Abstract
A 24-year-old patient with 7-week amenorrhoea consulted for vaginal bleeding without abdominal pain. Ultrasonography revealed a 7 × 4 cm solid right pelvic mass. There was no visible intrauterine gestational sac. The serum β-human chorionic gonadotropin (β-hCG) level was 11 998 IU/l. Emergency laparoscopy was performed for a presumptive diagnosis of ectopic pregnancy. At laparoscopy, the right ovary was enlarged with a non-haemorrhagic 7 × 4 cm solid lesion, which was resected. The histological diagnosis was a dysgerminoma with immunohistochemistry showing nests of syncytiotrophoblastic cells, which were the origin of the hCG production. There was no pregnancy, either intrauterine or ectopic. There was no evidence of metastasis from the dysgerminoma on the positron-emission tomography scanner. The patient underwent a second procedure for surgical staging of this ovarian germ-cell tumour. This ovarian dysgerminoma was staged FIGO 1A, and the patient did not receive adjuvant therapy. There was no recurrence at the last 8-month follow-up.
Background
The association of elevated serum human chorionic gonadotropin (hCG) and a pelvic mass is so common and usually associated with ectopic pregnancy that the gynaecologists who first managed this case overlooked alternative diagnoses. Nevertheless, retrospectively, the suspicion of a malignant ovarian tumour secreting hCG was high.
This case gives us the opportunity to discuss the differential diagnosis of the association of a pelvic mass on ultrasonography and elevated serum hCG level.
The differential diagnosis includes aetiologies that non-specialists may not be familiar with, and this causes most of the interest in this case, as the clinical picture of a pelvic mass and elevated hCG is commonly encountered.
Case presentation
A 24-year-old patient with no relevant prior medical history consulted for vaginal bleeding lasting for 4 weeks, without abdominal pain. There was a 7-week amenorrhoea and the patient assumed that she was pregnant. Right iliac fossa palpation was slightly painful, with no detected mass on bimanual examination. Serum β-human chorionic gonadotropin (β-hCG) level was 11 998 IU/l.
Investigations
Ultrasonography revealed a 7 × 4 cm solid right pelvic mass with some irregular internal echogenicity, which seemed distinct from the normal-appearing right ovary (figure 1). The left adnexa and the uterus were unremarkable; there was no visible intrauterine gestational sac and no free abdominal fluid.
Figure 1.
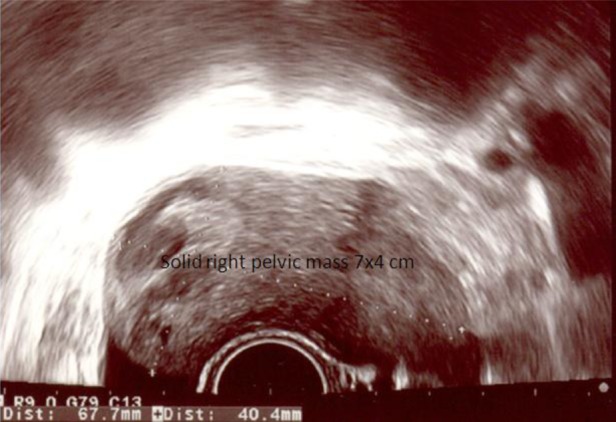
Ultrasonography showing a solid right pelvic mass with some irregular internal echogenicity.
Differential diagnosis of a pelvic mass on ultrasonography and elevated serum hCG level
Frequent: Ectopic tubal pregnancy; haemorrhagic corpus luteum associated with a pregnancy of unknown location; a pregnancy of unknown location with the incidental finding of a mature teratoma.
Rare: A pregnancy of unknown location or any cause of hCG elevation outside of pregnancy with any incidental pelvic mass; theca-lutein cyst associated with invasive mole or gestational choriocarcinoma; ovarian pregnancy.
Very rare: hCG-producing ovarian germ-cell tumour (dysgerminoma, mixed germ-cell tumour, ovarian choriocarcinoma and embryonal carcinoma); hCG-producing extragonadal germ-cell tumour with an incidental pelvic mass.
