Abstract
Background
Response time variability (RTV) is consistently increased in patients with attention-deficit/hyperactivity disorder (ADHD). A right-hemispheric frontoparietal attention network model has been implicated in these patients. The 3 main connecting fibre tracts in this network, the superior longitudinal fasciculus (SLF), inferior longitudinal fasciculus (ILF) and the cingulum bundle (CB), show microstructural abnormalities in patients with ADHD. We hypothesized that the microstructural integrity of the 3 white matter tracts of this network are associated with ADHD and RTV.
Methods
We examined RTV in adults with ADHD by modelling the reaction time distribution as an exponentially modified Gaussian (ex-Gaussian) function with the parameters μ, σ and τ, the latter of which has been attributed to lapses of attention. We assessed adults with ADHD and healthy controls using a sustained attention task. Diffusion tensor imaging–derived fractional anisotropy (FA) values were determined to quantify bilateral microstructural integrity of the tracts of interest.
Results
We included 100 adults with ADHD and 96 controls in our study. Increased τ was associated with ADHD diagnosis and was linked to symptoms of inattention. An inverse correlation of τ with mean FA was seen in the right SLF of patients with ADHD, but no direct association between the mean FA of the 6 regions of interest with ADHD could be observed.
Limitations
Regions of interest were defined a priori based on the attentional network model for ADHD and thus we might have missed effects in other networks.
Conclusion
This study suggests that reduced microstructural integrity of the right SLF is associated with elevated τ in patients with ADHD.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is characterized by a pervasive pattern of age-inappropriate inattentive behaviour and/or impulsiveness and hyperactivity. Although ADHD is often viewed as a childhood disorder, it also affects 2.5% of adults,1 which has profound negative implications for the patients themselves, their social environment and society. The pathophysiology of ADHD is still poorly understood owing in part to its substantial clinical and etiological heterogeneity.2
Increased response time variability (RTV) is one of the most common characteristics of patients with ADHD. Response time variability is the moment-to-moment fluctuation of performance in neuropsychological response time experiments and has been found to be increased in patients with ADHD across a number of different experimental paradigms, including sustained attention, flanker interference and working memory tasks (for reviews see the studies by Tamm and colleagues3 and Kofler and colleagues4). Recent studies suggest that increased performance variability in patients with ADHD might be a neurocognitive marker and may serve as a potential endophenotype for this disorder.5,6 In addition to capturing RTV through standard deviation (SD) of response time (RT), other quantitative measures of RTV have also been reported. The exponentially modified Gaussian (ex-Gaussian) method, for example, provides quantification of the shapes of RT frequency distributions in the form of 3 different parameters. Two parameters reflect the central tendency (μ) and variance (σ) of the Gaussian component. An exponential component, τ, provides information on the extent of the positive skew of RT distributions. Increased τ indicates more frequent excessively long RTs and is often attributed to lapses in attention.7–10 Increased τ has consistently been found in patients with ADHD across tasks.6–14 Compared with reports on τ, reports on alterations of μ and σ in patients with ADHD have been less consistent, and findings seem more task-dependent.10 The disorder has been associated with decreased μ,7,9,12 increased σ,7,9,12–14 or no differences in μ8,11,13,14 or σ.8,11 These studies show that ADHD-related RTV is predominantly determined by extremely long RTs, which are best captured by an elevation in τ. Studies disentangling the neurobiological substrates of τ are limited but may lead to a better understanding of attention processes in RT paradigms, especially in ADHD.
Russel and colleagues15 hypothesized that reduced myelination of white matter might be a neurobiological mechanism responsible for increased performance variability in patients with ADHD. Recently, RTV has been associated with intersubject variability in the microstructure of different white matter tracts. Using diffusion tensor imaging (DTI) and deriving fractional anisotropy (FA) coefficients reflecting the microstructural integrity of white matter,16 significant correlations were established between performance variability measured across different simple RT tasks and white matter microstructure.17,18 During the course of normal human postnatal development, RTV is known to change in a U-form fashion, with a decrease in variability throughout childhood and a later increase in adulthood.19 The reduction of RTV during adolescence has been partially linked to the maturation of white matter tracts in the brain.20 A recent study performed in young patients with ADHD and healthy controls showed that FA of the left cingulum bundle (CB) was negatively correlated with τ only in patients with ADHD.13 Importantly, the CB is part of the attentional network model as defined by Makris and colleagues.21 These researchers defined several neuroanatomical models of distributed network dysfunction that may lead to symptoms of ADHD. Brain regions in the attentional network are the right hemispheric frontoparietal cortices, the thalamus and the cerebellum. Besides the CB, 2 main fibre pathways connecting these regions are the superior longitudinal fasciculus (SLF) and the inferior longitudinal fasciculus (ILF). The SLF is a bidirectional link between regions in frontal and parietal cortices.21,22 White matter microstructure of the SLF has been shown to be compromised in children23,24 and in adults with ADHD,25 especially in the right-lateralized part.26,27 Another study showed that task-related measures of attentional performance in adults with ADHD correlated significantly with FA in the right SLF.28 The ILF connects the occipital with the temporal cortices,21 and bilateral regions of the ILF are also abnormal in children and adults with ADHD.27,29 Furthermore, the microstructural integrity of the right CB was found to be altered in adults with ADHD.26 Altogether, these findings suggest that the microstructural integrity of these fibre tracts, especially in the right hemisphere, might contribute to the complex etiology of attentional problems in patients with ADHD and subsequently may be partly responsible for a greater prevalence of extremely long RT in patients with ADHD, as reflected in the higher ex-Gaussian component τ in this group.
