Abstract
Introduction:
Over the last decade, high-flow nasal cannula (HFNC) therapy has become an increasingly important and popular mode of noninvasive respiratory support. HFNC facilitates delivery of humidified and heated oxygen at a high flow rate and generates positive airway pressure.
Methods:
We present five cases of children with OSA without adenotonsillar hypertrophy who were treated with HFNC.
Results:
We demonstrated a statistically significant improvement in apnea-hypopnea index and nadir oxygen saturation in this small cohort.
Conclusion:
We present our successful experience of treating severe OSA with HFNC in the home setting. Further randomized controlled trials are needed to determine whether HFNC could be considered as an established alternative for CPAP in OSA in children
Citation:
Joseph L, Goldberg S, Shitrit M, Picard E. High-flow nasal cannula therapy for obstructive sleep apnea in children. J Clin Sleep Med 2015;11(9):1007–1010.
Keywords: high-flow therapy, obstructive sleep apnea, home care, pediatrics
Over the last decade, high-flow nasal cannula (HFNC) therapy has become an increasingly important and popular mode of noninvasive respiratory support for acute and chronic respiratory failure in adults, children and neonates. HFNC is able to deliver very high flows because it heats and humidifies the air and gas mixture. The proposed mechanism of action of HFNC is several-fold: dead space of the upper airways are continuously flushed with oxygen; the flow rate is greater than the normal inspiratory flow rate, reducing upper airway collapse;2 and HFNC creates continuous positive pressure in the airways.1–3
In adults, HFNC is one of the options in the management of acute respiratory failure.4,5 In children, HFNC is an effective respiratory therapy for different diseases. Its use in RSV bronchiolitis prevents the need for intubation and reduces length of stay in the pediatric intensive care unit (PICU).6 It has also been successfully used in neonates with respiratory distress syndrome (RDS) for primary prevention of intubation or post-extubation care.7 HFNC seems to be at least as effective as nasal continuous positive airway pressure ventilation (CPAP) in neonates for the treatment of RDS.8
The standard treatment for obstructive sleep apnea (OSA) in children is adenoidectomy and/or tonsillectomy when indicated. In other cases CPAP is then proposed as recommended by recent evidence based guidelines.9 Unfortunately, many children, particularly those with developmental delay, cannot tolerate the masks required for delivering CPAP, necessitating the development of new masks.10 Because HFNC creates positive airway pressure, its use in OSA makes physiological sense. In the current study we tested the hypothesis that HFNC will improve OSA. We used the change in the apnea hypopnea index (AHI) and minimal oxygen saturation on therapy as the primary outcomes.
BRIEF SUMMARY
Current Knowledge/Study Rationale: High-flow nasal cannula (HFNC) therapy is used as respiratory support in many respiratory acute diseases and creates positive airway pressure. CPAP is the recommended first-line treatment for obstructive sleep apnea (OSA) not due to adenotonsillar hypertrophy but has very variable compliance.
Study Impact: We demonstrated significant improvement in polysomnography when using HFNC to treat OSA. This pilot study should pave the way for randomized studies of the use of HFNC as an alternative to CPAP.
METHODS
This was a retrospective review of all children under the age of 18 years who presented to our institution with OSA over a consecutive period of 30 months, with a pathological polysomnogram and required positive airway pressure ventilation. Children with OSA, requiring treatment with noninvasive ventilation, who could not tolerate CPAP, were assessed. Each case was reviewed and reported in detail including: previous medical history, upper respiratory pathology, flow and oxygen settings of the HFNC, and response to therapy as measured by AHI and nadir oxygen saturation. Polysomnography was performed using 2 of 3 different flow sensors (pressure, thermistor, or CO2), together with EEG, pulse oximetry and chest and abdominal excursion sensors. Flow rates for HFNC were chosen by the attending pulmonologist and were based upon clinical improvement in snoring seen at the bedside. Similar measurements were taken during repeat testing with HFNC. The constant nature of the background flow of the HFNC permitted good readings of the superimposed natural breathing on the pressure and thermistor transducers but did not allow an accurate reading of end-tidal CO2. Statistical analysis was performed using the one-tailed paired Student t-test comparing both AHI and nadir oxygen saturation with and without HFNC.