A positive pregnancy test with a pelvic mass and no intrauterine pregnancy on ultrasonography first raises the suspicion of an ectopic pregnancy. At serum β-hCG level above 2000 IU/l, a gestational sac should be visualised by ultrasound examination if an intrauterine pregnancy is present. In the cases of tubal ectopic pregnancy confirmed by histology, the most common preoperative finding on ultrasonography is an empty endometrial cavity with an inhomogeneous adnexal mass, and not the more specific hyperechoic ‘tubal ring’ or extrauterine visible embryo.1 2 The sonographic appearance of haemorrhagic corpus luteum cysts varies widely.3 4 Commonly, an early gestation cannot be localised on the initial transvaginal scan.5 So, the two most likely diagnoses are either an ectopic tubal pregnancy or a pregnancy of unknown location associated with a haemorrhagic corpus luteum. The most common ovarian tumour in pregnancy is the benign cystic mature teratoma (dermoid cyst),6–8 and this incidental finding in the work-up of a pregnancy of unknown location would make up the third most likely diagnosis.
Any other less common incidental pelvic mass may also be associated with a pregnancy of unknown location, mainly: ovarian cystadenoma, ovarian endometrioma, ovarian fibroma, uterine pedunculated myoma, paraovarian cyst and malignant ovarian tumour.6–9 The proportion of ovarian malignancy among large, complex, persisting masses during pregnancy ranges from 1–2%8 10 11 to 8.5%.6 9 12 Borderline epithelial tumours and germ-cell tumours (principally dysgerminoma and immature teratoma) together comprise the vast majority of these malignant tumours.10 13 Elevated serum tumour markers (α-feto protein, lactate dehydrogenase, placental alkaline phosphatase, and outside of pregnancy, hCG) are helpful in the work-up of a solid pelvic mass in a young woman, suggesting an ovarian germ-cell tumour; elevated CA-125 suggests an ovarian borderline or malignant epithelial tumour, although not sensitive, and in young women, not specific.
Any incidental pelvic mass may also be found with: a false-positive hCG result (most commonly anti-animal antibodies)14; quiescent gestational trophoblastic disease15; pituitary hCG found in perimenopausal and postmenopausal women14; exogenous hCG use in assisted reproductive technology or misuse in athletes or weight-loss programmes14 16; trophoblastic differentiation in any cancer (lung, biliary, bladder, sarcoma, gastrointestinal).17
Invasive moles and gestational choriocarcinomas penetrate the myometrium and may leave an empty uterine cavity.18 Associated multiseptated theca-lutein cysts are common. There may be increased myometrial echogenicity and vascularity.19
Ovarian pregnancies account for 3% of all ectopic pregnancies.20–22 The typical ultrasonographic appearance is a hyperechoic ring on or within the ovary23; however, the preoperative ultrasonographic distinction from a tubal pregnancy or corpus luteum is difficult.21 23
Various ovarian germ-cell tumours (some dysgerminomas and mixed germ-cell tumours, ovarian choriocarcinoma and embryonal carcinoma) produce hCG. Dysgerminoma is one of the most common ovarian malignancies in young women.10 24 Five per cent of the dysgerminomas contain isolated or nests of syncytiotrophoblastic cells (which do not justify a diagnosis of mixed germ-cell tumour) and produce hCG.25
Treatment
Emergency laparoscopy was performed for a presumptive diagnosis of ectopic pregnancy. At laparoscopy, the uterus, both tubes and left ovary, were normal; the right ovary was enlarged with a 7 × 4 cm reddish solid lesion with increased surface vascularity, but not haemorrhagic, which was considered an ovarian cyst (figure 2). Cystectomy was undertaken; this lesion turned out to be purely solid and was retrieved in two parts within an endobag.
Figure 2.
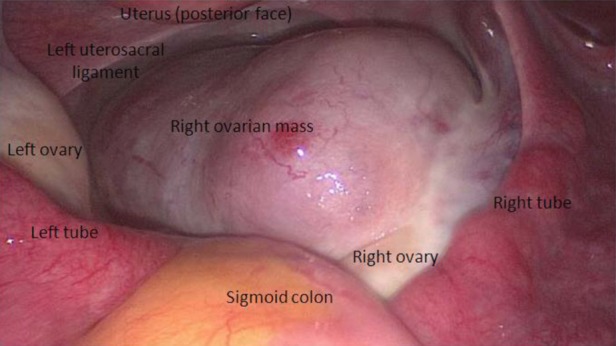
Laparoscopic view of the right ovarian mass.