Here, we aimed to advance existing knowledge on the association between ADHD, τ and microstructural integrity of the SLF, ILF and CB. Based on the white matter tracts described in the attention network model by Makris and colleagues,21 we used a hypothesis-driven region of interest (ROI) approach in a large sample of well-characterized adult patients with ADHD and healthy controls. We hypothesized that patients with ADHD would show elevated τ, especially linked to higher rates of inattention symptoms. Furthermore, we expected that ADHD and τ would be linked to reduced mean FA in the SLF, ILF and/or CB white matter tracts, with higher values on τ reflecting attention lapses associated with lower mean FA most prominently in right-lateralized white matter tracts.
Methods
Participants
We recruited individuals from the Dutch cohort of the International Multicentre persistent ADHD CollaboraTion (IMpACT; www.impactADHDgenomics.com)30 to participate in our study. The patients with ADHD were recruited from the Department of Psychiatry, Radboud University, Nijmegen, the Netherlands, and healthy controls were recruited through advertisements.
All participants underwent psychiatric assessments, neurocognitive tests and neuroimaging. Participants were assessed using the diagnostic interview for adult ADHD (DIVA).31 This interview focuses on the 18 DSM-IV symptoms of ADHD and uses concrete and realistic examples to investigate whether a symptom is currently present or whether it was present in childhood. We acquired supplementary information from parents and school reports whenever possible. In addition, we administered the ADHD Rating Scale-IV, a self-report questionnaire on current symptoms of attention and hyperactivity/impulsivity.32 Patients were included in the study if they met DSM-IV-TR criteria for ADHD in childhood as well as in adulthood. We used the Structured Clinical Interviews for DSM-IV (SCID-I and SCID-II)33,34 for comorbidity assessment. The assessments were carried out by trained professionals (i.e., psychiatrists or psychologists). Exclusion criteria were psychosis, alcohol or substance addiction in the 6 months preceding the study, current major depression, full-scale IQ estimate lower than 70 (prorated from the Block Design and Vocabulary components of the Wechsler Adult Intelligence Scale-III), neurologic disorders, sensorimotor disabilities, non-Caucasian race and use of medications other than psychostimulants or atomoxetine. Healthy controls who had a current neurologic or psychiatric disorder according to DIVA, SCID-I or SCID-II or who had any first-degree relatives with ADHD or another major psychiatric disorder were excluded. Because our study is part of a genetics project, all participants were Dutch and of European Caucasian ancestry to reduce potential genetic heterogeneity. The regional ethics committee (Centrale Commissie Mensgebonden Onderzoek: CMO Regio Arnhem — Nijmegen; protocol number III.04.0403) approved our study, and we obtained written informed consent from all participants.
Characterization of the RT frequency distribution in a sustained attention task
The ex-Gaussian parameters (μ, σ and τ) were derived from a sustained attention (SA)-dots task35 in which a pattern of 3, 4 or 5 dots was presented on a computer screen in a random order. Participants had to indicate as quickly and accurately as possible how many dots were present by pressing a button with their dominant (4 dots) or nondominant hands (3 and 5 dots). Owing to the duration (approximately 12 min consisting of 600 trials in total with 200 trials per condition) and complexity, this task forms a good basis for quantification of RTV with the ex-Gaussian method. All premature responses (RT < 150 ms) were excluded before the determination of the 3 ex-Gaussian parameters.11 In addition, we excluded error trials and the trial subsequent to errors, as these could reflect posterror slowing.36 An ex-Gaussian probability density function was then fit to the RT histogram for each participant. The optimal values for the 3 parameters (μ, σ and τ) of the ex-Gaussian function were determined with the Simplex search method described by Lacouture and Cousineau37 using MATLAB (Mathworks Inc.).
DTI acquisition
Whole brain imaging was performed with a 1.5 T MR scanner (Magnetom Avanto, Siemens Medical Systems) and a standard 8-channel head coil. We obtained a high-resolution T1-weighted magnetization-prepared rapid gradient-echo anatomic scan from each participant in which the inversion time (TI) was chosen to provide optimal grey matter–white matter T1 contrast (repetition time [TR] 2730 ms, echo time [TE] 2.95 ms, TI 1000 ms, flip angle 7°, field of view [FOV] 256 × 256 × 176 mm3, voxel size 1.0 × 1.0 × 1.0 mm3). The T1 images served as high-resolution reference images for diffusion imaging data. Transversely oriented diffusion-weighted images were acquired using a twice-refocused spin-echo-planar-imaging sequence that minimized imaging distortions from eddy currents.38 The diffusion imaging data were acquired using 2 different protocols. Sixty participants were scanned with the following protocol: TR 10 200 ms, TE 95 ms, FOV 320 × 320 × 160 mm3, voxel size 2.5 × 2.5 × 2.5 mm3, 6/8 partial Fourier. Four images without diffusion weighting (b = 0 s/mm2) and 30 images with diffusion weighting (b = 900 s/mm2, 34 diffusion directions) applied along noncollinear directions were acquired. The remaining 137 participants were scanned with an adapted second protocol that was implemented to reduce motion artifacts during scanning. Parameters that differed from the first protocol were TR (6700 ms), TE (85 ms) and FOV (220 × 220 × 140 mm3), and scans were acquired with full Fourier acquisition; other parameters were unchanged. For each slice, the diffusion weighting for the 30 images changed to b = 900 s/mm2. We corrected for possible variance introduced owing to the different protocols by including the diffusion-weighted acquisition protocol as a confound in the analyses.
