Abstract
The authors report the case of a 50-year-old alcoholic man with chronic hepatitis C virus infection, who presented to the emergency department with fever and exuberant ecchymoses and petechiae on both legs. After a careful examination and laboratory assessment, the not-so-obvious hypothesis of scurvy was disclosed. Simply with vitamin C replacement and nutritional advice, a dramatic improvement in his condition was observed. In modern societies, a generalised access to food renders scurvy as a rare disease, often misdiagnosed. A multidisciplinary approach is emphasised as the key to a more simple differential diagnosis, avoiding unnecessary exams and preventing serious complications, or even death, if left untreated.
Background
Focusing on a disease which was often mistakenly perceived as a thing of the past is a contribution to the accuracy of the 21st century medical practice.
Case presentation
In June 2011, a 50-year-old White man presented to the emergency department with a 2-week history of asthenia, occasional night fever, ecchymoses and petechiae on both legs. He described those lesions as initially violaceous plaques on the back of the knee and then purpuric, non-palpable lesions that have spread to the whole limbs. He denied any trauma or recent bleeding. He also denied weight loss, anorexia and sweating. He had no gastrointestinal complaints or other acute systemic symptoms.
His medical history was remarkable for hepatitis C, with no medical follow-up or treatment, and former intravenous drug abuse (heroin addiction), under replacement therapy with buprenorphine. He also had chronic obstructive pulmonary disease, depression, alcohol and tobacco abuse. He was currently taking mirtazapine, zolpidem, salbutamol and lactulosis. He denied use of aspirin, non-steroidal anti-inflammatory drugs or anticoagulants.
He was a divorced man, working as a security guard and resided alone at home. His family history was irrelevant. His dietary history revealed neither intake of fruits nor vegetables in the last 6 months.
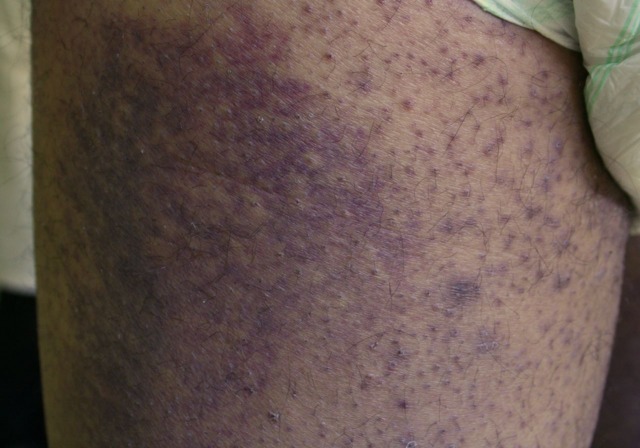
On clinical examination, we found an obese patient (body mass index, BMI=35), with a palpable non-tender liver, rotten teeth and infected hypertrophic gums (figure 1). Disseminated perifollicular petechiae on both legs and arms with palmar and plantar involvement were also found, with symmetric extensive haemorrhagic plaques on the back of the thighs (figure 2).
Figure 1.
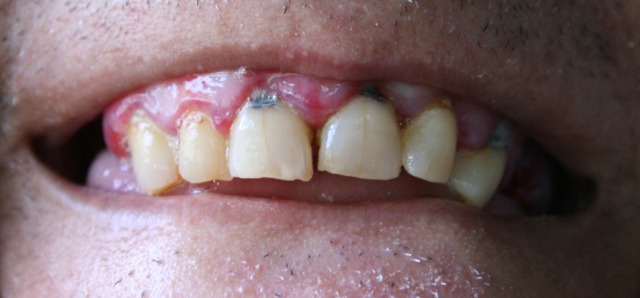
Rotten teeth and infected hypertrophic gums.
Figure 2.
Extensive haemorrhagic plaques on the back of the thighs.
Differential diagnosis included hepatitis C virus (HCV)-associated cryoglobulinaemia, malignancy, connective tissue, coagulation disorders or hypovitaminosis.
