Abstract
The X linked adrenoleukodystrophy (X-ALD) is a peroxisomal disease caused by defects of the ABCD1 gene on chromosome Xq28 leading to accumulation of very long chain fatty acids (VLCFA), progressive demyelination and adrenal insufficiency. An 8-year-old boy was referred to our paediatric endocrinology clinic due to fatigue and hyperpigmentation with onset at 2-years old. Blood tests revealed mineralocorticoid insufficiency. Serum adrenocorticotropic hormone and cortisol concentrations were compatible with adrenal insufficiency. Adrenal antibodies were negative. The elevated plasmatic concentration of VLCFA and the genotype analysis with sequencing of ABCD1 gene established the diagnosis of X-ALD. Brain MRI showed demyelination of white matter in the peritrigonal regions. Steroid replacement was started with good response. He initiated restriction of VLCFA by reducing the intake of fatty foods. The authors highlight the importance of suspecting of X-ALD in the aetiology of primary adrenal insufficiency as the first sign of the disease.
Background
X linked adrenoleukodystrophy (X-ALD, OMIM number 300100) is a genetic disease characterised by progressive demyelination within the central and peripheral nervous system, adrenal insufficiency (Addison's disease) and accumulation of very-long-chain fatty acids (VLCFA) in plasma, fibroblasts and tissues.1 X-ALD is caused by mutations in the ABCD1 gene, located at Xq28, which encodes the ALD protein which belongs to the subfamily D of ABC transporters (ATP-binding cassette). The ABC transporter helps form the channel through which VLCFAs move into the peroxisome, probably as Co-A esters. Therefore, any ABCD1 gene mutation causes a loss of function of ALD protein that is localised in the membrane of peroxisomes.2 The phenotype does not correlate with the genotype which suggests that of modifier genes or environmental/epigenetic/stochastic factors modulate the clinical outcome of the disease. Recent studies suggest for a ‘three-hit hypothesis’ for ALD. The first hit in the physiopathogenesis of axonal damage in X-ALD is proposed to be an oxidative burden as a direct consequence of peroxisomal metabolic impairment. The increased oxidative stress/damage and reduction in plasmalogens participate in the transition of VLCFA-mediated metabolic disease into neuroinflammatory disease. This transition is driven by the ‘second hit’. Studies also indicate that derangements in VLCFA and plasmalogens induce oxidative damage leading to expression of lipolytic enzymes and their products followed by enhanced expression of proinflammatory mediators. Finally, observations indicate a generalised loss of peroxisomes and thereby their functions in the inflammatory regions of central nervous system suggesting that a generalised loss of peroxisomal function participates in X-ALD neuropathology (third hit).3
In the adrenal gland, abnormal VLCFA may directly alter cellular function by inhibiting the effects of adrenocorticotropic hormone (ACTH) on the adrenocortical cells, or indirectly by initiating an autoimmune response. Usually, adrenocortical failure occurs along with irreversible degenerative neurological defects. Adrenal failure may predate, occur simultaneously with, or follow the onset of the neurological deterioration.4
The incidence of X-ALD has been estimated to be approximately 1:17 000 if one takes into account the homozygotes and symptomatic forms in heterozygotes females.1 The clinical spectrum is very broad but three main phenotypes can be distinguished including the childhood cerebral form, adrenomyeloneuropathy (AMN) and ‘Addison disease only’. According to Moser,5 childhood cerebral X-ALD occurs most frequently (37% of cases), followed by AMN (32%) and ‘Addison disease-only type’ (13%). The childhood cerebral form normally presents between 4 and 8 years of age. Initially resembles attention deficit hyperactivity disorder and may respond to stimulant medication. Progressive central demyelination occurs with neurological deterioration that includes impairment of cognition, behaviour, vision, hearing and motor function and often leading to total disability within 2 years and death within 5–10 years of diagnosis.6 AMN usually affects adults (second to fourth decade) and is characterised by a pure mylopathy and peripheral neuropathy. The neuropathological hallmark of AMN is an axonopathy, resembling spastic paraparesis or spastic paraplegia, without significant myelin degeneration or neuroinflammation. However, about 35% of AMN patients subsequently develop cerebral demyelination. These patients share the same poor prognosis as children with cerebral ALD. They manifest progressive paraparesis, sphincter disturbances, sexual