Image preprocessing
The diffusion-weighted data were preprocessed using an algorithm developed in house. In short, the diffusion-weighted images of each participant were realigned on the unweighted image using mutual information routines from SPM5. Next, we used an iteratively reweighted-least squares algorithm to correct for head and cardiac motion artifacts in the diffusion-weighted data.39 Using DTIFIT within the FMRIB Diffusion Toolbox (part of FMRIB’s Software Library [FSL]), FA images were created and subsequently fed into the tract-based spatial statistics (TBSS) pipeline.40 Here, all individual FA maps were nonlinearly registered to the FMRIB58_FA template using FSL’s nonlinear registration tool FNIRT and then affine-transformed into standard Montreal Neurological Institute (MNI) space. A mean FA image was created and thinned to create a mean FA skeleton, which represents the centres of all tracts common to the group. A threshold of 0.2 was used to avoid partial voluming effects. Individual FA skeleton images were then mapped onto this mean skeleton for statistical evaluation.
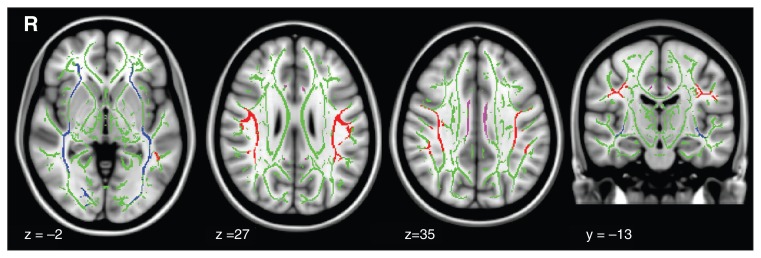
We used the Johns Hopkins University white matter tractography atlas41 with a probability threshold of 0.5 to identify regions on the mean FA skeleton corresponding to the left and right SLF and ILF. The ICBM-DTI-81 white matter labels atlas42 was used to identify regions on the mean FA skeleton corresponding to the left and right CB. For optimal accuracy, both atlases were nonlinearly registered to the FMRIB58 brain. To obtain mean FA values for each ROI for each participant, each mask was multiplied with the mapped individual’s FA skeleton images and averaged (Fig. 1).
Fig. 1.
Aligned and binarized masks of skeletonized white matter tracts for (blue) the left (1975 voxels) and right (2198 voxels) inferior longitudinal fasciculous, (red) the left (2780 voxels) and right (2248 voxels) superior longitudinal fasciculous, and (purple) the left (416 voxels) and right (401 voxels) cingulum bundle. The mean fractional anisotropy skeleton mask is shown in green.
Statistical analysis
The distributions of the 3 ex-Gaussian parameters were normalized by taking their natural logarithm. Outliers were defined as residuals with a larger or smaller value than 3 times the SD of the predicted value. One patient with ADHD had an extreme measurement of τ and was therefore excluded from all further analyses. We used an analysis of covariance (ANCOVA) model to assess whether the ex-Gaussian parameters (μ, σ and τ) were significantly different between groups, adjusting for age, sex and handedness. Then, separately for both groups, we performed ordinal regression analyses to examine the association of τ with self-reported symptoms of inattention and hyperactivity/impulsivity. These ordinal regressions were performed controlling for age, sex and handedness. To inspect the individual effects of each ROI on τ, we obtained standardized residuals adjusted for age, sex, handedness, DTI protocol and mean FA of the other ROIs. Those standardized and corrected residuals for each ROI were associated with τ in separate linear regressions. We then performed the same analyses separately for patients with ADHD and controls to address the specificity of the results for each group. Finally, logistic regression analyses were performed using the standardized residuals to inspect whether the mean FA of the 6 ROIs were associated with ADHD diagnosis. We applied Bonferroni correction to adjust for multiple comparisons. Given that we performed 15 independent statistical tests (i.e., 3 analyses including the ex-Gaussian parameters, 6 for the association between the ROIs and τ and 6 for the association between the ROIs and ADHD), we considered results to be significant at p < 0.0033.
Results
Demographic and clinical characteristics
In total, we recruited 197 participants (101 adults with ADHD and 96 healthy controls); we excluded 1 outlier, yielding a final sample of 100 adults with ADHD and 96 controls. Demographic characteristics of the study sample are presented in Table 1. There were no significant differences between patients with ADHD and controls with respect to age (p = 0.58, d = 0.03) or estimated IQ (p = 0.58, d = 0.10). Patients with ADHD and controls showed the expected differences in self-reported inattention (p < 0.001, d = −3.631) and hyperactive/impulsive symptoms (p < 0.001, d = −2.647). Groups were equally distributed with respect to sex (χ2 = 0.450, p = 0.56), handedness (χ2 = 0.925, p = 0.40) and DTI acquisition protocols (χ2 = 3.922, p = 0.06). There were more women than men in our sample (63 women and 38 men in the ADHD group, and 55 women and 41 men in the control group).
Table 1.
Demographic and clinical characteristics of the sample
| Group; mean ± SD or no. | ||
|---|---|---|
|
|
||
| Characteristic | ADHD, n = 101 | Control, n = 96 |
| Sex, male:female | 38:63 | 41:55 |
| Age, yr | 35.83 ± 11.16 | 36.17 ± 11.17 |
| IQ estimate* | 108.08 ± 14.92 | 109.68 ± 15.34 |
| Inattentive symptoms† | 6.38 ± 2.04¶ | 0.55 ± 0.993¶ |
| Hyperactive/impulsive symptoms† | 5.56 ± 2.24¶ | 0.79 ± 1.21¶ |
| No. of errors | 27.8 ± 20.08¶ | 16.94 ± 11.26¶ |
| Medication-naive | 16 | — |
| Current medication | ||
| Amphetamine | 10 | — |
| Methylphenidate | 56 | — |
| Non-stimulant | 3 | — |
| Past treatment | 16 | — |
| ≥ 1 depressive episode (remitted)‡ | 44 | 9 |
| Anxiety disorder (remitted)‡ | 23 | 5 |
| Substance abuse (remitted)‡ | 18 | 7 |
| Borderline personality disorder‡ | 9 | 0 |
| Antisocial‡ | 3 | 0 |
| Right-handed | 85 | 86 |
| DTI acquisition protocol 1§ | 64 | 73 |
ADHD = attention-deficit/hyperactivity disorder; DTI = diffusion tensor imaging; SD = standard deviation.
