Abstract
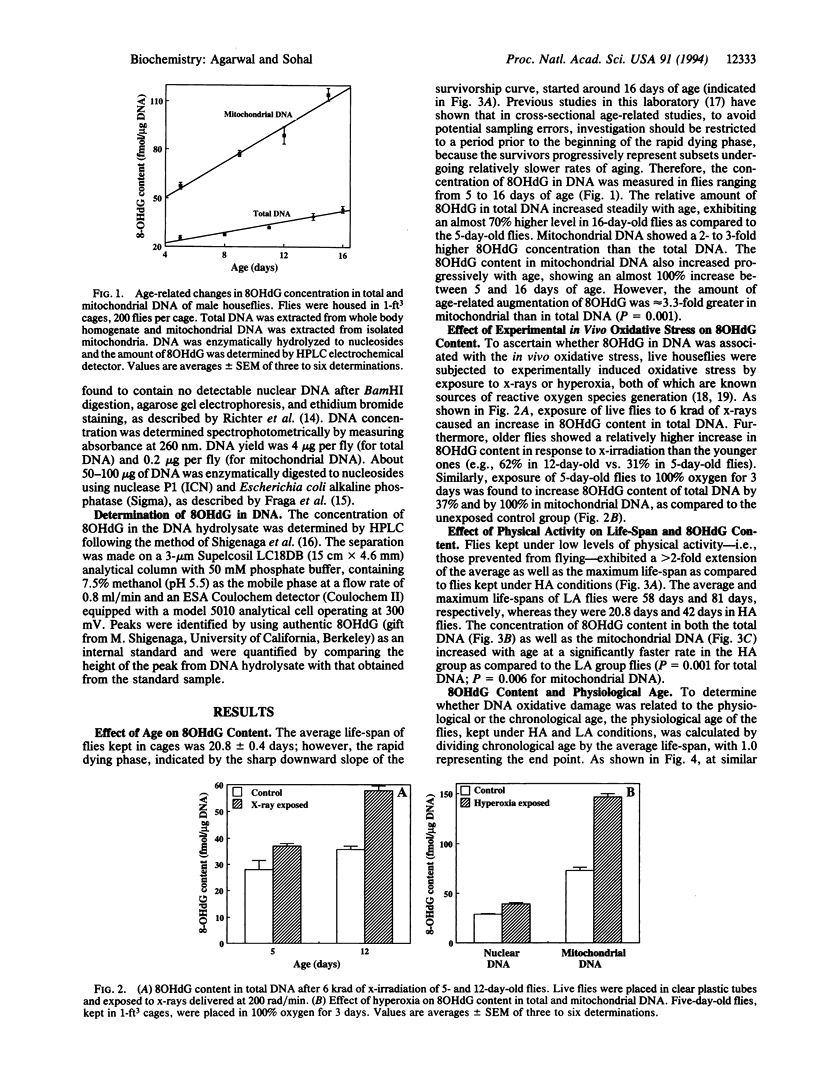
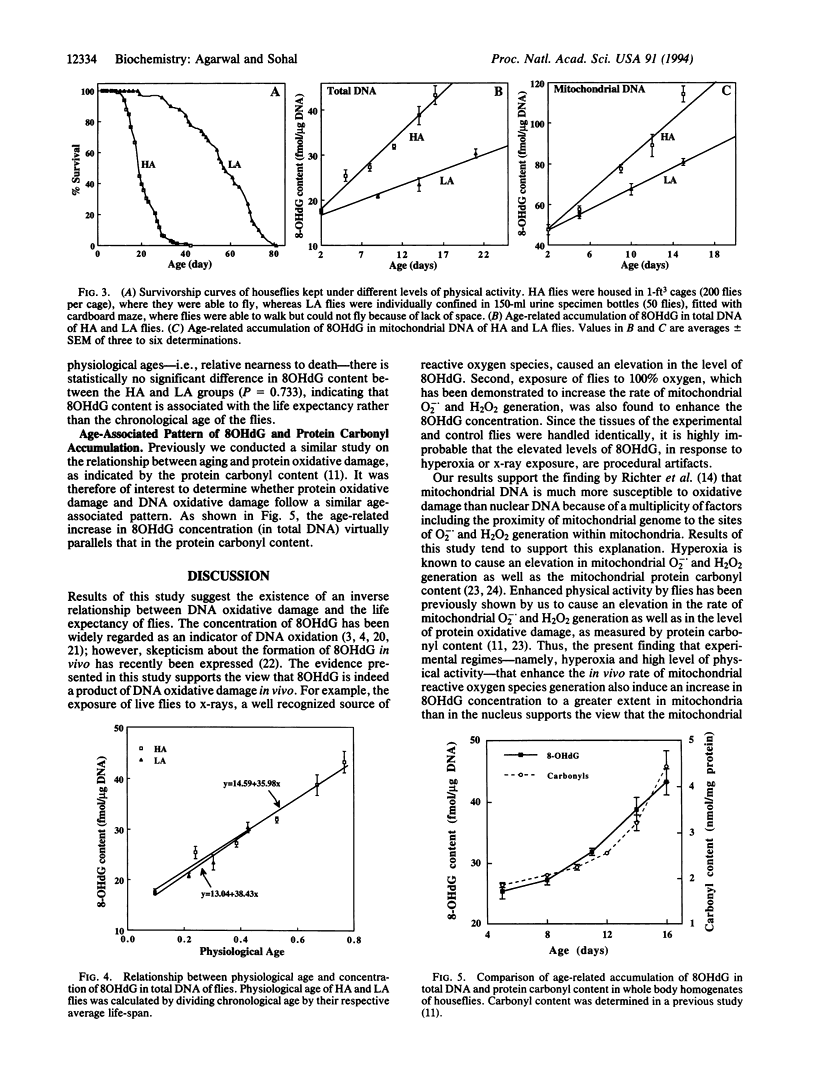
The objective of this study was to explore the relationship between oxidative molecular damage and the aging process by determining whether such damage is associated with the rate of aging, using the adult housefly as the experimental organism. Because the somatic tissues in the housefly consist of long-lived postmitotic cells, it provides an excellent model system for studying cumulative age-related cellular alterations. Rate of aging in the housefly was manipulated by varying the rate of metabolism (physical activity). The concentration of 8-hydroxydeoxyguanosine (80HdG) was used as an indicator of DNA oxidation. Exposure of live flies to x-rays and hyperoxia elevated the level of 8OHdG. The level of 8OHdG in mitochondrial as well as total DNA increased with the age of flies. Mitochondrial DNA was 3 times more susceptible to age-related oxidative damage than nuclear DNA. A decrease in the level of physical activity of the flies was found to prolong the life-span and corresponding reduce the level of 8OHdG in both mitochondrial and total DNA. Under all conditions examined, mitochondrial DNA exhibited a higher level of oxidative damage than total DNA. The 8OHdG levels were found to be inversely associated with the life expectancy of houseflies. The pattern of age-associated accrural of 8OHdG was virtually identical to that of protein carbonyl content. Altoghether, results of this study support the hypothesis that oxidative molecular damage is a causal factor in senescence.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal S., Sohal R. S. Relationship between aging and susceptibility to protein oxidative damage. Biochem Biophys Res Commun. 1993 Aug 16;194(3):1203–1206. doi: 10.1006/bbrc.1993.1950. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Endogenous oxidative DNA damage, aging, and cancer. Free Radic Res Commun. 1989;7(3-6):121–128. doi: 10.3109/10715768909087933. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Shigenaga M. K., Hagen T. M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato H., Jr, Hoselton M. A., Sohal R. S. An analysis of the effects of individual variation and selective mortality on population averages in aging populations. Exp Gerontol. 1979;14(3):133–140. doi: 10.1016/0531-5565(79)90028-7. [DOI] [PubMed] [Google Scholar]