dysfunction, and not infrequently, impaired adrenocortical function. All symptoms are progressive over decades.7 The third phenotype, ‘Addison disease only’, presents in males between age 2 years and adulthood, but usually before 7.5 years, without evidence of neurological abnormality. Signs include unexplained vomiting, weakness, coma or hyperpigmentation due to increased ACTH secretion. Biochemical evidence of adrenal insufficiency can be present for up to 2 years before the development of clinical signs. Some men with ABCD gene mutations are reported to have biochemical evidence but no clinical features of adrenal insufficiency.8 Most individuals who present with isolated adrenal insufficiency develop AMN by middle age. Female carriers may be symptomatic depending upon the pattern of X-chromosome inactivation. Approximately 50% develop neurological manifestations similar to AMN but have a later onset (age ≥35 years), a milder disease, cerebral disturbance is uncommon and adrenal insufficiency is rare.9
The diagnosis of X-ALD may be raised by the above clinical signs or symptoms, including isolated adrenal insufficiency and is commonly achieved by laboratory evaluation of increased concentrations of VLCFA in plasma. Testing typically includes three VLCFA parameters: the level of hexacosanoic acid (C26:0), and the ratio of hexacosanoic acid to tetracosanoic acid (C26:0/C24:0), and to docosanoic acid (C26:0/C22:0).7
We report a case of a patient with X-ALD presenting with adrenal insufficiency and highlight the importance of testing for ALD in males with idiopathic Addison's disease.
Case presentation
An 8-year-old boy was referred to our paediatric endocrinology clinic due to progressive fatigue and generalised hyperpigmentation beginning at 2-years old. He had no episodes of loss of consciousness, salt-craving or gastrointestinal complaints of nausea and vomiting. The patient attended the third grade and had been retained because of learning disabilities. There were no other relevant facts of his medical history. He was the third child of unrelated healthy parents with uneventful family history, namely no relatives with identified psychiatric, neurological or endocrine disorders. On clinical examination, he presented with prominent adynamia and muscle weakness. He had generalised skin hyperpigmentation, especially of palmar creases and of the buccal mucosa. Pubic hair growth was absent and testicular volume was estimated inferior to 4 ml bilaterally (Tanner stage I). There was no hypotension and growth parameters (weight on 10th–25th percentile, stature on 10th percentile) were normal for sex and age. His mental status and neurological evaluation were normal. There were no other significant findings in the remaining clinical examination.
Investigations
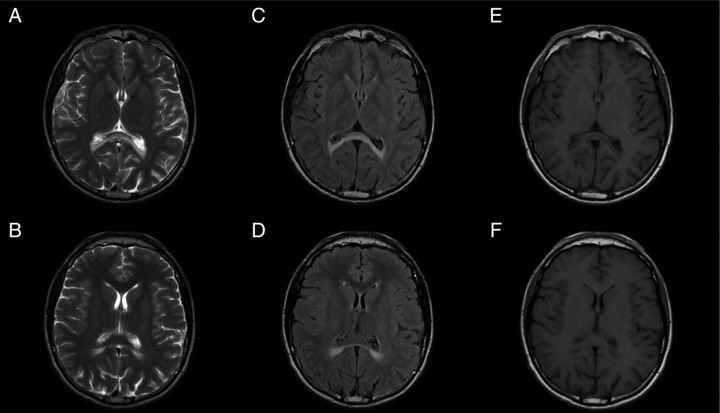
Laboratory results showed hyponatraemia (124 mmol/l, normal range 135–145 mmol/l) and hyperkalaemia (6.5 mmol/l, normal range 3.5–4.6 mmol/l). Serum concentrations for creatinine, blood urea nitrogen, calcium and complete blood count were within normal limits. Plasma renin activity (46.5 ng/ml/h, normal range 0.9–1.9 ng/ml/h) and aldosterone (15 pg/ml, normal range 40–760 pg/ml) serum concentrations confirmed mineralocorticoid deficiency. The measurement of morning cortisol (2.9 μg/dl, normal range 5.0–11.0 μg/dl) and ACTH levels (9279.0 pg/ml, normal range 0.0–46.0 pg/ml) established the diagnosis of adrenal insufficiency. Antiadrenal antibody levels were negative excluding autoimmune adrenal insufficiency. Testosterone concentrations (552 ng/dl, normal range 166–811 ng/dl), dehydroepiandrosterone sulphate (61.4 μg/dl, normal range 70.2–492.0 μg/dl), serum follicle-stimulating hormone and luteinising hormone (3.16 mUI/ml and 2.58 mUI/ml, respectively) were within normal limits. Given the laboratory findings concentrations of VLCFA in the plasma were determined and proved to be increased (C26:0, 0.8 μg/ml (normal 0.16–0.57 μg/ml), C24:0/C22:0 ratio 1.48 (normal ratio 0.63–1.10), C26:0/C22:0, 0.047 (normal ratio 0.004–0.022)) establishing the diagnosis of X-ALD. Abdominal MRI showed no structural abnormalities. Brain MRI revealed symmetrical T2-weighted signal intensities of the posterior peritrigonal regions bilaterally, and involvement of the splenium of the corpus callosum (Loes pattern 1), without calcification areas (figure 1). Spinal cord and cervical MRI was normal. The diagnosis was confirmed by molecular genetic testing of the ABCD1 gene locus which detected the presence, in hemozygosity, the c.1534G>A (p.G512S) mutation, described as causal for X-ALD. The family tree was provided for genetic assessment in order to allow the family genetic counselling.