Scores represent the average of the standard scores for the block design and vocabulary assessments of the Wechsler Adult Intelligence Scale-III.
As measured using the ADHD-DSM-IV self rating scale.
As measured using the Structured Clinical Interview for DSM-IV for axis I and axis II disorders.
First version of DTI acquisition protocol.
Significant difference between patients with ADHD and controls.
Case–control analysis of ex-Gaussian parameters (μ, σ, and τ) and association with self-reported symptoms
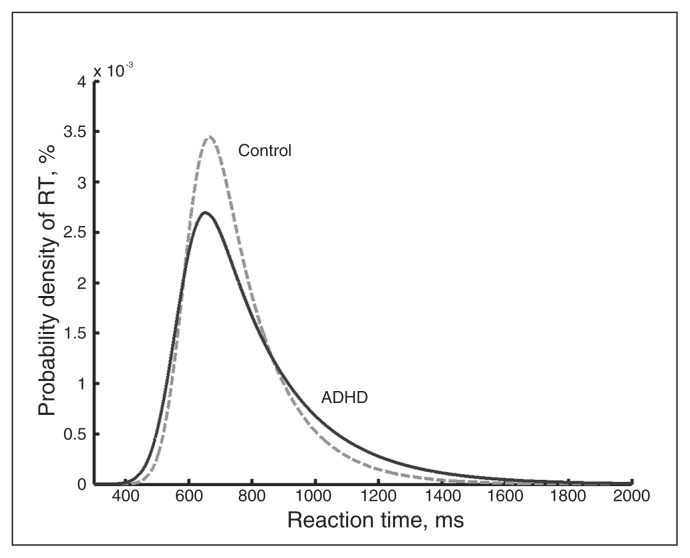
The ex-Gaussian parameter μ was significantly lower in patients with ADHD than in controls (F1,191 = 6.73, p = 0.010). No differences were found for values of σ (F1,191 = 3.71, p = 0.06). The strongest effect was for τ, which was significantly increased in patients with ADHD compared with controls (F1, 191 = 24.96, p < 0.001; Table 2). After correcting for multiple testing, only τ remained significant. The probability density function was more positively skewed in patients than in controls (Fig. 2). The estimated mean log-likelihood values for the goodness of fit of the ex-Gaussian probability density function in patients and controls were 3.586e+03 ± 0.253e+03 and 3.594e+03 ± 0.201e+03, respectively. This difference was not significant (t194, p = 0.80). Ordinal regressions showed that in patients with ADHD the parameter τ was significantly associated with self-reported symptoms of inattention (Nagelkerke R2 = 0.097, p = 0.002; Appendix 1, Table S1, available at jpn.ca), and this association was not observed in controls (p = 0.32; Appendix 1, Table S1). Hyperactive/impulsive symptoms were not associated with τ in the ADHD group (p = 0.51) or in the control group (p = 0.97; Appendix 1, Table S1). Correction for IQ did not change the results.
Table 2.
ANCOVA analyses of exponentially modified Gaussian parameters for differences between patients with ADHD and controls
| Group, mean ± SD | |||||
|---|---|---|---|---|---|
|
|
|||||
| Variable | ADHD, n = 100 | Control, n = 96 | Statistic* | p value | Effect size* |
| μ | 568.70 ± 79.67 | 596.70 ± 75.10 | F1,191 = 6.73 | 0.010 | 0.034 |
| σ | 64.04 ± 18.76 | 58.85 ± 16.03 | F1,191 = 3.71 | 0.06 | 0.019 |
| τ | 215.16 ± 95.99 | 158.46 ± 55.03 | F1,191 = 24.96 | < 0.001 | 0.116 |
ADHD = attention-deficit/hyperactivity disorder; SD = standard deviation.
Corrected for age, sex and handedness.
Fig. 2.
The exponentially modified Gaussian probability density function (PDF) across patients with ADHD and controls. Patients with ADHD have a more positive skewed PDF than controls. RT = response time.