Laboratory data revealed a macrocytic anaemia (Hg 8.5 g/dl) with normal white blood cell (WBC) and platelet count. Folic acid measurement was low (2.1 ng/ml) and vitamin B12 was normal; iron studies were also normal. Cholesterol fractions were normal with high triglycerides (261 mg/dl). Blood clot factors and coagulation studies were normal. Erythrocyte sedimentation rate was 62 mm/h and reactive C protein (RCP) was 6.6 mg/dl. Serum electrolytes, liver function tests and alpha-fetoprotein were normal; gamma-glutamyltransferase was slightly elevated (340 U/l). Blood and urine cultures were negative. HIV and syphilitic serologies were negative; HCV immunoglobulin (Ig) G was positive with low viral load; hepatitis B surface antigen (HBsAg) and hepatitis B core (HBc) IgG were positive. Immunology profile, including anti-neutrophil cytoplasmic antibodies, cryoglobulins, anti-nuclear antibody (ANA), double-stranded DNA and complement were negative/normal. A vitamin C level was requested but the results were not available for several days. Thoracoabdominopelvic CT scan only revealed fatty liver. Skin biopsy (haemorrhagic plaque on the left thigh) revealed mild acanthosis with scarce superficial perivascular infiltrate, mild basal layer hyperpigmentation, erythrocyte extravasation and no signs of vasculitis.
According to these results, hypovitaminosis C—scurvy—emerged as the most likely diagnosis. The patient was given oral ascorbic acid 1000 mg daily; within a few days, a reduction in leg and thigh oedema was noted, as well as a regression of the cutaneous lesions. One week later, the ascorbic acid level result disclosed as being in the low normal range (0.7 mg/dl).
The patient came for a follow-up consultation a month later and total regression of the cutaneous lesions and previous complaints were found.
Differential diagnosis
The differential diagnosis of scurvy is extensive and we considered the most likely diseases regarding this particular patient. A complete blood count with peripheral smear, coagulation studies, biochemistry and liver function test eliminated platelet disorders and coagulopathies. A negative immunology rendered vascular collagen diseases unlikely.
Normal WBC count, normal protein electrophoresis and thoracoabdominopelvic CT scan were negative for malignancy.
Owing to the fact that this patient had a history of HCV infection, and presented to the clinics with fatigue, myalgia and purpura mainly on dependent areas, cryoglobulinaemia was considered too. Laboratorial investigations were negative for cryoglobulins, complement levels were normal and rheumatoid factor was negative. HCV viral load was also low. Likewise, polyarteritis nodosa related to coinfection with hepatitis B was also excluded.
Treatment
Ascorbic acid of 2000 mg was given orally once daily for 3 days; 1000 mg ascorbic acid orally id for 20 days and then oral multivitamins complex. Diet rich in fruits and vegetables.
Outcome and follow-up
The above-mentioned therapy showed long-term efficacy, except for the lost teeth, with total remission of symptoms within a few days and physical signs within 3 weeks. An appropriate diet also resulted in significant weight loss and alcohol withdrawal was promoted.
Discussion
Scurvy is a disease resulting from the deficiency in ascorbic acid—vitamin C. Since humans are unable to synthesise this vitamin, adequate intake of fruits and vegetables is crucial.1
Scurvy is an ancient disease, first described in 1500 BC in Ebers papyrus, and frequently seen among sailormen due to poor dietary intake during long sea trips.2 Its recognition has changed throughout the years, as it became rare in the developed world and its clinical course can mimic a series of other serious disorders. These include haematological abnormalities, meningococcemia and systemic lupus erythematosus, for which a fast diagnostic approach is mandatory.