Figure 1.
T2 weighted images of the brain showing hyperintensities (and corresponding hypointense signal on T1) in the peritrigonal regions, bilaterally and symmetrical, involving the splenium of the corpus callosum (Loes pattern 1), without recoverable expansion or mass effect (a, b - axial T2 FSE; c, d - axial FLAIR; e,f - axial T1 FSE).
Differential diagnosis
Even though X-ALD is an important cause of primary adrenocortical insufficiency in males with Addison disease, if primary adrenal insufficiency is diagnosed, further testing should be performed to exclude other conditions. These may include autoimmune polyglandular syndromes, congenital adrenal hypoplasia, tuberculosis and, less commonly, Allgrove syndrome (achalasia, alacrima and adrenal hypoplasia). The differential diagnosis for individuals presenting with learning disabilities, behaviour problems and other signs of neurological deterioration with cerebral involvement includes other leukodystrophies such as metachromatic leukodystrophy and Krabbe disease. Multiple sclerosis and other dementing disorders such as juvenile neuronal ceroid-lipofuscinosis (Batten disease) may also be discarded.7 Nevertheless, these late conditions are less common and primarily thought usually when childhood cerebral form is the main phenotype.
Treatment
Glucocorticoid replacement therapy was started with clinical improvement. The patient became more active and cheerful, with increased appetite and decreased hyperpigmentation and improved academic performance. Restriction of dietary VLCFA was accomplished by reducing the intake of fatty foods although it is of our knowledge that this approach does not decrease the VLCFA concentration because endogenous synthesis continues. ‘Lorenzo's oil’, a 4:1 mixture of glyceryl trioleate and glyceryl trierucate, combined with moderate reduction of fat in the diet, normalises or significantly lowers the levels of VLCFA in plasma within 4 weeks, but its clinical efficacy and clinical indications for its use have been controversial for more than 15 years.10 The limited evidence suggests that ‘Lorenzo's oil’ may delay the progression of disease in individuals with early or mild ALD and perhaps also for AMN.11 Therefore, it should be offered to individuals with presymptomatic ALD, this is, those with laboratory studies diagnostic of ALD, but without abnormal neurological or MRI findings, and those with ‘Addison disease only’ and normal MRI, as many of the patients with this phenotype will become symptomatic. Even though our patient has no evidence of neurological deficits to date, taking into account his brain MRI abnormalities, we decided not to start dietary therapy with ‘Lorenzo's oil’. Another suggested therapeutic agent for X-ALD is lovastatin given its ability to reduce VLCFA concentration in ALD fibroblasts. However, it was concluded that lovastatin should not be prescribed as a therapy to lower levels of VLCFA in patients with X-ALD and has no clinical benefit for the neurological symptoms.12 This therapeutic agent was not considered for our patient. At present, haematopoietic cell transplantation (HCT) is emerging as the treatment of choice for patients with early stages of cerebral involvement in X-ALD. There are a variety of haematological sources from which stem cells can be harvested, including peripheral blood, bone marrow and umbilical cord blood. The ideal candidates for HCT are males with neurological deficits and abnormalities on brain MRI presenting early in their disease course. For those without MRI evidence of cerebral involvement or advanced neurological disease, HCT is not recommended because almost half of the first group will remain free of neurological disease and there is scarce evidence that HCT provides clinical improvement for patients included in this second group.10 Based on the neurological examination and neuroimaging assessment, we concluded that our patient is an appropriate candidate for HCT. Thus, the patient was referred for psychology evaluation to define the follow-up strategy.