Association analysis of mean FA of the SLF, ILF and CB with τ
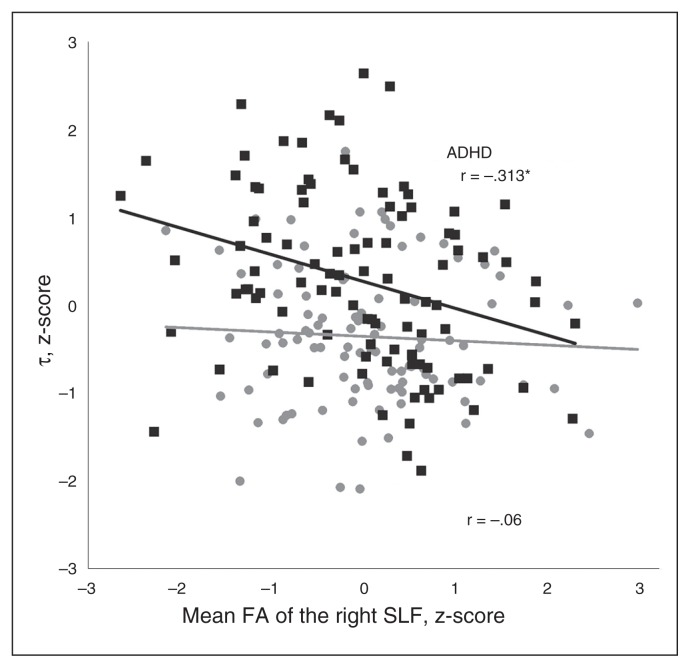
The mean FA value of the right SLF was associated with τ (R2 = 0.044, p = 0.003), with higher mean FA associated with lower values of τ (Table 3). This finding remained significant after correcting for multiple testing. Sensitivity analyses for each ROI showed that the effects remain stable when not controlling for the other ROIs (Appendix 1, Table S2). Furthermore, we performed extra sensitivity analyses to inspect whether the effect remained stable if all nonerror trials were included to determine the ex-Gaussian parameters (Appendix 1, Table S3), if all trials were included to determine the ex-Gaussian parameters (Appendix 1, Table S4) and if controlling for the number of errors in our analyses (Appendix 1, Table S5). All separate sensitivity analyses were consistent with the effects found in the main analyses. Separate analyses for patients with ADHD and controls showed that the association between the mean FA value of the right SLF and τ was specific to the ADHD group (R2 = 0.098, p = 0.002), as there was no association in controls (p = 0.56; Fig. 3 and Appendix 1, Table S6). None of the other 5 ROIs was associated with τ (all p > 0.05; Table 3), and no direct association was observed between ADHD diagnosis and mean FA in the 6 ROIs (all p > 0.05; Appendix 1, Table S7). Correction for IQ did not change the results. Furthermore, we performed sensitivity analyses to show that the direction of effects across DTI protocols remained the same (Appendix 1, Table S8).
Table 3.
Single regression analyses associating τ with mean fractional anisotropy values of bilateral regions of interests corrected for age, sex, handedness and DTI protocol*
| Dependent variable: τ | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| FA residuals | No. | b ± SE | Statistic | p value | β | R2 |
| Left SLF | 196 | 1.282 ± 2.974 | T195 = 0.431 | 0.67 | 0.031 | 0.001 |
| Right SLF | 196 | −8.675 ± 2.910 | T195 = −2.981 | 0.003 | −0.209 | 0.044 |
| Left ILF | 196 | −0.813 ± 2.975 | T195 = −0.273 | 0.79 | −0.020 | 0.001 |
| Right ILF | 196 | 3.053 ± 2.967 | T195 = 1.029 | 0.31 | 0.074 | 0.005 |
| Left CB | 196 | 1.682 ± 2.973 | T195 = 0.566 | 0.57 | 0.041 | 0.002 |
| Right CB | 196 | 0.234 ± 2.975 | T195 = 0.079 | 0.94 | 0.006 | 0.001 |
CB = cingulum bundle; DTI = diffusion tensor imaging; FA = fractional anisotropy; ILF = inferior longitudinal fasciculus; ROI = region of interest; SE = standard error; SLF = superior longitudinal fasciculus.
For each ROI, the effects of age, sex, handedness, DTI protocol and FA values of the other ROIs were regressed out and the standardized residuals were used in the analyses.
Fig. 3.
Semi-partial correlation between τ and mean fractional anisotropy (FA) of the right superior longitudinal fasciculus (SLF), separately for patients with ADHD and controls. Both variables were standardized to z-scores. *Significant correlation between FA of the right SLF and τ.
Discussion
In this study we investigated the association of the shape of RT distribution with white matter integrity of a priori ROIs within the attention network model in a large sample of adults with ADHD. We found decreased μ and increased τ in patients with ADHD compared with controls. Consistent with our hypothesis, the strongest effect was found for τ, and increased levels of τ were also associated with increased symptoms of inattention. We found τ to be linked to the microstructural white matter integrity of the right SLF, with increased levels of τ being associated with lower mean FA. This effect was specific to the patients with ADHD.
Several studies in children and adolescents have applied ex-Gaussian analyses to RT data and have shown consistently that increased τ is associated with ADHD.6–14 To our knowledge, the present study is the first of this size performed in adults that used ex-Gaussian analyses to demonstrate that these childhood findings may represent a persistent deficit in patients with ADHD. In addition, the correlation of τ with inattention symptoms supports earlier literature suggesting that excessively long RTs represent lapses of attention.7–10
We showed that increased τ is associated with lower FA values in the right SLF. Thus, within the attentional network model,21 the white matter microstructure of the SLF might be a particularly important mechanism underlying poor attention in patients with ADHD. We could not replicate an earlier finding by Lin and colleagues,13 who showed that the CB was associated with μ and τ in patients with ADHD; this could be explained by sample and/or methodological differences between our studies. Lin and colleagues investigated children and adolescents (n = 56) and used individual-based tractography, which is different from our atlas-based DTI method. The present study did confirm our expectation that the association between mean FA and τ is lateralized to the right hemisphere. Asymmetry in the brain substrates of attention has been the subject of much research,21,43 and 2 recent meta-analyses of functional MRI studies have suggested that altered brain activity in a right-hemispheric network underlies attention problems in patients with ADHD.44,45 Several studies have pointed out that the right-hemispheric dominance for attentional functions has an anatomic basis.26,43,46 Children and adults with ADHD show attention difficulties for the left visual field47–49 and an altered architecture of right-hemispheric attentional mechanisms, and more specifically, deficits in the right anterior region have been implicated.48 Our results extend these findings by pinpointing reduced white matter microstructural integrity in the right SLF as a potential locus for impaired response stability as measured with τ. This finding may thus contribute to a better understanding of lateralized attentional deficits in patients with ADHD.