In western countries, the incidence appears to be rising.3 Data from the National Health and Nutrition Examination Survey (NHANES 2003–2004) found that men aged 20–39 and those older than 60 years had a higher prevalence of deficiency than similarly aged women. Overall, 8.2% of men and 6% of women were deficient in vitamin C.4 Scurvy affects all ages, and groups at particular risk include isolated elderly patients, diet faddists, the institutionalised, patients with cancer, those with chronic abdominal pain and malabsorption syndromes or on renal dialysis—since vitamin C is not protein bound, it is lost during dialysis. Scurvy is also associated with psychiatric and behavioural disorders, alcoholism being the most common.1 Alcohol abuse can lead to scurvy in several ways: alcohol beverages contain no vitamin C and alcoholic eat poorly, often consuming little else; also, alcohol decreases the intestinal absorption of vitamin C.5 One particular group includes ‘bachelor’ or ‘widower’ scurvy, men who prepare their own meals or dine in restaurants, not ordering fresh fruits or vegetables.6
Vitamin C, also known as ascorbic acid, is a water-soluble necessary cofactor in collagen biosynthesis. Failure in collagen synthesis results in impaired wound healing, deficient osteoblast and fibroblast function and defective tooth formation.7 Vitamin C is absorbed in the small intestine and its half-life is of approximately 30 min.8 There is no storage site in the body and the total pool reaches 1500–2500 mg. Normal population's requirement is about 60–90 mg/day, but smokers require higher doses of vitamin C (110–125 mg/day) because of increased demand as a result of increased oxidative stress.9 The US Food and Drug Administration recommends a daily dietary allowance of vitamin C of 75 mg for women and 90 mg for men. Within 8–12 weeks of inadequate intake, or when the body pool falls below 350 mg, clinical manifestations arise. The most frequent signs and symptoms of scurvy are due to decreased production and increased fragility of collagen. Then, signs of scurvy are secondary to capillary fragility and include intradermal, perifollicular and gingival haemorrhage, bone pain from subperiosteal haemorrhage and loosening of the teeth from decreased dentine.10 Other signs and symptoms include weakness, lassitude, depression, arthralgias, petechiae, follicular hyperkeratosis, corkscrew hairs, non-palpable purpura, ecchymoses, mainly in legs and thighs, gingival swelling and halitosis.7 11 Late findings include dyspnoea, severe jaundice, fever, seizures, peripheral oedema, sicca syndrome and femoral neuropathy. If left untreated, patients may die from infection or sudden death from cerebral haemorrhage or haemopericardium.3
The diagnosis of scurvy is essentially a clinical one, based on a suggestive dietary history and the clinical manifestations described above. Plasma or leucocyte ascorbic acid levels may confirm the clinical diagnosis, but this level tends to reflect the recent dietary intake, rather than the actual tissue levels of vitamin C. An Oral Ascorbic Acid Tolerance Test was proposed by Dutra de Oliveira et al, in 1959, but it is important to emphasise that signs of scurvy can occur with low-normal serum levels of ascorbic acid.12 13 The strongest confirmation of disease is the resolution of manifestations after the administration of ascorbic acid.9
Laboratory findings include anaemia, which correlates with the severity and duration of the scurvy.1 14 15 Anaemia is caused by three main mechanisms: haemorrhage into tissue or loss into the gastrointestinal tract (normochromic normocytic); inadequate folic acid dietary consumption (macrocytic), as it was seen in the present reported case; or, rarely, iron deficiency, as its absorption depends on ascorbic acid (microcytic).1 Other laboratory findings occur in a minority of patients; in one-third leucopaenia is present, but platelet count and function remain normal.16
Skeletal x-ray changes are mostly seen at the end of long bones and include atrophy and disappearance of trabeculae causing a ‘ground glass’ roentgenographic appearance.7 The Fraenkel line, or scurvy line, appears when the cortex is thin and an irregular white line is seen at the metaphysis. Other changes include epiphysial shell with a central lucency (Wimberger's sign of scurvy), metaphyseal excrescences of the beaks, sub-epiphysial infractions an increased density of periostitis.17
The prognosis of scurvy is excellent, as the response to vitamin C replacement is often dramatic. The recommendations concerning ascorbic acid doses in oral treatment for scurvy are not consensual.3 7 10 11 18–20 Ascorbic acid of 1000 mg twice daily per os for 3 days, followed by 20 days of 500 mg twice daily was given to the presented patient, as well as a diet rich in fruits and vegetables. As mentioned above, other treatment dosages could also be considered. Symptoms usually disappear within 3–5 days, with most physical findings resolving within 1–2 weeks.