RESULTS
There were 5 patients who received HFNC as treatment for obstructive sleep apnea, and each child had at least one polysomnogram before and after initiation of treatment. The following is a brief description of each of these patients:
Case 1
A 22-month-old boy was brought to the pediatric emergency room because of severe nocturnal snoring with oxygen desatu-ration. He was a second twin born at 26 weeks gestation with a birth weight of 900 g. He was diagnosed with mild bronchopulmonary dysplasia, discharged from the neonatal intensive care unit without supplemental oxygen and had no need for chronic respiratory treatment. At 12 months of age he started snoring and underwent adenoidectomy with temporary improvement of his symptoms. Over the following months, the snoring became more severe, causing frequent nocturnal wakening. A flexible, drug-induced sleep endoscopy (DISE) was performed and demonstrated severe pharyngeal collapse without evidence of tonsillar or adenoidal hypertrophy. On neurological examination he had mild generalized hypotonia and brain MRI was normal. Polysomnography demonstrated an AHI of 46 per hour.
Nasal CPAP was initiated but the child rejected it repeatedly. HFNC therapy at a flow of 10 L/min and FiO2 of 30% was initiated, resulting in resolution of both snoring and hypoxia. He was discharged with nocturnal HFNC. Polysomnography while receiving HFNC demonstrated an AHI of 1.9/h with a single episode of oxygen desaturation to 86%. His weight increased from 8.2 Kg (z score −3.27) at presentation to 11.5 kg (z score −1.53) over 23 months of treatment.
Case 2
A 15-year-old boy with severe psychomotor retardation presented to our outpatient clinic due to worsening snoring and arousals during sleep. His tonsils were assessed by direct visualization and his adenoids were assessed by fiberoptic laryngoscopy and found to be not enlarged. A polysomnogram showed severe OSA with an AHI of 29/h. Despite significant attempts on the part of the family, he was unable to adapt to any of the CPAP masks because of his mental status. He was hospitalized preoperatively for a tracheostomy but required bridging therapy with HFNC at a flow of 10 L/min and FiO2 of 21%. He persistently maintained good oxygen saturation during sleep. The tracheostomy was postponed and he was discharged on a home HFNC device with the same parameters. Polysomnography on the above treatment demonstrated an AHI of 6/h.
Case 3
A 3-year-old girl born at term with multiple anomalies, including situs inversus totalis, microcephaly, and microphthalmia presented to the pulmonology clinic with inspiratory stridor. Initial examination showed that her weight had dropped from the 10th percentile at birth to below the 3rd percentile. DISE demonstrated very severe laryngomalacia with complete collapse of a very small epiglottis into the larynx during inspiration. A sleep study demonstrated an AHI 18.7/h. These caused periodic hypercapnia to 52 mm Hg and oxygen desaturation down to 61%. A trial with CPAP failed mainly due to her face anomalies. Low flow oxygen therapy partially corrected the hypoxia but not the hypoventilation. With a flow of 6 L/min and FiO2 of 21%, only one event of desaturation was observed. She was discharged home with HFNC therapy and no desaturations were noted for 3 months. Polysomnography on HFNC demonstrated a substantial improvement with an AHI of 8.8/h, the flow was increased to 8 L/min, and repeat polysomnography is planned.
Case 4
A 2-month-old girl with generalized hypotonia and poor weight gain was admitted because of apneas. On examination she had retrognathia, a protruding tongue, and unilateral cho-anal stenosis. She had constant stridor, and respiratory rate was 12 breaths per minute. Her reflexes were generally increased but brain MRI was normal. She had intermittent hypoxia and her blood gas showed partially compensated respiratory acidosis with pCO2 55–63 mm Hg; pH 7.32–7.40; and HCO3 31–35 mmol/L. DISE showed pharyngo-laryngo-tracheomalacia. Polysomnography showed mixed obstructive and central apneas with an AHI 15/h and end-tidal CO2 as high as 55 mm Hg. She was treated with HFNC therapy at a flow of 5 L/min and FiO2 of 21%. After 2 months of HFNC therapy her hypotonia had resolved and physical development was normal for her age. Her weight increased from under the second percentile to 7th percentile. Venous blood gas using HFNC showed pCO2 41.3 mm Hg with pH 7.38 and HCO3 23 mmol/L. Polysomnography with HFNC showed primarily obstructive events with an AHI of 5.6/h.
Case 5
A 3-year-old boy with Treacher Collins syndrome underwent tracheostomy placement at 2 weeks of age due to upper airway obstruction. A mandibular advancement operation was performed followed by adenotonsillectomy and decannulation. DISE demonstrated significant airway obstruction at the level of the tongue and soft palate. Polysomnography was performed because of nocturnal snoring and showed an AHI of 6.2/h with oxygen desaturations under 90% for 3% of the time. Meanwhile, he had a significant clinical deterioration with witnessed nocturnal apneas and desaturations down to 50% and presented urgently to the emergency department. HFNC therapy at a flow of 10 L/min and FiO2 of 21% was started with immediate clinical improvement. Polysomnography while using the device demonstrated AHI 2.7/h and saturations under 90% for 0.2% of the night.