Outcome and follow-up
On follow-up visits there was complete improvement of adrenal insufficiency symptoms. The patient maintains glucocorticoid replacement therapy with hydrocortisone (30 mg/m2). Presently, at 17-years old, there is no evidence of neurological deficits and no new demyelinating lesions in other areas of the brain at follow-up MRI imaging.
Discussion
Primary adrenal insufficiency, also known as Addison's disease, results from disease intrinsic to the adrenal cortex and is caused by either genetic defects or acquired disease that affect the adrenal function. Its incidence is unknown but a retrospective review of 103 children with primary adrenal insufficiency identified congenital adrenal hyperplasia (73%) as the most prevalent cause followed by autoimmune adrenal failure (13%) and peroxisomal disorders (5%), including adrenoleukoystrophy.13
While in the 1940s tuberculosis was responsible for most cases, ALD is currently considered as an aetiological factor of primary adrenocortical insufficiency for up to 20% of boys with idiopathic Addison's disease.14 However, adrenocortical insufficiency is present in at least 50% of patients with cerebral-ALD/AMN, but may be the only clinical manifestation of X-ALD in up to 10% of cases.15 This case report highlights the challenges of diagnosing this rare peroxisomal disorder especially in patients presenting with ‘Addison disease-only’ type. It also emphasises that confirmation or exclusion of X-ALD in males presenting with primary adrenal insufficiency is mandatory since the late diagnosis compromises the management of both patients and their extended families.
Assay of plasma VLCFA is the most frequently used test for diagnosis of X-ALD and is accurate in almost all cases. For patients with high index suspicion but normal or borderline plasma VLCFA, concentrations should also be measured in the cultured skin fibroblasts before excluding the diagnosis of X-ALD.7 In our patient the plasma VLCFA levels established the diagnosis and molecular analysis of ABCD1 gene confirmed the presence of a causal mutation of X-ALD. The definitive test for suspected female carriers and asymptomatic individuals is a mutation analysis.16
All individuals with confirmed ALD/AMN complex, including symptomatic female heterozygotes, should undergo neuroimaging to determine if cerebral involvement is present and testing of adrenal function.16 Our patient's brain MRI showed Loes pattern 1 imaging findings consisting of bilateral symmetric involvement of the parieto-occipital white matter (66% of cases, mainly in children) and follow-up MRI imaging revealed stabilisation of lesions. Eleven years after disease onset and although the patient presents with identified brain lesions in neuroimaging, he maintains no deficit neurological symptoms. It is important to refer that symptoms and MRI imaging findings do not always match since some patients with demyelinating lesions at MRI imaging may be asymptomatic.16 It also must be remembered that the delay from onset of adrenal insufficiency to the development of neurological disability is highly variable and may be as long as 32 years.17 There is still no clear explanation for the highly variable course of this disease, but it is proven that the individuals with the ‘Addison disease only’ phenotype are at risk of developing neurological impairment. The lack of disease progression from the metabolic abnormalities and axonal degeneration to cerebral demyelination and neuroinflammation suggest that in addition to loss of ALD protein function and VLCFA excess, it is likely that other factors play a critical role for X-ALD disease expression. Further studies on the molecular mechanisms of X-ALD are needed in order to understand this monogenetic but multifactorial disease.
It is also noteworthy that prompt evaluation for X-ALD and careful monitoring are essential since early diagnosis is likely to improve outcomes from HCT before overt neurological involvement. Given the patient's brain involvement and normal neurological examination, we decided to refer him for HCT. There is little understanding regarding the mechanisms that alter the course of the disease but probably there is an underlying immunosuppression combined with replacement of microglia by donor-derived cells.10 A preliminary study suggests that haematopoietic stem cell gene therapy with a lentiviral vector can provide clinical benefits in X-ALD. This therapy corrected VLCFA metabolism for several months in cultured skin fibroblasts obtained from patients with X-ALD.18 Therefore, it is hoped that in the near future gene therapy may become available for those affected with this potentially devastating disease.
Finally, the family study is essential for appropriate genetic counselling for carrier screening and antenatal diagnosis. In addition to providing the option of early treatment, it may avoid premature deaths of affected males.
Indeed, we emphasise that the different phenotypic presentations of X-ALD are not definitive and changes may occur, sometimes rapidly evolving, for which one should be alert. For this reason, endocrine, neurological and neuroimaging follow-up of these patients is essential.