The histological diagnosis of the ovarian mass was a dysgerminoma with syncitiotrophoblastic cells. Microscopically, the aspect was typical of dysgerminoma. Immunohistochemistry showed placental-like alkaline phosphatase (PLAP) positivity, which is characteristic of various ovarian germ-cell tumours and organic cation transporter 4 positivity, which is more specific for dysgerminoma. Immunostaining with leucocyte common antigen (a marker of lymphoma) was negative, but that with β-hCG revealed syncitiotrophoblastic cells (figure 3). These giant multinucleated cells did not show frequent or atypical mitoses. They were isolated or aggregated in small nests. In rare cases, some of them formed larger structures without a cytotrophoblastic component. The syncitiotrophoblastic elements were estimated to be 5% of the tumour. These cells were the origin of the hCG production; there was no pregnancy, either intrauterine or ectopic.
Figure 3.
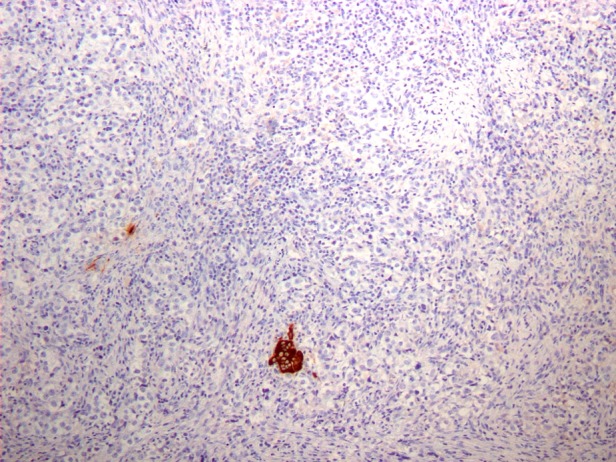
Immunostaining with β-hCG revealing syncitiotrophoblastic cells.
Only malignant ovarian germ-cell tumours produce hCG (some dysgerminomas and mixed germ-cell tumours, ovarian non-gestational choriocarcinomas, embryonal carcinomas, polyembryomas). If such a suspicion is raised preoperatively or peroperatively, the case should be managed by a gynaecological oncologist.26 Laparoscopic enucleation of the intact lesion within an endobag and without spillage would be adequate.27 The patient should be informed that a second procedure might be necessary in case of malignancy. Frozen section may be used, so as to complete the surgical treatment within a single procedure: accuracy is above 90% for ovarian tumours as a whole, although it is less for borderline and mucinous epithelial tumours, and not established but likely high for dysgerminoma.28 29 Should the suspicion of malignancy be confirmed, unilateral salpingo-oophorectomy would be the minimal surgical resection.30 31 Peritoneal washing and biopsies, omental and contralateral ovarian biopsies and locoregional lymphadenectomy are generally recommended,32 33 although they are debated in ovarian germ-cell tumours with no extraovarian macroscopic disease.30 31 33 Fertility-sparing surgery (conservation of the uterus and contralateral adnexa) is very well established in young women with early-stage ovarian germ-cell tumours.30–34 As dysgerminoma is highly chemosensitive, fertility-sparing of the uterus and contralateral adnexa could also be considered even in advanced-stage disease, in young women.30 31 Patients with dysgerminoma limited to one ovary with intact capsule and negative peritoneal washing (stage IA) do not need chemotherapy.30–34 Recurrences can be effectively salvaged with chemotherapy.30
Outcome and follow-up
Serum tumour markers were done upon reception of the histological diagnosis: α-feto protein, lactate dehydrogenase, phosphatase alkaline and CA-125 levels were normal.
