and psychosis in a prospective cohort study: cause, effect, and directionality. Am J Psychiatry. 2013;170:734–41. doi: 10.1176/appi.ajp.2012.12091169. [DOI] [PubMed] [Google Scholar]
- 4.Heins M, Simons C, Lataster T, et al. Childhood trauma and psychosis: a case-control and case-sibling comparison across different levels of genetic liability, psychopathology, and type of trauma. Am J Psychiatry. 2011;168:1286–94. doi: 10.1176/appi.ajp.2011.10101531. [DOI] [PubMed] [Google Scholar]
- 5.Larsson S, Andreassen OA, Aas M, et al. High prevalence of childhood trauma in patients with schizophrenia spectrum and affective disorder. Compr Psychiatry. 2013;54:123–7. doi: 10.1016/j.comppsych.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367:1040–2. doi: 10.1016/S0140-6736(06)68450-X. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein BI, Shamseddeen W, Axelson DA, et al. Clinical, demographic, and familial correlates of bipolar spectrum disorders among offspring of parents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:388–96. [PMC free article] [PubMed] [Google Scholar]
- 8.Aas M, Dazzan P, Fisher HL, et al. Childhood trauma and cognitive function in first-episode affective and non-affective psychosis. Schizophr Res. 2011;129:12–9. doi: 10.1016/j.schres.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Maziade M, Gilbert E, Berthelot N, et al. Findings and concepts from children at genetic risk that may transform prevention research and practice in schizophrenia and mood disorders. In: Raynaud J-P, Hodes M, Gau SS-F, editors. From Research to Practice in Child and Adolescent Mental Health. Maryland: Rowman & Littlefield; 2014. [Google Scholar]
- 10.Nieto RG, Castellanos FX. A meta-analysis of neuropsychological functioning in patients with early onset schizophrenia and pediatric bipolar disorder. J Clin Child Adolesc Psychol. 2011;40:266–80. doi: 10.1080/15374416.2011.546049. [DOI] [PubMed] [Google Scholar]
- 11.Simonsen C, Sundet K, Vaskinn A, et al. Neurocognitive dysfunction in bipolar and schizophrenia spectrum disorders depends on history of psychosis rather than diagnostic group. Schizophr Bull. 2011;37:73–83. doi: 10.1093/schbul/sbp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maziade M, Rouleau N, Merette C, et al. Verbal and visual memory impairments among young offspring and healthy adult relatives of patients with schizophrenia and bipolar disorder: selective generational patterns indicate different developmental trajectories. Schizophr Bull. 2011;37:1218–28. doi: 10.1093/schbul/sbq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meijer J, Simons CJ, Quee PJ, et al. Cognitive alterations in patients with non-affective psychotic disorder and their unaffected siblings and parents. Acta Psychiatr Scand. 2012;125:66–76. doi: 10.1111/j.1600-0447.2011.01777.x. [DOI] [PubMed] [Google Scholar]
- 14.Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70:1107–12. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- 15.Maziade M, Paccalet T. A protective-compensatory model may reconcile the genetic and the developmental findings in schizophrenia. Schizophr Res. 2013;144:9–15. doi: 10.1016/j.schres.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Maziade M, Rouleau N, Gingras N, et al. Shared neurocognitive dysfunctions in young offspring at extreme risk for schizophrenia or bipolar disorder in eastern quebec multigenerational families. Schizophr Bull. 2009;35:919–30. doi: 10.1093/schbul/sbn058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schretlen DJ, Cascella NG, Meyer SM, et al. Neuropsychological functioning in bipolar disorder and schizophrenia. Biol Psychiatry. 2007;62:179–86. doi: 10.1016/j.biopsych.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glahn DC, Almasy L, Barguil M, et al. Neurocognitive endophenotypes for bipolar disorder identified in multiplex multigenerational families. Arch Gen Psychiatry. 2010;67:168–77. doi: 10.1001/archgenpsychiatry.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gur RE, Calkins ME, Gur RC, et al. The Consortium on the Genetics of Schizophrenia: neurocognitive endophenotypes. Schizophr Bull. 2007;33:49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gould F, Clarke J, Heim C, et al. The effects of child abuse and neglect on cognitive functioning in adulthood. J Psychiatr Res. 2012;46:500–6. doi: 10.1016/j.jpsychires.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majer M, Nater UM, Lin JM, et al. Association of childhood trauma with cognitive function in healthy adults: a pilot study. BMC Neurol. 2010;10:61. doi: 10.1186/1471-2377-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koenen KC, Moffitt TE, Caspi A, et al. Domestic violence is associated with environmental suppression of IQ in young children. Dev Psychopathol. 2003;15:297–311. doi: 10.1017/s0954579403000166. [DOI] [PubMed] [Google Scholar]