In contrast to our expectations, we could not replicate a direct association between FA in the 6 ROIs with the categorical diagnosis of ADHD. The absence of a significant association between FA values and diagnosis in our study, despite earlier findings of such a link,25–27 might be explained by the heterogeneity of the ADHD phenotype.2 ADHD is likely to have multiple neurobiological causes,21,50 and this might lead to difficulties finding a direct association between ADHD and a specific neurobiological marker consistently. Variations at the neural system level might be more closely linked to τ than the category ADHD as such. Thus, reducing phenotypic heterogeneity at the clinical diagnostic level by studying ADHD-linked quantitative traits at the neurocognitive level, which are more biologically based (such as τ), could help to elucidate the neurobiological substrates underlying specific behavioural aspects of ADHD.
Our study might have implications beyond ADHD, as elevated τ has been observed not only in patients (children and adults) with ADHD, but also in patients with autism,11 schizophrenia51 and bipolar disorder.52 Further research on the microstructural white matter integrity of the SLF might also be interesting in these disorders. Moreover, mean FA of the SLF is considerably heritable (58%)53 and may be useful as an endophenotype explaining genetic effects on ADHD and other psychiatric disorders as it is potentially more directly linked to gene expression than a clinical diagnosis.5,21
Limitations
In addition to the clear strengths of this study, which lie in the size and the deep phenotyping of our adult sample, several limitations must also be taken into account when considering the present findings. First, we used an ROI approach to control for type I error by limiting the number of statistical tests instead of correcting for the large number of voxels in the brain. As our a priori hypothesis entailed that τ reflects attentional processes, we based our ROIs on the attentional network model of Makris and colleagues21 and on previous work by Lin and colleagues13 and Konrad and colleagues.28 However, while providing the largest statistical power, our ROI approach did not allow us to formulate novel hypotheses about other regions important for attention and ADHD; whole brain analyses in larger samples will be necessary. Similarly, as we used the diagnostic measure only to link FA values, we might have missed more specific links with quantitative measures of inattention and hyperactivity/impulsivity. A further potential limitation of our study might lie in the SLF consisting of 4 separate components, the SLF I, SLF II, SLF III and the arcuate fascicle.22 Specifically, the SLF II has been associated with FA reductions in adults with ADHD.26 We quantified the FA in the entire SLF after skeletonization in order to account for realignment issues. However, with this procedure the subcomponents of the SLF could not be evaluated. Given that the SLF components could have different functional affiliations, we may have missed more regionally localized effects. In future research, it might be interesting to inspect other diffusion parameters, such as mean, axial or radial diffusivity,54 which we did not investigate in the present study. Furthermore, a combination of diffusion imaging with electrophysiological methodologies might provide more insight into the mechanisms underlying moment by moment fluctuations in performance, which might be associated with the energy metabolism in the brain.15 Such an explanation of RT variability would be well in line with the cognitive-energetic model of ADHD.55 A combination of these methodologies would allow inspecting electrophysiological correlates of attention over time and their link to the microstructure of the right SLF in patients with ADHD. A final potential limitation of this study is that diffusion imaging data were acquired using 2 different protocols. This might have reduced the power of our analyses, but should not have influenced our results in other ways. First, we performed all analyses controlling for scanner acquisition protocol even though group and sex representation did not differ significantly across scanner acquisition protocols. Second, performing the association analysis of mean FA of the SLF, ILF and CB with τ and ADHD diagnosis for the 2 scan protocols separately confirmed the same pattern of results in both (Appendix 1, Table S8).
Conclusion
The present results shed more light on the complex association between performance variability (as indexed by more frequent excessively long RTs), attention problems and microstructural white matter abnormalities of the right SLF in adults with ADHD.
Acknowledgements
The authors thank Paul Gaalman for technical assistance with MRI scanning and Janneke Dammers for assistance with recruitment and testing. The authors also thank all of the participants of this study. This study was funded in part by grants awarded to B. Franke from the Brain & Cognition Excellence Program of the Netherlands Organization for Scientific Research (NWO, grant 433-09-229), the Netherlands Brain Foundation (Nederlandse Hersenstichting, grant 15F07[2]27), and by a Vici grant from NWO (grant 016.130.669). The research leading to these results has also received funding from the European Community‘s Seventh Framework Programme (FP7/2007–2013) under grant 60245 (IMAGEMEND). The funders had no further role in study design; in the collection, analysis and interpretation of data; in writing the report; or in the decision to submit the paper for publication. The sample used in this study is part of the international multicentre persistent ADHD collaboration (IMpACT). IMpACT unites major research centers working on the biology of ADHD persistence across the lifespan and has participants in the Netherlands, Germany, Spain, Norway, the United Kingdom, Sweden, the United States and Brazil. Principal investigators of IMpACT are Barbara Franke (chair), Andreas Reif (vice-chair), Stephen V. Faraone, Jan Haavik, Bru Cormand, Antoni Ramos Quiroga, Philip Asherson, Klaus-Peter Lesch, Jonna Kuntsi, Claiton Bau, Jan Buitelaar, Stefan Johansson, Henrik Larsson, Alysa Doyle, and Eugenio Grevet.
Footnotes
Competing interests: C. Kan was a paid member of the European Adult ADHD Advisory Board of Eli Lilly in 2011 and 2012 and was a paid lecturer at the Adult ADHD Academy. J. Buitelaar has served as a consultant, advisory board member and speaker for Bristol-Myers Squibb, Janssen Cilag BV, Eli Lilly, Novartis, Schering-Plough, Shire, Servier, Lundbeck and UCB. He is not a stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents, and royalties. No other competing interests declared.