A positron-emission tomography scanner was performed that showed suspicious hypermetabolic mediastinal enlarged lymph nodes and a hypermetabolic 7 mm lung lesion. Bronchoscopy was inconclusive because of lack of tolerance to this procedure; mediastinal lymph node biopsy by thoracoscopy and sputum examination showed no malignancy but the presence of Mycobacterium tuberculosis. Lung and mediastinal nodal tuberculosis was diagnosed, and there was no radiological evidence of metastasis from the dysgerminoma. The patient underwent a second procedure for surgical staging by laparoscopy with peritoneal washing and biopsies, right salpingo-oophorectomy, omental biopsy, and right pelvic and paraaortic lymphadenectomy. No malignancy was found in these tissues. This ovarian dysgerminoma was then staged FIGO 1A, and the patient did not receive adjuvant therapy. There was no recurrence at the last 8-month follow-up.
Discussion
In cases of tubal ectopic pregnancy confirmed by histology with the preoperative finding on ultrasonography of an inhomogeneous adnexal mass, the mean serum hCG level is 2267 IU/l1; the higher hCG level in the case reported here is usually associated with an extrauterine visible embryo without cardiac activity.1
Adnexal masses associated with ectopic pregnancies usually are smaller than the 7 × 4 cm reported here on ultrasonography and at laparoscopy. Moreover, the ultrasonography found a solid mass with some irregular internal echogenicity, which is suggestive of a dysgerminoma in a young woman,35 and not an inhomogeneous lesion (a mixture of hyperechoic and anechoic tissues), which is suggestive of an ectopic pregnancy.
On colour Doppler sonography, ectopic pregnancies usually present a peripheric blood flow,36 whereas moderate or abundant internal colour content is indicative of a dysgerminoma in a young woman.35
MRI is more specific for ovarian malignancy than ultrasonography and computerised tomography scan.37 38 Therefore, it is most helpful in young women, in the investigation of a suspicious pelvic mass.
In the case reported here, colour Doppler sonography and MRI were not performed, because the patient was operated on in emergency, for a presumptive diagnosis of ectopic pregnancy.
Finally, at laparoscopy, ovarian pregnancy typically presents as a haemorrhagic dark-blue cystic lesion,22 39 and not as a solid reddish mass with no haemorrhage as described here.
Based on the preoperative and operative findings discussed above, the suspicion of a malignant ovarian tumour secreting hCG was high.
There are a few similar cases of ovarian tumours secreting hCG mistaken for ectopic pregnancy in the literature, mostly ovarian non-gestational choriocarcinomas and some dysgerminomas.40–43
Learning points.
Ovarian lesions that are purely solid on ultrasonography and at laparoscopy should raise the suspicion of a malignant tumour, some of which secrete hCG and may mimic ectopic pregnancy.
Whenever operating on an ovarian solid or partly solid lesion, one should consider the likelihood of malignancy and resect this lesion without spillage.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Condous G, Okaro E, Khalid A, et al. The accuracy of transvaginal ultrasonography for the diagnosis of ectopic pregnancy prior to surgery. Hum Reprod 2005;20:1404–9. [DOI] [PubMed] [Google Scholar]
- 2.Dialani V, Levine D. Ectopic pregnancy: a review. Ultrasound Q 2004;20:105–17. [DOI] [PubMed] [Google Scholar]
- 3.Swire MN, Castro-Aragon I, Levine D. Various sonographic appearances of the hemorrhagic corpus luteum cyst. Ultrasound Q 2004;20:45–58. [DOI] [PubMed] [Google Scholar]
- 4.Durfee SM, Frates MC. Sonographic spectrum of the corpus luteum in early pregnancy: gray-scale, color, and pulsed Doppler appearance. J Clin Ultrasound 1999;27:55–9. [DOI] [PubMed] [Google Scholar]
