Abstract
Congenital chylous ascites (CCA) is a rare disease that results from maldevelopment of the intra-abdominal lymphatic system. Few cases have been described and no gold standard treatment has been defined so far. Octreotide, a somatostatin analogue, has been used for the treatment of CCA, but always after a failed conservative approach with fasting, total parenteral nutrition (TPN) or medium chain triglyceride (MCT) feeds. We report the case of a newborn with CCA treated by fasting, TPN and octreotide for a period of 15 days until the abdominal distension was successfully reduced at which point treatment was switched to an MCT formula. On day 25 the patient was breastfed and was discharged on day 33. No recurrence of chylous ascites was noted. Our experience highlights the successful treatment with TPN and octreotide as the first step for the conservative approach of CCA in a newborn, reducing the length of treatment and hospitalisation.
Background
Chylous ascites is defined as the accumulation of chyle within the peritoneal cavity. Many pathological conditions can result in this disease, including malignant neoplasms, infections, liver cirrhosis, blunt abdominal trauma and surgery injury.
Congenital chylous ascites (CCA) is a rare condition resulting from maldevelopment of the intra-abdominal lymphatic system.1
Treatment of CCA is usually conservative and includes fasting, total parenteral nutrition (TPN) and medium-chain triglycerides (MCT) diet. Surgery is reserved to cases that are unresponsive to prolonged conservative treatment.
Octreotide, a synthetic peptide analogue of somatostatin, has been used safely in the treatment of chylothorax; whereas, only a few cases of CCA treated with octreotide are reported. However, there is no standard therapeutic approach for CCA due to the rarity of this condition. Therefore, no protocols exist on the timing and dosage of octreotide. Here, we describe a case of a newborn with CCA successfully treated with TPN and octreotide.
Case presentation
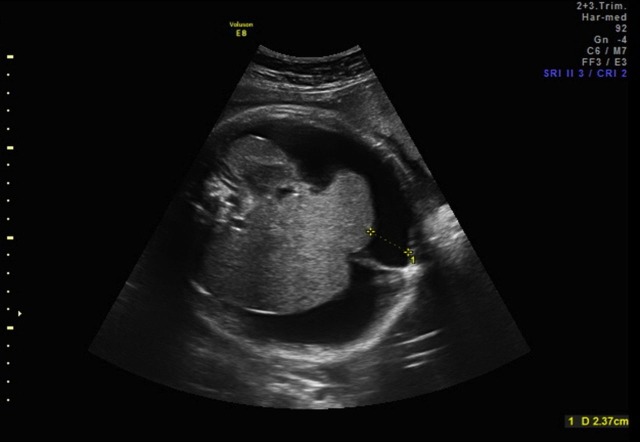
A 3.58 kg female neonate was born at 38 weeks’ gestation to a 34-year-old gravida 2, para 1 mother. Fetal ascites was detected by prenatal ultrasound examination at 20 weeks’ gestation. Fetal paracentesis was performed at 35 weeks and 640 ml of fluid were aspirated. Laboratory analysis proved the fluid to be chylous and cultures were negative for congenital infections. The persistence of the ascites was detected at repeated ultrasound at 36 weeks (figure 1), when fetal abdominal circumference was 41 cm (above the 95th percentile for gestational age). At birth, Apgar score was 5 and 7 at 1 and 5 min, respectively. The abdomen was massively distended (girth 43 cm) and dull to percussion.
Figure 1.
Ultrasound examination of the fetal abdomen at 36 weeks showing a massive fluid collection surrounding the liver.
Investigations
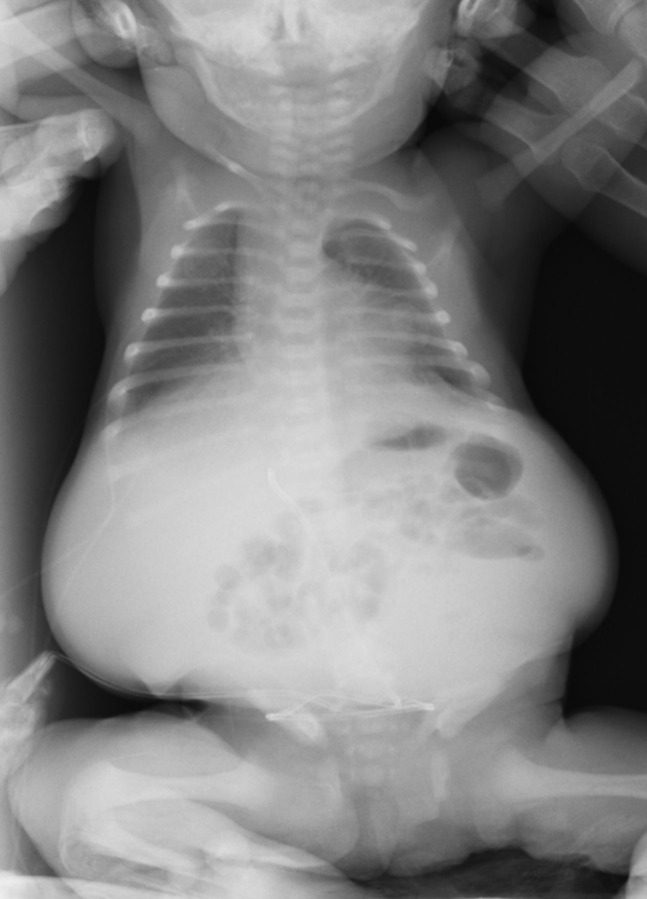
Owing to the severe respiratory compromise, a paracentesis was performed immediately at birth and 280 ml of straw-coloured fluid were aspirated. Analysis of the peritoneal fluid showed an elevated white blood cell count with 93% lymphocytes, confirming the diagnosis of chylous ascites. Ascitic fluid culture for viral and bacterial infections was negative. Echocardiogram revealed no cardiac abnormality. Abdominal x-ray confirmed massive ascites with centrally located bowel loops (figure 2). Abdominal ultrasound also showed a large amount of ascites; the liver and spleen were normal.