Contributors: M. Onnink, M. Zwiers, M. Hoogman, D. Slaats-Willemse, J. Buitelaar and B. Franke designed the study. T. Wolfers, M. Onnink, M. Hoogman, J. Mostert, C. Kan and B. Franke acquired the data, which T. Wolfers, M. Onnink, M. Zwiers, A. Arias-Vasquez, M. Hoogman, C. Kan, J. Buitelaar and B. Franke analyzed. T. Wolfers, M. Onnink, M. Hoogman, C. Kan and B. Franke wrote the article, which all authors reviewed and approved for publication.
References
- 1.Simon V, Czobor P, Balint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194:204–11. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- 2.Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry. 2005;57:1215–20. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Tamm L, Narad ME, Antonini TN, et al. Reaction time variability in ADHD: a review. Neurotherapeutics. 2012;9:500–8. doi: 10.1007/s13311-012-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kofler MJ, Rapport MD, Sarver DE, et al. Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clin Psychol Rev. 2013;33:795–811. doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Frazier-Wood AC, Bralten J, Arias-Vasquez A, et al. Neuropsychological intra-individual variability explains unique genetic variance of ADHD and shows suggestive linkage to chromosomes 12, 13, and 17. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:131–40. doi: 10.1002/ajmg.b.32018. [DOI] [PubMed] [Google Scholar]
- 6.Kuntsi J, Klein C. Intraindividual variability in ADHD and its implications for research of causal links. Curr Top Behav Neurosci. 2012;9:67–91. doi: 10.1007/7854_2011_145. [DOI] [PubMed] [Google Scholar]
- 7.Hervey AS, Epstein JN, Curry JF, et al. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychol. 2006;12:125–40. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- 8.Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychol (Amst) 2000;104:167–90. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- 9.Gu S-L, Gau SS-F, Tzang S-W, et al. The ex-Gaussian distribution of reaction times in adolescents with attention-deficit/hyperactivity disorder. Res Dev Disabil. 2013;34:3709–19. doi: 10.1016/j.ridd.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Epstein JN, Langberg JM, Rosen PJ, et al. Evidence for higher reaction time variability for children with ADHD on a range of cognitive tasks including reward and event rate manipulations. Neuropsychology. 2011;25:427–41. doi: 10.1037/a0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geurts HM, Grasman RP, Verte S, et al. Intra-individual variability in ADHD, autism spectrum disorders and Tourette’s syndrome. Neuropsychologia. 2008;46:3030–41. doi: 10.1016/j.neuropsychologia.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Buzy WM, Medoff DR, Schweitzer JB. Intra-individual variability among children with ADHD on a working memory task: an ex-Gaussian approach. Child Neuropsychol. 2009;15:441–59. doi: 10.1080/09297040802646991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin HY, Gau S, Huang-Gu S, et al. Neural substrates of behavioral variability in attention deficit hyperactivity disorder: based on ex-Gaussian reaction time distribution and diffusion spectrum imaging tractography. Psychol Med. 2014;44:1751–64. doi: 10.1017/S0033291713001955. [DOI] [PubMed] [Google Scholar]
- 14.Vaurio RG, Simmonds DJ, Mostofsky SH. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47:2389–96. doi: 10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell VA, Oades RD, Tannock R, et al. Response variability in attention-deficit/hyperactivity disorder: a neuronal and glial energetics hypothesis. Behav Brain Funct. 2006;2:30. doi: 10.1186/1744-9081-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion‐weighted images. NMR Biomed. 1995;8:333–44. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 17.Fjell AM, Westlye LT, Amlien IK, et al. Reduced white matter integrity is related to cognitive instability. J Neurosci. 2011;31:18060–72. doi: 10.1523/JNEUROSCI.4735-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moy G, Millet P, Haller S, et al. Magnetic resonance imaging determinants of intraindividual variability in the elderly: combined analysis of grey and white matter. Neuroscience. 2011;186:88–93. doi: 10.1016/j.neuroscience.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 19.Williams BR, Hultsch DF, Strauss EH, et al. Inconsistency in reaction time across the life span. Neuropsychology. 2005;19:88–96. doi: 10.1037/0894-4105.19.1.88. [DOI] [PubMed] [Google Scholar]
- 20.Tamnes CK, Fjell AM, Westlye LT, et al. Becoming consistent: developmental reductions in intraindividual variability in reaction time are related to white matter integrity. J Neurosci. 2012;32:972–82. doi: 10.1523/JNEUROSCI.4779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makris N, Biederman J, Monuteaux MC, et al. Towards conceptualizing a neural systems-based anatomy of attention-deficit/hyperactivity disorder. Dev Neurosci. 2009;31:36–49. doi: 10.1159/000207492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–69. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence KE, Levitt JG, Loo SK, et al. White matter microstructure in attention-deficit/hyperactivity disorder subjects and their siblings. J Am Acad Child Adolesc Psychiatry. 2013;52:431–440. doi: 10.1016/j.jaac.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Ewijk H, Heslenfeld DJ, Zwiers MP, et al. Different mechanisms of white matter abnormalities in attention-deficit/hyperactivity disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2014;53:790–9. doi: 10.1016/j.jaac.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton LS, Levitt JG, O’Neill J, et al. Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuroreport. 2008;19:1705–8. doi: 10.1097/WNR.0b013e3283174415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makris N, Buka SL, Biederman J, et al. Attention and executive systems abnormalities in adults with childhood ADHD: a DT-MRI study of connections. Cereb Cortex. 2008;18:1210–20. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- 27.Cortese S, Imperati D, Zhou J, et al. White matter alterations at 33-year follow-up in adults with childhood attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74:591–8. doi: 10.1016/j.biopsych.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konrad A, Dielentheis TF, El Masri D, et al. Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. Eur J Neurosci. 2010;31:912–9. doi: 10.1111/j.1460-9568.2010.07110.x. [DOI] [PubMed] [Google Scholar]