- 5.Kirk E, Papageorghiou AT, Condous G, et al. The diagnostic effectiveness of an initial transvaginal scan in detecting ectopic pregnancy. Hum Reprod 2007;22:2824–8. [DOI] [PubMed] [Google Scholar]
- 6.Schmeler KM, Mayo-Smith WW, Peipert JF, et al. Adnexal masses in pregnancy: surgery compared with observation. Obstet Gynecol 2005;105 (5 Pt 1):1098–103. [DOI] [PubMed] [Google Scholar]
- 7.Platek DN, Henderson CE, Goldberg GL. The management of a persistent adnexal mass in pregnancy. Am J Obstet Gynecol 1995;173:1236–40. [DOI] [PubMed] [Google Scholar]
- 8.Bernhard LM, Klebba PK, Gray DL, et al. Predictors of persistence of adnexal masses in pregnancy. Obstet Gynecol 1999;93:585–9. [DOI] [PubMed] [Google Scholar]
- 9.Whitecar MP, Turner S, Higby MK. Adnexal masses in pregnancy: a review of 130 cases undergoing surgical management. Am J Obstet Gynecol 1999;181:19–24. [DOI] [PubMed] [Google Scholar]
- 10.Leiserowitz GS, Xing G, Cress R, et al. Adnexal masses in pregnancy: how often are they malignant? Gynecol Oncol 2006;101:315–21. [DOI] [PubMed] [Google Scholar]
- 11.Bromley B, Benacerraf B. Adnexal masses during pregnancy: accuracy of sonographic diagnosis and outcome. J Ultrasound Med 1997;16:447–52. [DOI] [PubMed] [Google Scholar]
- 12.Hess LW, Peaceman A, O'Brien WF, et al. Adnexal mass occurring with intrauterine pregnancy: report of fifty-four patients requiring laparotomy for definitive management. Am J Obstet Gynecol 1988;158:1029–34. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal P, Kehoe S. Ovarian tumours in pregnancy: a literature review. Eur J Obstet Gynecol Reprod Biol 2011;155:119–24. [DOI] [PubMed] [Google Scholar]
- 14.Braunstein GD. False-positive serum human chorionic gonadotropin results: causes, characteristics, and recognition. Am J Obstet Gynecol 2002;187:217–24. Review. [DOI] [PubMed] [Google Scholar]
- 15.Cole LA, Khanlian SA, Giddings A, et al. Gestational trophoblastic diseases: 4. Presentation with persistent low positive human chorionic gonadotropin test results. Gynecol Oncol 2006;102:165–72. [DOI] [PubMed] [Google Scholar]
- 16.Olsen TG, Barnes AA, King JA. Elevated HCG outside of pregnancy—diagnostic considerations and laboratory evaluation. Obstet Gynecol Surv 2007;62:669–74; quiz 691. [DOI] [PubMed] [Google Scholar]
- 17.Demirtas E, Krishnamurthy S, Tulandi T. Elevated serum beta-human chorionic gonadotropin in nonpregnant conditions. Obstet Gynecol Surv 2007;62:675–9. [DOI] [PubMed] [Google Scholar]
- 18.Golfier F, Frappart L, Schott AM, et al. A plea for the creation of trophoblastic disease reference centers in France. J Gynecol Obstet Biol Reprod 2000;29:538–47. [PubMed] [Google Scholar]
- 19.Jain KA. Gestational trophoblastic disease: pictorial review. Ultrasound Q 2005;21:245–53. [DOI] [PubMed] [Google Scholar]
- 20.Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10 year population-based study of 1800 cases. Hum Reprod 2002;17:3224–30. [DOI] [PubMed] [Google Scholar]
- 21.Raziel A, Schachter M, Mordechai E, et al. Ovarian pregnancy-a 12-year experience of 19 cases in one institution. Eur J Obstet Gynecol Reprod Biol 2004;114:92–6. [DOI] [PubMed] [Google Scholar]
- 22.Odejinmi F, Rizzuto MI, Macrae R, et al. Diagnosis and laparoscopic management of 12 consecutive cases of ovarian pregnancy and review of literature. J Minim Invasive Gynecol 2009;16:354–9. [DOI] [PubMed] [Google Scholar]
- 23.Comstock C, Huston K, Lee W. The ultrasonographic appearance of ovarian ectopic pregnancies. Obstet Gynecol 2005;105:42–5. [DOI] [PubMed] [Google Scholar]
- 24.Smith HO, Berwick M, Verschraegen CF, et al. Incidence and survival rates for female malignant germ cell tumors. Obstet Gynecol 2006;107:1075–85. [DOI] [PubMed] [Google Scholar]
