Abstract
The amplitude-integrated EEG (aEEG) is used to detect neonatal seizures in neonates with asphyxia, and despite limited data-information compared with standard EEG, aEEG is increasingly used as routine monitoring in neonatal intensive care units due to the logistic advantages. In addition, the aEEG background is of prognostic value in these infants. However, aEEG artefacts can lead to an erroneous interpretation of the background pattern. We report a full-term infant with severe perinatal asphyxia with diaphragm spasms that caused a significant alteration in aEEG background pattern. After administration of a neuromuscular blocker, the aEEG background transformed from discontinuous low-voltage pattern to a flat trace. The aEEG background pattern can be misinterpreted by electrical activity of non-cerebral origin. Administration of neuromuscular blockers is a rapid method to differentiate between cerebral and muscular activity on the aEEG when EEG is not (yet) available.
Background
The amplitude-integrated EEG (aEEG) is used to detect neonatal seizures in neonates with asphyxia, and despite limited data-information compared with standard EEG, aEEG is increasingly used as routine monitoring in neonatal intensive care units (NICUs) due to the logistic advantages. The change in background pattern of the aEEG has prognostic value in these infants.1 2
However, artefacts can lead to an erroneous interpretation of the background pattern, possibly resulting in overtreatment or undertreatment of patients.
This case demonstrates the possible pitfalls in aEEG interpretation and a method to differentiate between aEEG patterns caused by artefacts and those representing cerebral electric activity.
Case presentation
A primigravida with an uneventful full-term pregnancy was admitted during nightshift to our centre because of diminished fetal movements. Cardiotocography showed a fetal tachycardia with a sudden onset of severe bradycardia. An emergency caesarean was performed. A female infant was born with Apgar scores of 0, 0 and 2 after 1, 5 and 10 min, respectively.
Cardiopulmonary resuscitation (CPR) with intubation, ventilation and administration of adrenaline was instituted. Heart rhythm was detected after 10 min of CPR. The infant was transferred to ourNICU. The cause of the severe perinatal asphyxia was unrevealed. Blood gas analysis during CPR showed a pH < 6.6, base excess <−25 mmol/l and a serum lactate over 25 mmol/kg. Physical examination at 1 and 12 h after birth showed a hypotonic infant with absent brain stem reflexes, consistent with Sarnat stage 3.3
Investigations
During mechanical ventilation, frequent diaphragm spasms were noted. Routine aEEG was started, awaiting standard EEG. The aEEG monitoring (figure 1) showed a background pattern that matched the criteria of discontinuous low-voltage (DLV) pattern without signs of neonatal seizures.1 The raw EEG showed severely suppressed brain activity without signs of neonatal seizures.
Figure 1.
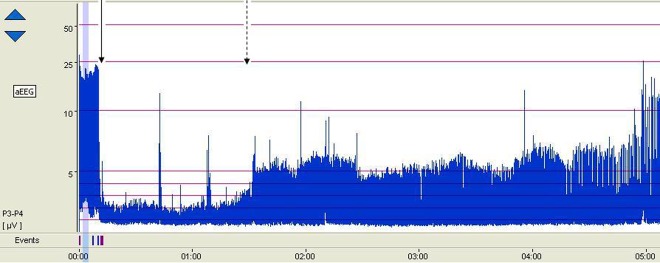
The aEEG background tracing of the patient. At 10 min after the start of the recording, rocuronium was administered (solid arrow). The aEEG background changed from discontinuous voltage pattern to flat trace pattern. After 1 h the background changed gradually to low-voltage pattern (dotted arrow).
As the diaphragm spasms interfered with mechanical ventilation a single dose of 0.3 mg/kg rocuronium was administered intravenously. Subsequently, the diaphragm spasms disappeared. In addition, immediately after administration of the rocuronium the aEEG background pattern changed from a DLV pattern to a flat trace (FT) (figure 2). At 1 h after rocuronium administration the background pattern gradually changed from an FT to continuous low-voltage (CLV) pattern.
Figure 2.
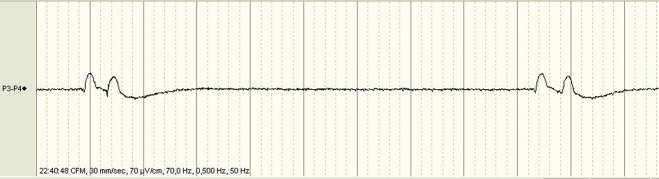
The raw EEG tracing during the first 10 min of the aEEG recording showing a flat line with isolated signal changes of relatively high amplitude and low frequency, which were in phase with the diaphragm spasms.