- 29.Silk TJ, Vance A, Rinehart N, et al. White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum Brain Mapp. 2009;30:2757–65. doi: 10.1002/hbm.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franke B, Vasquez AA, Johansson S, et al. Multicenter analysis of the SLC6A3/DAT1 VNTR haplotype in persistent ADHD suggests differential involvement of the gene in childhood and persistent ADHD. Neuropsychopharmacology. 2010;35:656–64. doi: 10.1038/npp.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kooij JJS. Adult ADHD: diagnostic assessment and treatment. Springer; 2012. [Google Scholar]
- 32.Kooij JJS, Buitelaar JK, van den Oord EJ, et al. Internal and external validity of attention-deficit hyperactivity disorder in a population-based sample of adults. Psychol Med. 2005;35:817–27. doi: 10.1017/s003329170400337x. [DOI] [PubMed] [Google Scholar]
- 33.Groenestijn MAC, Akkerhuis G, Kupka R, et al. Gestructureerd klinisch interview voor de vaststelling van DSM-IV as I stoornissen (SCID-I) [Structured Clinical Interview for DSM-IV Axis I disorders (SCID-I)]. Lisse, The Netherlands: Swets & Zeitlinger; 1999. [Google Scholar]
- 34.Weertman A, Arntz A, Kerkhofs M. Gestructureerd diagnostisch interview voor DSM-IV persoonlijkheidsstoornissen (SCID II) [Structural clinical interview for DSM-IV personality disorders (SCID II)]. Lisse, The Netherlands: Swets & Zeitlinger; 2000. [Google Scholar]
- 35.De Sonneville L. Amsterdam neuropsychological tasks: a computer-aided assessment program. Computers in psychology. 1999;6:187–203. [Google Scholar]
- 36.Epstein JN, Hwang ME, Antonini T, et al. Examining predictors of reaction times in children with ADHD and normal controls. J Int Neuropsychol Soc. 2010;16:138–47. doi: 10.1017/S1355617709991111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacouture Y, Cousineau D. How to use MATLAB to fit the ex-Gaussian and other probability functions to a distribution of response times. Tutor Quant Methods Psychol. 2008;4:35–45. [Google Scholar]
- 38.Reese TG, Heid O, Weisskoff RM, et al. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–82. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 39.Zwiers MP. Patching cardiac and head motion artefacts in diffusion-weighted images. Neuroimage. 2010;53:565–75. doi: 10.1016/j.neuroimage.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 41.Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–47. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–82. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thiebaut de Schotten M, Dell’Acqua F, Forkel SJ, et al. A lateralized brain network for visuospatial attention. Nat Neurosci. 2011;14:1245–6. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- 44.Cortese S, Kelly C, Chabernaud C, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038–55. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hart H, Radua J, Mataix-Cols D, et al. Meta-analysis of fMRI studies of timing in attention deficit hyperactivity disorder (ADHD) Neurosci Biobehav Rev. 2012;36:2248–56. doi: 10.1016/j.neubiorev.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Shinoura N, Suzuki Y, Yamada R, et al. Damage to the right superior longitudinal fasciculus in the inferior parietal lobe plays a role in spatial neglect. Neuropsychologia. 2009;47:2600–3. doi: 10.1016/j.neuropsychologia.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Carter CS, Krener P, Chaderjian M, et al. Asymmetrical visual-spatial attentional performance in ADHD: evidence for a right hemispheric deficit. Biol Psychiatry. 1995;37:789–97. doi: 10.1016/0006-3223(94)00217-Q. [DOI] [PubMed] [Google Scholar]
- 48.Epstein JN, Conners CK, Erhardt D, et al. Asymmetrical hemispheric control of visual-spatial attention in adults with attention deficit hyperactivity disorder. Neuropsychology. 1997;11:467–73. doi: 10.1037/0894-4105.11.4.467. [DOI] [PubMed] [Google Scholar]
- 49.ter Huurne N, Onnink M, Kan C, et al. Behavioral consequences of aberrant alpha lateralization in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74:227–33. doi: 10.1016/j.biopsych.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Franke B, Faraone SV, Asherson P, et al. The genetics of attention deficit/hyperactivity disorder in adults, a review. Mol Psychiatry. 2012;17:960–87. doi: 10.1038/mp.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaiser S, Roth A, Rentrop M, et al. Intra-individual reaction time variability in schizophrenia, depression and borderline personality disorder. Brain Cogn. 2008;66:73–82. doi: 10.1016/j.bandc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Bora E, Vahip S, Akdeniz F. Sustained attention deficits in manic and euthymic patients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1097–102. doi: 10.1016/j.pnpbp.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 53.Kochunov P, Glahn DC, Lancaster JL, et al. Genetics of microstructure of cerebral white matter using diffusion tensor imaging. Neuroimage. 2010;53:1109–16. doi: 10.1016/j.neuroimage.2010.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–29. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sergeant J. The cognitive-energetic model: an empirical approach to attention-deficit hyperactivity disorder. Neurosci Biobehav Rev. 2000;24:7–12. doi: 10.1016/s0149-7634(99)00060-3. [DOI] [PubMed] [Google Scholar]