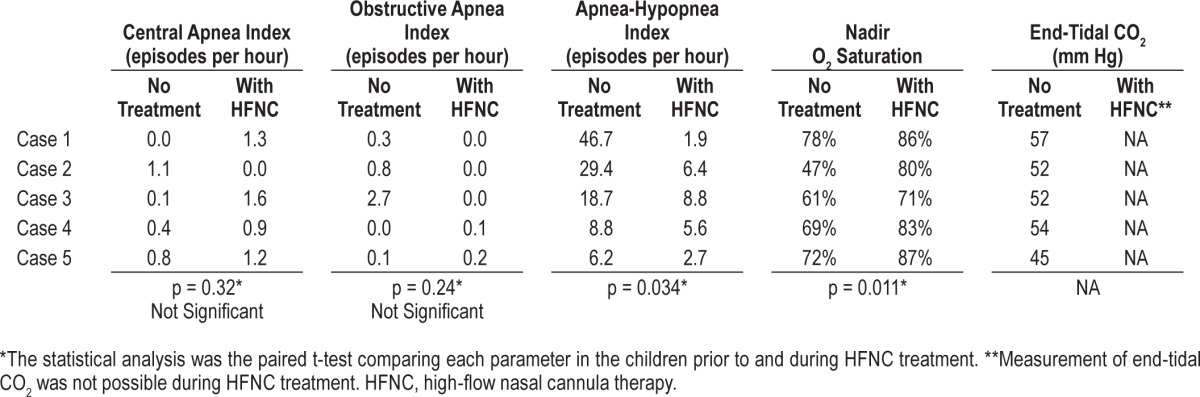
Taking all cases together, while on HFNC, mean AHI decreased from 22.98/h to 5/h (p = 0.034) and mean nadir oxygen saturation increased from 65% to 81.4% (p = 0.011) (Table 1, Figure 1). Details regarding other parameters of the polysomnography are presented in Table 2.
Table 1.
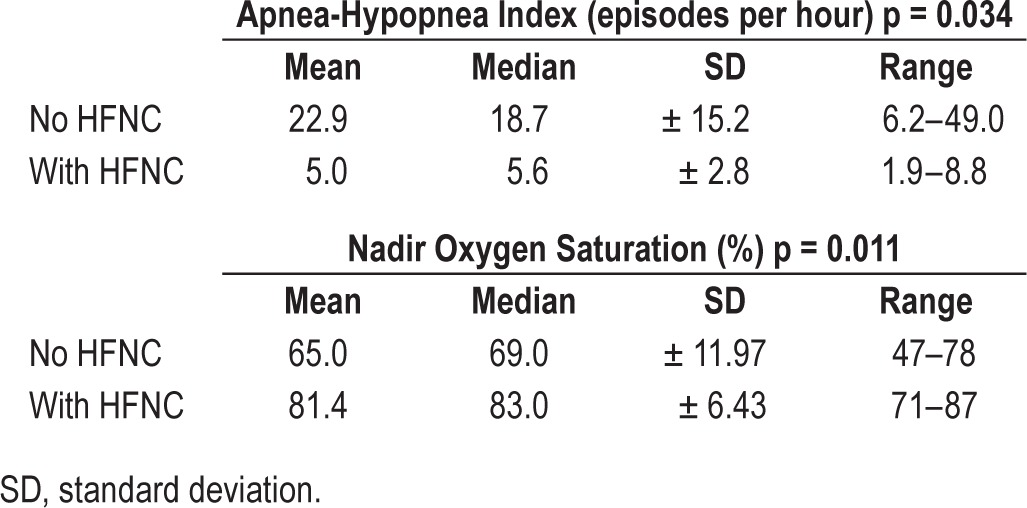
Figure 1. Apnea-hypopnea index and minimal oxygen saturation during polysomnography with and without HFNC treatment for each of the five cases.
The thick line represents the mean change.
Table 2.