Learning points.
X linked adrenoleukodystrophy (X-ALD) should always be suspected in males with primary adrenal insufficiency.
Prompt diagnosis of X-ALD is essential because haematopoietic stem cells transplantation is the standard option treatment for those with early neuropsychological deterioration.
The clinical course of X-ALD is highly variable and its phenotypes not definitive requiring close endocrine, neurological and neuroimaging follow-up.
Early diagnosis brings the possibility of genetic counselling, carrier detection and antenatal diagnosis.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Aubourg P. Adrénoleucodystrophie liée à l'X. Ann Endocrinol (Paris) 2007;68:403–11. [DOI] [PubMed] [Google Scholar]
- 2.van Roermund CW, Visser WF, Ijlst L, et al. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB J 2008;22:4201. [DOI] [PubMed] [Google Scholar]
- 3.Singh I, Pujol A. Pathomechanisms underlying X-adrenoleukodystrophy: a three-hit hypothesis. Brain Pathol 2010;20:838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hein S, Schönfeld P, Kahlert S, et al. Toxic effects of X-linked adrenoleukodystrophy-associated, very long chain fatty acids on glial cells and neurons from rat hippocampus in culture. Hum Mol Genet 2008;17:1750. [DOI] [PubMed] [Google Scholar]
- 5.Moser HW. Adrenoleukodystrophy: phenotype, genetics, pathogenesis and therapy. Brain 1997;120:1485–508. [DOI] [PubMed] [Google Scholar]
- 6.Cappa M, Bizarri C, Vollono C, et al. Adrenoleukodystrophy. Endocr Dev 2011;20:149–60. [DOI] [PubMed] [Google Scholar]
- 7.Moser HW, Moser AB, Steinberg SJ. X-linked adrenoleukodystrophy. GeneReviews 2009. http://www.ncbi.nlm.nih.gov/books/NBK1315/ (accessed 18 July 2012).. [Google Scholar]
- 8.Dubey P, Raymond GV, Moser AB, et al. Adrenal insufficiency in asymptomatic adrenoleukodystrophy patients identified by very long-chain fatty acid screening. J Pediatr 2005;146:528. [DOI] [PubMed] [Google Scholar]
- 9.Ronghe MD, Barton J, Jardine PE, et al. The importance of testing for adrenoleucodystrophy in males with idiopathic Addison's disease. Arch Dis Child 2002;86:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger J, Pujol A, Aubourg P, et al. Current and future pharmacological treatment strategies in X-Linked adrenoleukodystrophy. Brain Pathol 2010;20:845–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moser HW, Moser AB, Hollandsworth K, et al. ‘Lorenzo's oil’ therapy for X-linked adrenoleukodystrophy: rationale and current assessment of efficacy. J Mol Neurosci 2007;33:105. [DOI] [PubMed] [Google Scholar]
- 12.Engelen M, Ofman R, Dijkgraaf MG, et al. Lovastatin in X-linked adrenoleukodystrophy. N Engl J Med 2010;362:276–7. [DOI] [PubMed] [Google Scholar]
- 13.Perry R, Kecha O, Paquette J, et al. Primary adrenal insufficiency in children: twenty years experience at the Sainte-Justine Hospital, Montreal. J Clin Endocrinol Metab 2005;90:3243. [DOI] [PubMed] [Google Scholar]
- 14.Laureti S, Casucci G, Santeusanio F, et al. X-linked adrenoleukodystrophy is a frequent cause of idiopathic Addison's disease in young adult male patients. J Clin Endocrinol Metab 1996;81:470. [DOI] [PubMed] [Google Scholar]
- 15.Van Geel BM, Bezman L, Loes DJ, et al. Evolution of phenotypes in adult male patients with X-linked adrenoleukodystrophy. Ann Neurol 2001;49:186–94. [DOI] [PubMed] [Google Scholar]
- 16.Kim FH, Kim HF. Childhood X-linked adrenoleukodystrophy: clinical-pathologic overview and MR imaging manifestations at initial evaluation and follow-up. RadioGraphics 2005;25:619–31. [DOI] [PubMed] [Google Scholar]
- 17.Moser HW, Bergin A, Naidu S, et al. Adrenoleukodystrophy. Endocrinol Metab Clin North Am 1991;20:297–318. [PubMed] [Google Scholar]
- 18.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 2009;326:818. [DOI] [PubMed] [Google Scholar]