- Farmer K. J., Sohal R. S. Relationship between superoxide anion radical generation and aging in the housefly, Musca domestica. Free Radic Biol Med. 1989;7(1):23–29. doi: 10.1016/0891-5849(89)90096-8. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Wong P. K., Altmiller D. H., Rickard R. C. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun. 1986;1(3):163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- Fraga C. G., Shigenaga M. K., Park J. W., Degan P., Ames B. N. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C. Nonrandom binding of the carcinogen N-hydroxy-2-acetylaminofluorene to repetitive sequences of rat liver DNA in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6943–6947. doi: 10.1073/pnas.81.22.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa M., Sugiyama S., Hattori K., Takasawa M., Ozawa T. Age-associated damage in mitochondrial DNA in human hearts. Mol Cell Biochem. 1993 Feb 17;119(1-2):95–103. doi: 10.1007/BF00926859. [DOI] [PubMed] [Google Scholar]
- Hayakawa M., Torii K., Sugiyama S., Tanaka M., Ozawa T. Age-associated accumulation of 8-hydroxydeoxyguanosine in mitochondrial DNA of human diaphragm. Biochem Biophys Res Commun. 1991 Sep 16;179(2):1023–1029. doi: 10.1016/0006-291x(91)91921-x. [DOI] [PubMed] [Google Scholar]
- Kasai H., Crain P. F., Kuchino Y., Nishimura S., Ootsuyama A., Tanooka H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986 Nov;7(11):1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993 Apr 22;362(6422):709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- Oliver C. N., Ahn B. W., Moerman E. J., Goldstein S., Stadtman E. R. Age-related changes in oxidized proteins. J Biol Chem. 1987 Apr 25;262(12):5488–5491. [PubMed] [Google Scholar]
- Orr W. C., Sohal R. S. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994 Feb 25;263(5150):1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- Ragland S. S., Sohal R. S. Ambient temperature, physical activity and aging in the housefly, Musca domestica. Exp Gerontol. 1975;10(5):279–289. doi: 10.1016/0531-5565(75)90005-4. [DOI] [PubMed] [Google Scholar]
- Ragland S. S., Sohal R. S. Mating behavior, physical activity and aging in the housefly, Musca domestica. Exp Gerontol. 1973 Jun;8(3):135–145. doi: 10.1016/0531-5565(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Richter C., Park J. W., Ames B. N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenaga M. K., Ames B. N. Assays for 8-hydroxy-2'-deoxyguanosine: a biomarker of in vivo oxidative DNA damage. Free Radic Biol Med. 1991;10(3-4):211–216. doi: 10.1016/0891-5849(91)90078-h. [DOI] [PubMed] [Google Scholar]