DISCUSSION
We have presented our experience of five children with OSA who did not tolerate noninvasive CPAP. The use of HFNC, in both the hospital and home settings, led to significant improvement of symptoms. Four of the five cases had severe OSA (AHI ≥ 15/h), and they all improved to mild-moderate severity (AHI < 10/h). Where hypoventilation was assessed, there was reduced evidence of chronic CO2 retention after treatment with HFNC. While some polysomnogram results remained pathological, the overall improvement in AHI and oxygen saturation was thought to be clinically significant. HFNC is better tolerated because its warm humidified nature decreases the sensation of flow in the patients' nose which leads to increased compliance.11 In addition, HFNC therapy is delivered via the nose in a similar way to standard oxygen therapy, potentially eliminating the mid-face hypoplasia seen in some children after prolonged use of CPAP.12,13 To the best of our knowledge, this is the first report using HFNC at home for the treatment of OSA. Previously, McGinley et al. have used a similar nasal cannula system with insufflation of warm humidified air to treat adults14 and children15 with OSA; however, this was a hospital-based sleep lab report. Our findings support the recent statement by Tan et al that “HFNC may be a viable and possibly more child-friendly alternative” when compared to CPAP.16
Mechanism of Action in OSA
HFNC treatment is based upon a fixed flow rate via nasal cannulae facilitating airflow through narrow airways. The pressures that are subsequently generated do not explain this effect alone. It has been hypothesized that HFNC exceeds the inspiratory flow rate, reducing the negative pressure generated during inspiration2 and reducing the tendency of the airways to collapse.17 This was demonstrated in adults,14 when the endexpiratory supraglottic pressure became less negative after the addition of high flow therapy and inspiratory airflow no longer showed a flow-limited pattern.
Positive pressure in the upper and lower airways are not regulated and are dependent upon several factors such as the amount of leak around the nasal cannula and whether the mouth is opened or closed. Pressures have been measured by several authors at the nasopharynx or esophagus. Arora et al.3 studied children with viral bronchiolitis and found a linear increase in pressure up to a flow of 6 L/min and a lesser gradient from 6–8 L/min, reaching a maximum of 5 cm H2O. Similarly, Kubicka et al.18 measured flow rates of up to 5 L/min in neonates over 1,500 g current weight and the highest pressure measured was 4.5 cm H2O. Pressures achieved with mouth open were much smaller. It seems that the pressures generated by HFNC will not exceed pressures used therapeutically in CPAP, are sufficient to keep the airways open during sleep, and hence are able to treat OSA.
Safety Concerns
Airway pressure is not regulated when HFNC is used, and there is no mechanism in place to prevent sudden increases in pressure. This may result in air leak including pneumothorax or pneumomediastinum when in the outpatient setting. In addition, the high flows in HFNC tend to increase end expiratory lung volume, increasing this risk.19 Hedge et al. described three cases of pneumothorax or pneumomediastinum that occurred while HFNC therapy was being administered in a 2-month old with bronchiolitis, a 22-month-old child post trauma and mechanical ventilation, and a 16-year-old patient post anesthesia.20 Flow rates ranged from 6–20 L/min at the time of the complication. They all had significant hyperinflation at the time of the development of air leak. The risk of generating excessive pulmonary pressures in children with OSA is reduced because the patients are asleep; hence tidal breathing is shallower while heated humidified air tends to prevent broncho-spasm. Moreover, these patients generally do not suffer from obstructive lung diseases. Hence we speculate that the risk of using HFNC in OSA is probably low.
A second concern raised in the use of HFNC is that of increased incidence of Gram-negative bacteremia, specifically due to Ralstonia pickettii. One brand of hospital-based devices was temporarily removed from the market. After appropriate methods of infection control were initiated, the devices were reapproved for public use.21
CONCLUSION
HFNC seems to be a viable alternative therapy for children with severe OSA for whom CPAP is not appropriate. Our report should stimulate the inception of randomized controlled trials to determine whether HFNC could be considered as an established alternative for CPAP in OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. Dr. Joseph was the lead physician in the first and fourth cases and wrote the manuscript. Dr. Goldberg was the lead physician in the second and last cases and critically reviewed the manuscript. Mrs. Shitrit was the respiratory technician who facilitated the discharge of each child and is closely involved in their follow up. Dr. Picard was the lead physician in the third case and critically reviewed the manuscript. Drs. Joseph and Goldberg contributed equally to this paper.