Figure 2.
Orthostatic abdominal x-ray performed at birth after the paracentesis.
Treatment
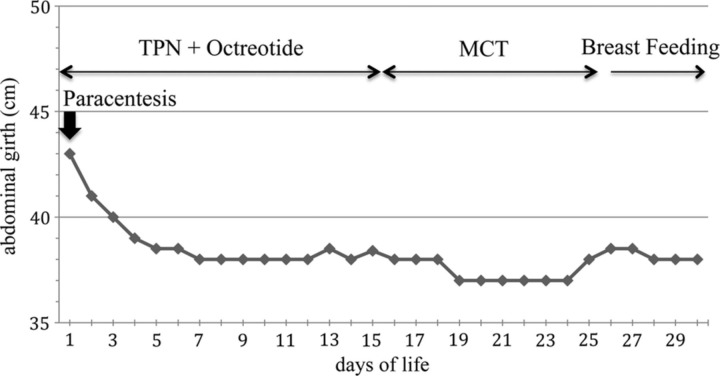
The infant was immediately treated with fasting, TPN and intravenous administration of octreotide at the dose of 3 μg/kg/h. Abdominal girth was measured daily and no subsequent paracentesis were performed. The abdominal girth gradually decreased to 38 cm and at day 15 octreotide therapy was stopped and enteral feeding with 90% MCT-based formula (Monogen; Nutricia Advanced Nutrition, the Netherlands) was introduced.
Outcome and follow-up
No recurrence of ascites was detected at repeat ultrasound examination and by day 25 the baby was exclusively breastfed and blood tests were normal. The infant was discharged on day 33. Abdominal sonography 1 month later showed no recurrence of ascites. At a follow-up of 6 months, the patient was doing well.
Discussion
CCA is a relatively rare entity, accounting for only 4% of cases of neonatal ascites, and it is primarily related to congenital abnormalities of the lymphatics.
Neonates with CCA usually present with abdominal distension and respiratory distress.2 In these patients, paracentesis is not only a diagnostic but also a therapeutic tool.3 4 However, this procedure should not be repeated if the baby shows no respiratory distress during conservative treatment, due to the inherent risk of developing hypoproteinemia and accidental perforation of a bowel loop. In our case only one paracentesis was needed at birth due to worsening respiratory distress related to abdominal distension.
The treatment of CCA is primarily conservative and is intended to decrease the lymph flow, allowing the gut to rest and decreasing the intestinal secretions. Conservative management includes TPN and enteral feeding with a formula high in MCT, low in long-chain triglycerides and enhanced in protein content.
However, these treatment modalities usually take many weeks to be effective.2 5
Surgical approach, consisting of resection of the macroscopic localised anomaly or ligation of the leaking lymphatic vessels, is considered if 1–2 months of conservative treatment fails.
Octreotide, a somatostatin analogue, has recently been used for treating chylothorax and chylous ascites caused by various disorders in infants.6 Octreotide is known to decrease gastric, pancreatic and intestinal secretions and to reduce the splanchnic blood flow, which may contribute to decrease lymph flow.
To the best of our knowledge, only six case reports have been published on somatostatin analogue treatment of CCA.1 2 6–9 Of these, in two cases the treatment with octreotide failed resulting in surgical intervention.8 9 Therefore, only five cases of CCA including our case were successfully treated with octreotide. In addition, variations regarding octreotide dosing regimens (0.5–8 mcg/kg/h), modes of drug administration (intravenous or subcutaneous), commencement and duration of treatment have been reported in the literature (table 1).
Table 1.