- 25.Roth LM, Talerman A. Recent advances in the pathology and classification of ovarian germ cell tumors. Int J Gynecol Pathol 2006;25:305–20. Review. [DOI] [PubMed] [Google Scholar]
- 26.Vernooij F, Heintz P, Witteveen E, et al. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: a systematic review. Gynecol Oncol 2007;105:801–12. [DOI] [PubMed] [Google Scholar]
- 27.Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet 2001;357:176–82. [DOI] [PubMed] [Google Scholar]
- 28.Ilvan S, Ramazanoglua R, Akyildizb EU, et al. The accuracy of frozen section (intraoperative consultation) in the diagnosis of ovarian masses. Gynecol Oncol 2005;97:395–9. [DOI] [PubMed] [Google Scholar]
- 29.Baker P, Oliva E. Review: a practical approach to intraoperative consultation in gynecological pathology. Int J Gynecol Pathol 2008;27:353–65. [DOI] [PubMed] [Google Scholar]
- 30.Parkinson CA, Hatcher HM, Earl HM, et al. Multidisciplinary management of malignant ovarian germ cell tumours. Gynecol Oncol 2011;121:625–36. [DOI] [PubMed] [Google Scholar]
- 31.Morice P, Denschlag D, Rodolakis A, et al. Recommendations of the Fertility Task Force of the European Society of Gynecologic Oncology about the conservative management of ovarian malignant tumors. Int J Gynecol Cancer 2011;21:951–63. [DOI] [PubMed] [Google Scholar]
- 32.Mangili G, Sigismondi C, Lorusso D, et al. Is surgical restaging indicated in apparent stage IA pure ovarian dysgerminoma? The MITO group retrospective experience. Gynecol Oncol 2011;121:280–4. [DOI] [PubMed] [Google Scholar]
- 33.Patterson DM, Rustin GJ. Controversies in the management of germ cell tumours of the ovary. Curr Opin Oncol 2006;18:500–6. [DOI] [PubMed] [Google Scholar]
- 34.Patterson DM, Murugaesu N, Holden L, et al. A review of the close surveillance policy for stage I female germ cell tumors of the ovary and other sites. Int J Gynecol Cancer 2008;18:43–50. [DOI] [PubMed] [Google Scholar]
- 35.Guerriero S, Testa AC, Timmerman D, et al. Imaging of gynecological disease (6): clinical and ultrasound characteristics of ovarian dysgerminoma. Ultrasound Obstet Gynecol 2011;37:596–602. [DOI] [PubMed] [Google Scholar]
- 36.Mégier P, Desroches A. Color and pulsed Doppler ultrasonography imaging of tubal ectopic pregnancy: study of 100 cases. J Radiol 2003;84(11 Pt 1):1753–6. [PubMed] [Google Scholar]
- 37.Bell DJ, Pannu HK. Radiological assessment of gynecologic malignancies. Obstet Gynecol Clin N Am 2011;38:45–68. [DOI] [PubMed] [Google Scholar]
- 38.Kinkel K, Lu Y, Mehdizade A, et al. Indeterminate ovarian mass at US: incremental value of second imaging test for characterization—meta-analysis and Bayesian analysis. Radiology 2005;236:85–94. [DOI] [PubMed] [Google Scholar]
- 39.Priya S, Kamala S, Gunjan S. Two interesting cases of ovarian pregnancy after in vitro fertilization-embryo transfer and its successful laparoscopic management. Fertil Steril 2009;92:394.e17–19. [DOI] [PubMed] [Google Scholar]
- 40.Chen YX, Xu J, Lv WG, et al. Primary ovarian choriocarcinoma mimicking ectopic pregnancy managed with laparoscopy—case report. Eur J Gynaecol Oncol 2008;29:174–6. [PubMed] [Google Scholar]
- 41.Gerson RF, Lee EY, Gorman E. Primary extrauterine ovarian choriocarcinoma mistaken for ectopic pregnancy: sonographic imaging findings. AJR Am J Roentgenol 2007;189:W280–3. [DOI] [PubMed] [Google Scholar]
- 42.Freij MA, Saleh H, Hamouda T, et al. Primary ovarian choriocarcinoma presenting with acute abdomen mimicking an ectopic pregnancy. Eur J Gynaecol Oncol 2011;32:425–6. [PubMed] [Google Scholar]
- 43.Schmitt C, Lamblin G, Buenerd A, et al. High-level HCG in non-gravidic situations: about two cases. J Gynecol Obstet Biol Reprod (Paris) 2010;39:675–8. [DOI] [PubMed] [Google Scholar]