ABBREVIATIONS
- AHI
apnea hypopnea index
- CPAP
continuous positive airway pressure ventilation
- DISE
drug-induced sleep endoscopy
- HFNC
high flow nasal cannula
- NP
nasopharyngeal
- OSA
obstructive sleep apnea
- PICU
pediatric intensive care unit
- RDS
respiratory distress syndrome
REFERENCES
- 1.Frizzola M, Miller TL, Rodriguez ME, et al. High-flow nasal cannula: impact on oxygenation and ventilation in an acute lung injury model. Pediatr Pulmonol. 2011;46:67–74. doi: 10.1002/ppul.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spentzas T, Minarik M, Patters AB, Vinson B, Stidham G. Children with respiratory distress treated with high-flow nasal cannula. J Intensive Care Med. 2009;4:323–8. doi: 10.1177/0885066609340622. [DOI] [PubMed] [Google Scholar]
- 3.Arora B, Mahajan P, Zidan MA, Sethuraman U. Nasopharyngeal airway pressures in bronchiolitis patients treated with high-flow nasal cannula oxygen therapy. Pediatr Emerg Care. 2012;28:1179–84. doi: 10.1097/PEC.0b013e318271a671. [DOI] [PubMed] [Google Scholar]
- 4.Ricard JD. High flow nasal oxygen in acute respiratory failure. Minerva Anesthesiol. 2012;78:836–41. [PubMed] [Google Scholar]
- 5.Lenglet H, Sztrymf B, Leroy C, Brun P, Dreyfuss D, Ricard JD. Humidified high flow nasal oxygen during respiratory failure in the emergency department: feasibility and efficacy. Respir Care. 2012;57:1873–8. doi: 10.4187/respcare.01575. [DOI] [PubMed] [Google Scholar]
- 6.McKiernan C, Chua LC, Visintainer PF, et al. High flow nasal cannulae therapy in infants with bronchiolitis. J Pediatr. 2010;156:634–8. doi: 10.1016/j.jpeds.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 7.Ojha S, Gridley E, Dorling J. Use of heated humidified high-flow nasal cannula oxygen in neonates: a UK wide survey. Acta Paediatr. 2013;102:249–53. doi: 10.1111/apa.12090. [DOI] [PubMed] [Google Scholar]
- 8.Yoder BA, Stoddard RA, Li M, King J, Dirnberger DR, Abbasi S. Heated, humidified high-flow nasal cannula versus nasal CPAP for respiratory support in neonates. Pediatrics. 2013;131:e1482–90. doi: 10.1542/peds.2012-2742. [DOI] [PubMed] [Google Scholar]
- 9.Marcus CL, Brook LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–84. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 10.Kushida CA, Halbower AC, Kryger MH, et al. Evaluation of a new pediatric positive airway pressure mask. J Clin Sleep Med. 2014;10:979–84. doi: 10.5664/jcsm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55:408–13. [PubMed] [Google Scholar]
- 12.Li KK, Riley RW, Guilleminault C. An unreported risk in the use of home nasal continuous positive airway pressure and home nasal ventilation in children: mid-face hypoplasia. Chest. 2000;117:916–8. doi: 10.1378/chest.117.3.916. [DOI] [PubMed] [Google Scholar]
- 13.Villa MP, Pagani J, Ambrosio R, Ronchetti R, Bernkopf E. Mid-face hypoplasia after long-term nasal ventilation. Am J Respir Crit Care Med. 2002;166:1142–3. doi: 10.1164/ajrccm.166.8.257c. [DOI] [PubMed] [Google Scholar]
- 14.McGinley BM, Patil SP, Kirkness JP, Smith PL, Schwartz AR, Schneider H. A nasal cannula can be used to treat obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:194–200. doi: 10.1164/rccm.200609-1336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGinley BM, Halbower A, Schwartz AR, Smith PL, Patil SP, Schneider H. Effect of a high-flow open nasal cannula system on obstructive sleep apnea in children. Pediatrics. 2009;124:179–88. doi: 10.1542/peds.2008-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan HL, Gozal D, Kheirandish-Gozal L. Obstructive sleep apnea in children: a critical update. Nature Sci Sleep. 2013;5:109–23. doi: 10.2147/NSS.S51907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103:1400–5. doi: 10.1016/j.rmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Kubicka ZJ, Limauro J, Darnall RA. Heated, humidified high-flow nasal cannula therapy: yet another way to deliver continuous positive airway pressure? Pediatrics. 2008;121:82–8. doi: 10.1542/peds.2007-0957. [DOI] [PubMed] [Google Scholar]
- 19.Hough JL, Pham TMT, Schibler A. Physiological effects of high flow nasal cannula in infants with bronchiolitis. Pediatr Crit Care Med. 2014;15:e214–9. doi: 10.1097/PCC.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 20.Hegde S, Prodhan P. Serious air leak syndrome complicating high-flow nasal cannula therapy: a report of 3 cases. Pediatrics. 2013;131:e939–44. doi: 10.1542/peds.2011-3767. [DOI] [PubMed] [Google Scholar]
- 21.Dani C, Pratesi S, Migliori C, Bertini G. High flow nasal cannula therapy as respiratory support in the preterm infant. Pediatr Pulmonol. 2009;44:629–34. doi: 10.1002/ppul.21051. [DOI] [PubMed] [Google Scholar]