This case illustrates how scurvy, still being present in developed nations, should be considered as part of an expanded differential diagnosis.
Learning points.
Scurvy may be easily misdiagnosed as its clinical features often mimic other serious diseases; a high index of suspicion level is essential the specific populations at risk.
Serious complications may be the presenting complaint of a scurvy patient in the emergency room.
Dietary habits should always be included in a complete medical history.
A multidisciplinary approach may be the key to an early diagnosis, avoiding expensive and lengthy laboratory work-up, as well as invasive procedures.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol 1999;41:895–906. [DOI] [PubMed] [Google Scholar]
- 2.Major RH. A history of medicine. In: Thomas CC. ed Octavo: Bannerstone House, Springfield, 1954:5. [Google Scholar]
- 3.De Luna R, Colley BJ, Smith K, et al. Scurvy: an often forgotten cause of bleeding. Am J Hematol 2003;73:85–7. [DOI] [PubMed] [Google Scholar]
- 4.Hampl JS, Taylor CA, Johnston CS. Vitamin C deficiency and depletion in the United States: the Third National Health and Nutrition Examination Survey, 1988 to 1994. Am J Public Health 2004;94:870–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fazio V, Flint DM, Wahlqvist ML. Acute effects of alcohol on plasma ascorbic acid in healthy subjects. Am J Clin Nutr 1981;34:2394–6. [DOI] [PubMed] [Google Scholar]
- 6.Connelly TJ, Becker A, McDonald JW. Bachelor scurvy. Int J Dermatol 1982;21:209–11. [DOI] [PubMed] [Google Scholar]
- 7.Algahtani HA, Abdu AP, Khojah IM, et al. Inability to walk due to scurvy: a forgotten disease. Ann Saudi Med 2010;30:325–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olmedo JM, Yiannias JA, Windgassen EB, et al. Scurvy: a disease almost forgotten. Int J Dermatol 2006;45:909–13. [DOI] [PubMed] [Google Scholar]
- 9.Velandia B, Centor RM, McConnell V, et al. Scurvy still present in developed countries. J Gen Intern Med 2008;23:1281–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamura Y, Welch DC, Zic JA, et al. Scurvy presenting as painful gait with bruising in a young boy. Arch Pediatr Adolesc Med 2000;154:732–5. [DOI] [PubMed] [Google Scholar]
- 11.Lim KH, Rajasoorya C, Chew LS. Simulated bleeding—a forgotten disease in a land of plenty. Singapore Med J 1996;37:157–9. [PubMed] [Google Scholar]
- 12.World Health Organization/NHD 99.11. Scurvy and its prevention and control in major emergencies. World Health Organization; http://www.who.int/nutrition/publications/emergencies/WHO_NHD_99.11/en/ (accessed 27 Dec 2011). [Google Scholar]
- 13.Oliveira JE, Pearson WN, Darby WJ. Clinical usefulness of the oral ascorbic acid tolerance test in scurvy. Am J Clin Nutr 1959;7:630–3. [DOI] [PubMed] [Google Scholar]
- 14.Bronte-Stewart B. The anemia of adult scurvy. QJM 1953;22:309–29. [PubMed] [Google Scholar]
- 15.Reuler JB, Broudy VC, Cooney TG. Adult scurvy. JAMA 1985;253:805–7. [PubMed] [Google Scholar]
- 16.Cox EV, Meynell MJ, Cooke , et al. Scurvy and anaemia. Am J Med 1962;32:240–50. [DOI] [PubMed] [Google Scholar]
- 17.Choi SW, Park SW, Kwon YS, et al. MR imaging in a child with scurvy: a case report. Korean J Radiol 2007;8:443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pimentel L. Scurvy: historical review and current diagnostic approach. Am J Emerg Med 2003;21:328–32. [DOI] [PubMed] [Google Scholar]
- 19.Dubé Mark MD. Scurvy in a man with schizophrenia. CMAJ 2011;183:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maltos AL, Silva LL, Junior AG, et al. Scurvy in a patient with AIDS. Case Rep 2011;44:122–3. [DOI] [PubMed] [Google Scholar]