Treatment
Although the patient met the current criteria for therapeutic hypothermia, because at this time this was not yet standard therapy this treatment was not started.
Outcome and follow-up
Based on the findings on aEEG and the clinical condition, severe brain damage with adverse neurological outcome was diagnosed. Intensive care treatment was withdrawn and the patient died subsequently.
Discussion
The aEEG is important in monitoring the neurological condition and assessing the prognosis in perinatal asphyxia. However, we address that the distinctive patterns obtained by the aEEG algorithm may be influenced by confounding electrical activity of non-cerebral origin, as demonstrated in this patient. Immediately following paralysing the infant, the discontinuous pattern changed to an FT. The FT pattern was consistent with the neurological condition. We interpreted the erroneously monitored discontinuous background pattern as an artefact attributed to diaphragm muscle activity.
The EEG showed a flat line with isolated signal changes of relatively high amplitude and low frequency, which were in phase with the diaphragm spasms (figure 2). To eliminate artefacts in the aEEG, the original EEG signal is filtered by an asymmetric band pass filter, which strongly attenuates electrical activity containing frequencies below 2 Hz and above 15 Hz.4 The observed electrical changes in EEG caused by the diaphragm spasms were not filtered, as their frequency was above 2 Hz, resulting in an erroneously amplitude-integrated signal. These artefacts have been described previously by Hellstrom-Westas and coworkers.5 6
The pharmacological properties of rocuronium correspond to the clinical course and aEEG finding over time: rocuronium provides a swift neuromuscular block within minutes and lasts for approximately 60 min.7 Indeed, after a few minutes the diaphragm spasms disappeared and after 1 h reappeared with the aEEG background pattern gradually changing towards a low-voltage pattern.
Our considerations for the diaphragm spasms were cortical epileptic activity manifested as hiccups, diaphragm contractions as a hyperexcitable response to mechanical ventilation or loss of autonomous regulation. Epileptic activity or myoclonic status epilepticus originating from the deep basal nuclei may not be traced with a one- or two-channel (a)EEG and might have caused the diaphragm movements. Myoclonic status epilepticus is sometimes observed in adults after severe hypoxia and has been associated with poor outcome.8 As neuromuscular blocking agents eliminate electric activity resulting from muscular contractions and do not alter cerebral electric activity, it may differentiate between cerebral and muscular electric activities on the aEEG.
Hence, although standard EEG remains the gold standard, aEEG with its limitations in mind, such as artefacts as described above, can offer rapid information on neonatal brain function.
Learning points.
Amplitude-integrated EEG (aEEG) has an important role in monitoring neonatal seizures and background pattern with prognostic value.
aEEG has logistic advantages over standard EEG, despite the latter being more comprehensive.
aEEG background pattern may be erroneously classified due to a non-cerebral origin; in particular, when the aEEG is not in agreement with the clinical condition, the aEEG background must be interpreted simultaneously with the original EEG signal.
Differentiation between cerebral and muscular electrical activity on the aEEG when standard EEG is not readily available, administration of neuromuscular blockers can be useful.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.de Vries LS, Toet MC. Amplitude integrated electroencephalography in the full-term newborn. Clin Perinatol 2006;33:619–32. [DOI] [PubMed] [Google Scholar]
- 2.Hagmann CF, Robertson NJ, Azzopardi D. Artifacts on electroencephalograms may influence the amplitude-integrated EEG classification: a qualitative analysis in neonatal encephalopathy. Pediatrics 2006;118:2552–4. [DOI] [PubMed] [Google Scholar]
- 3.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 1976;33:696–705. [DOI] [PubMed] [Google Scholar]
- 4.Rosen I. The physiological basis for continuous electroencephalogram monitoring in the neonate. Clin Perinatol 2006;33:593–611. [DOI] [PubMed] [Google Scholar]
- 5.Hellstrom-Westas L, Rosen I, de Vries LS, et al. Amplitude-integrated EEG classification and interpretation in preterm and term infants. Neoreviews 2006;7:e76. [Google Scholar]
- 6.Hellstrom-Westas L, de Vries LS, Rosen I. An atlas of amplitude-integrated EEGs in the newborn. London: Parthenon, Ch 3, 2003: 41. [Google Scholar]
- 7.Rapp HJ, Altenmueller CA, Waschke C. Neuromuscular recovery following rocuronium bromide single dose in infants. Paediatr Anaesth 2004;14:329–53. [DOI] [PubMed] [Google Scholar]
- 8.Wijdicks EF, Hijdra A, Young GB, et al. Prediction of outcome in comatose survivors after cardiopulmonary resuscitation. Neurology 2006;67:203–10. [DOI] [PubMed] [Google Scholar]