- 23.Mills R, Alati R, O’Callaghan M, et al. Child abuse and neglect and cognitive function at 14 years of age: findings from a birth cohort. Pediatrics. 2011;127:4–10. doi: 10.1542/peds.2009-3479. [DOI] [PubMed] [Google Scholar]
- 24.Shannon C, Douse K, McCusker C, et al. The association between childhood trauma and memory functioning in schizophrenia. Schizophr Bull. 2011;37:531–7. doi: 10.1093/schbul/sbp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savitz JB, van der Merwe L, Stein DJ, et al. Neuropsychological task performance in bipolar spectrum illness: genetics, alcohol abuse, medication and childhood trauma. Bipolar Disord. 2008;10:479–94. doi: 10.1111/j.1399-5618.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- 26.Scala S, Pousada A, Stone WS, et al. Verbal and visual-spatial memory impairment in youth at familial risk for schizophrenia or affective psychosis: a pilot study. Schizophr Res. 2013;144:122–8. doi: 10.1016/j.schres.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seidman LJ, Giuliano AJ, Smith CW, et al. Neuropsychological functioning in adolescents and young adults at genetic risk for schizophrenia and affective psychoses: results from the Harvard and Hillside Adolescent High Risk Studies. Schizophr Bull. 2006;32:507–24. doi: 10.1093/schbul/sbj078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hans SL, Marcus J, Nuechterlein KH, et al. Neurobehavioral deficits at adolescence in children at risk for schizophrenia: the Jerusalem Infant Development Study. Arch Gen Psychiatry. 1999;56:741–8. doi: 10.1001/archpsyc.56.8.741. [DOI] [PubMed] [Google Scholar]
- 29.Fioravanti M, Bianchi V, Cinti ME. Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. BMC Psychiatry. 2012;12:64. doi: 10.1186/1471-244X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mesholam-Gately RI, Giuliano AJ, Goff KP, et al. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–36. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- 31.McDonald C. The Maudsley Family Study of Psychosis: A Quest for Intermediate Phenotypes. New York, NY: Psychology Press; 2008. [Google Scholar]
- 32.Bora E, Lin A, Wood SJ, et al. Cognitive deficits in youth with familial and clinical high risk to psychosis: a systematic review and meta-analysis. Acta Psychiatr Scand. 2014;130:1–15. doi: 10.1111/acps.12261. [DOI] [PubMed] [Google Scholar]
- 33.Arseneault L, Cannon M, Fisher HL, et al. Childhood trauma and children’s emerging psychotic symptoms: a genetically sensitive longitudinal cohort study. Am J Psychiatry. 2011;168:65–72. doi: 10.1176/appi.ajp.2010.10040567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Nierop M, Lataster T, Smeets F, et al. Psychopathological mechanisms linking childhood traumatic experiences to risk of psychotic symptoms: analysis of a large, representative population-based sample. Schizophr Bull. 2014;40(Suppl 2):S123–30. doi: 10.1093/schbul/sbt150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–6. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 36.Bifulco A, Bernazzani O, Moran PM, et al. The childhood experience of care and abuse questionnaire (CECA.Q): validation in a community series. Br J Clin Psychol. 2005;44:563–81. doi: 10.1348/014466505X35344. [DOI] [PubMed] [Google Scholar]
- 37.Maziade M, Roy MA, Fournier JP, et al. Reliability of best-estimate diagnosis in genetic linkage studies of major psychoses: results from the Quebec pedigree studies. Am J Psychiatry. 1992;149:1674–86. doi: 10.1176/ajp.149.12.1674. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 39.Spitzer RL, Williams JB, Gibbon M, et al. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–9. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 40.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition: DSM-IV-TR. American Psychiatric Publisher; 2000. [Google Scholar]
- 41.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–97. [PubMed] [Google Scholar]
- 42.Gilbert E, Merette C, Jomphe V, et al. Cluster analysis of cognitive deficits may mark heterogeneity in schizophrenia in terms of outcome and response to treatment. Eur Arch Psychiatry Clin Neurosci. 2014;264:333–43. doi: 10.1007/s00406-013-0463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slick DJ. Psychometric in neuropsychological assessment. In: Strauss ES, Spreen O, editors. Compendium of neuropsychological tests: Administration, norms, and commentary. 3rd edition ed. New York: Oxford University Press; 2006. pp. 3–43. [Google Scholar]
- 44.Diaz A, Petersen AC. Institute of Medicine report: new directions in child abuse and neglect research. JAMA Pediatr. 2014;168:101–2. doi: 10.1001/jamapediatrics.2013.4560. [DOI] [PubMed] [Google Scholar]