- Shigenaga M. K., Park J. W., Cundy K. C., Gimeno C. J., Ames B. N. In vivo oxidative DNA damage: measurement of 8-hydroxy-2'-deoxyguanosine in DNA and urine by high-performance liquid chromatography with electrochemical detection. Methods Enzymol. 1990;186:521–530. doi: 10.1016/0076-6879(90)86146-m. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Agarwal S., Dubey A., Orr W. C. Protein oxidative damage is associated with life expectancy of houseflies. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7255–7259. doi: 10.1073/pnas.90.15.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal R. S., Dubey A. Mitochondrial oxidative damage, hydrogen peroxide release, and aging. Free Radic Biol Med. 1994 May;16(5):621–626. doi: 10.1016/0891-5849(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Müller A., Koletzko B., Sies H. Effect of age and ambient temperature on n-pentane production in adult housefly, Musca domestica. Mech Ageing Dev. 1985 Mar;29(3):317–326. doi: 10.1016/0047-6374(85)90071-5. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Sohal B. H. Hydrogen peroxide release by mitochondria increases during aging. Mech Ageing Dev. 1991 Feb;57(2):187–202. doi: 10.1016/0047-6374(91)90034-w. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Toy P. L., Farmer K. J. Age-related changes in the redox status of the housefly, Musca domestica. Arch Gerontol Geriatr. 1987 Jul;6(2):95–100. doi: 10.1016/0167-4943(87)90001-x. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Oliver C. N. Metal-catalyzed oxidation of proteins. Physiological consequences. J Biol Chem. 1991 Feb 5;266(4):2005–2008. [PubMed] [Google Scholar]
- Stadtman E. R. Protein oxidation and aging. Science. 1992 Aug 28;257(5074):1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Starke-Reed P. E., Oliver C. N., Carney J. M., Floyd R. A. Protein modification in aging. EXS. 1992;62:64–72. doi: 10.1007/978-3-0348-7460-1_7. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Freeman B. A., Crapo J. D. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch Biochem Biophys. 1982 Sep;217(2):411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Freeman B. A., Levitt J. G., Crapo J. D. The effect of hyperoxia on superoxide production by lung submitochondrial particles. Arch Biochem Biophys. 1982 Sep;217(2):401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]