Case reports from the literature of congenital chylous ascites treated with octreotide
In all neonates with CCA, octreotide was used as a second-line treatment starting at 18 days, 20 days, 36 days and 6 months, respectively, when only TPN or MCT feeds failed in resolving chylous ascites. Our case is the first reported case in which CCA was successfully treated with octreotide started on the first day of life. After paracentesis the abdominal girth decreased to 41 cm and no reaccumulation of fluid was detected after the starting of the therapy. Owing to the presence of residual ascites a combined therapy was decided upon (fasting, TPN and octreotide) in order to reduce to a minimum the risks of relapse; this approach can possibly reduce the duration of therapy and avoid a new paracentesis. We used octreotide at the dose of 3 μg/kg/h for a period of 15 days. A rapid decrease of abdominal girth was observed within 7 days after the start of the treatment (figure 3). Abdominal ultrasound performed on day 25 showed no recurrence of chylous effusion. None of the reported side effects of octreotide, such as diarrhoea, fatty stools, liver dysfunction and transient glucose disturbance were noted in our patient.
Figure 3.
Changes of abdominal girth during the treatment.
Currently, no guidelines exist concerning the duration of therapy which may vary depending on the single patient. Although Karagol et al6 proposed MCT diet as a first option for the treatment of CCA, we suggest that octreotide along with TPN should be started soon after birth. This approach could be more effective in reducing treatment duration and length of hospitalisation. We believe TPN is much better than enteral feeding with MCT diet, because the bowel is bypassed and the association with octreotide allows a rapid resolution of the lymphatic leakage due to the reduction of lymph flow. Early and aggressive treatment aims at reducing the risk of complications related to prolonged fasting and TPN and could possibly avoid surgery. In our case MCT diet was introduced only when intra-abdominal fluid had completely disappeared and after octreotide infusion had been stopped. Switching to breast milk was done on day 25.
It can be argued that the real efficacy of octreotide cannot be assessed when it is used in association with TPN. According to the literature, TPN allows resolution of chylous ascites within 2–6 weeks5 10; whereas in all the cases in which octreotide treatment was successful, the duration of therapy ranged from 1 to 3 weeks.1 2 6 7
In conclusion, we believe that treatment with fasting and TPN plus octreotide treatment should be the first-line therapy for CCA after diagnostic paracentesis. We recommend intravenous octreotide infusion at the dosage of 3 mcg/kg/h as effective treatment for CCA in a newborn. However, further multicentre-controlled trials are needed to assess the most appropriate duration of treatment with octreotide and its optimal dosage in infants with CCA.
Learning points.
Intravenous infusion of octreotide associated with fasting and total parenteral nutrition (TPN) is an effective conservative approach for congenital chylous ascites (CCA).
Newborn with CCA should be treated with octreotide as soon as possible, shortening of TPN duration, hospital stay and avoiding surgery.
A dosage of 3 mcg/kg/h of octreotide is an effective dose in a neonate with CCA.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Huang Y, Zhuang S, Li Y, et al. Successful management of congenital chylous ascites in a premature infant using somatostatin analogue. Indian J Pediatr 2011;78:345–7. [DOI] [PubMed] [Google Scholar]
- 2.Caty MG, Hilfiker ML, Azizkhan RG, et al. Successful treatment of congenital chylous ascites with a somatostatin analogue. Pediatr Surg Int 1996;11:396–7. [DOI] [PubMed] [Google Scholar]
- 3.Aalami OO, Allen DB, Organ CH., Jr Chylous ascites: a collective review. Surgery 2000;128:761–8. [DOI] [PubMed] [Google Scholar]
- 4.Machmousch M, Amin A, Lanjaoui I, et al. Congenital chylous ascites: report of four cases and review of the literature. Ann Saudi Med 2000;20:436–9. [DOI] [PubMed] [Google Scholar]
- 5.Man DKW, Spitz L. The management of chylous ascites in children. J Pediatr Surg 1985;20:72–5. [DOI] [PubMed] [Google Scholar]
- 6.Karagol BS, Zenciroglu A, Gokce S, et al. Therapeutic management of neonatal chylous ascites: report of a case and review of the literature. Acta Pediatr 2010;99:1307–10. [DOI] [PubMed] [Google Scholar]
- 7.Mishra R, Kumar S. Octreotide in congenital chylous ascites an avoid requirement of total parenteral nutrition. Indian J Gastroenterol 2007;26:299–300. [PubMed] [Google Scholar]
- 8.te Pas AB, vd Ven K, Stokkel MP, et al. Intractable congenital chylous ascites. Acta Pediatr 2004;93:1403–5. [DOI] [PubMed] [Google Scholar]
- 9.Melo-Filho AA, Souza IJ, Leite CA, et al. Refractory congenital chylous ascites. Indian J Pediatr 2010;77:1335–7. [DOI] [PubMed] [Google Scholar]
- 10.Leibovitch I, Mor Y, Golomb J, et al. The diagnosis and management of postoperative chylous ascites. J Urol 2002;167:449–57. [DOI] [PubMed] [Google Scholar]