- 45.Teicher MH, Samson JA. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry. 2013;170:1114–33. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cicchetti D, Rogosch FA, Howe ML, et al. The effects of maltreatment and neuroendocrine regulation on memory performance. Child Dev. 2010;81:1504–19. doi: 10.1111/j.1467-8624.2010.01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pederson CL, Maurer SH, Kaminski PL, et al. Hippocampal volume and memory performance in a community-based sample of women with posttraumatic stress disorder secondary to child abuse. J Trauma Stress. 2004;17:37–40. doi: 10.1023/B:JOTS.0000014674.84517.46. [DOI] [PubMed] [Google Scholar]
- 48.Reichenberg A, Caspi A, Harrington H, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167:160–9. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meier MH, Caspi A, Reichenberg A, et al. Neuropsychological decline in schizophrenia from the premorbid to the postonset period: evidence from a population-representative longitudinal study. Am J Psychiatry. 2014;171:91–101. doi: 10.1176/appi.ajp.2013.12111438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maziade M, Rouleau N, Cellard C, et al. Young offspring at genetic risk of adult psychoses: the form of the trajectory of IQ or memory may orient to the right dysfunction at the right time. PLoS ONE. 2011;6:e19153. doi: 10.1371/journal.pone.0019153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Snellenberg JX, de Candia T. Meta-analytic evidence for familial coaggregation of schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2009;66:748–55. doi: 10.1001/archgenpsychiatry.2009.64. [DOI] [PubMed] [Google Scholar]
- 52.Cullen AE, Fisher HL, Roberts RE, et al. Daily stressors and negative life events in children at elevated risk of developing schizophrenia. Br J Psychiatry. 2014;204:354–60. doi: 10.1192/bjp.bp.113.127001. [DOI] [PubMed] [Google Scholar]
- 53.Afifi TO, Mather A, Boman J, et al. Childhood adversity and personality disorders: results from a nationally representative population-based study. J Psychiatr Res. 2011;45:814–22. doi: 10.1016/j.jpsychires.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 54.Scher CD, Forde DR, McQuaid JR, et al. Prevalence and demographic correlates of childhood maltreatment in an adult community sample. Child Abuse Negl. 2004;28:167–80. doi: 10.1016/j.chiabu.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 55.Holzer L, Urben S, Passini CM, et al. A randomized controlled trial of the effectiveness of computer-assisted cognitive remediation (CACR) in adolescents with psychosis or at high risk of psychosis. Behav Cogn Psychother. 2014;42:421–34. doi: 10.1017/S1352465813000313. [DOI] [PubMed] [Google Scholar]
- 56.Puig O, Penades R, Baeza I, et al. Cognitive remediation therapy in adolescents with early-onset schizophrenia: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2014;53:859–68. doi: 10.1016/j.jaac.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Chaffin M, Friedrich B. Evidence-based treatments in child abuse and neglect. Child Youth Serv. 2004;26:1097–113. [Google Scholar]
- 58.Reupert AE, Maybery DJ, Kowalenko NM. Children whose parents have a mental illness: prevalence, need and treatment. Med J Aust. 2012;1(Suppl 1):7–9. doi: 10.5694/mja11.11200. [DOI] [PubMed] [Google Scholar]
- 59.Creamer M, McFarlane AC, Burgess P. Psychopathology following trauma: the role of subjective experience. J Affect Disord. 2005;86:175–82. doi: 10.1016/j.jad.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 60.Ostiguy CS, Ellenbogen MA, Walker CD, et al. Sensitivity to stress among the offspring of parents with bipolar disorder: a study of daytime cortisol levels. Psychol Med. 2011;41:2447–57. doi: 10.1017/S0033291711000523. [DOI] [PubMed] [Google Scholar]
- 61.Niemi LT, Suvisaari JM, Tuulio-Henriksson A, et al. Childhood developmental abnormalities in schizophrenia: evidence from high-risk studies. Schizophr Res. 2003;60:239–58. doi: 10.1016/s0920-9964(02)00234-7. [DOI] [PubMed] [Google Scholar]
- 62.Byrne M, Hodges A, Grant E, et al. Neuropsychological assessment of young people at high genetic risk for developing schizophrenia compared with controls: preliminary findings of the Edinburgh High Risk Study (EHRS) Psychol Med. 1999;29:1161–73. doi: 10.1017/s0033291799001002. [DOI] [PubMed] [Google Scholar]
- 63.Klimes-Dougan B, Ronsaville D, Wiggs EA, et al. Neuropsychological functioning in adolescent children of mothers with a history of bipolar or major depressive disorders. Biol Psychiatry. 2006;60:957–65. doi: 10.1016/j.biopsych.2006.03.031. [DOI] [PubMed] [Google Scholar]