Abstract
Objective:
To evaluate the effect of sedation depth on drug-induced sleep endoscopy (DISE).
Methods:
Ninety patients with obstructive sleep apnea (OSA) and 18 snorers underwent polysomnography and DISE under bispectral index (BIS)-guided propofol infusion at two different sedation levels: BIS 65–75 (light sedation) and 50–60 (deep sedation).
Results:
For the patients with OSA, the percentages of velopharynx, oropharynx, hypopharynx, and larynx obstructions under light sedation were 77.8%, 63.3%, 30%, and 33.3%, respectively. Sedation depth was associated with the severity of velopharynx and oropharynx obstruction, oropharynx obstruction pattern, tongue base obstruction, epiglottis anteroposterior prolapse and folding, and arytenoid prolapse. In comparison, OSA severity was associated with the severity of velopharynx obstruction, severity of oropharynx obstruction, and arytenoid prolapse (odds ratio (95% confidence interval); 14.3 (4.7–43.4), 11.7 (4.2–32.9), and 13.2 (2.8–62.3), respectively). A good agreement was noted between similar DISE findings at different times and different observers (kappa value 0.6 to 1, respectively). A high percentage of arytenoid prolapse (46.7% among the patients with OSA under light sedation) was noted.
Conclusions:
Greater sedative depth increased upper airway collapsibility under DISE assessment. DISE under BIS-guided propofol infusion, and especially a level of 65–75, offers an objective and reproducible method to evaluate upper airway collapsibility. Some findings were induced by drug sedation and need careful interpretation. Specific arytenoid prolapse patterns were noted for which further investigations are warranted.
Clinical Trials Registration:
http://www.clinicaltrials.gov, identifier: NCT01100554
Commentary:
A commentary on this article appears in this issue on page 965.
Citation:
Lo YL, Ni YL, Wang TY, Lin TY, Li HY, White DP, Lin JR, Kuo HP. Bispectral index in evaluating effects of sedation depth on drug-induced sleep endoscopy. J Clin Sleep Med 2015;11(9):1011–1020.
Keywords: bispectral index, drug-induced sleep endoscopy, laryngeal obstruction, obstructive sleep apnea
Obstructive sleep apnea (OSA) involves multiple segments of the upper airway. Various methods including reconstructed computed tomography (CT) or magnetic resonance imaging (MRI), nasofibroscopy under Müller maneuver and cephalometry have been applied to determine the most suitable treatment when continuous positive airway pressure (CPAP) could not be used. However, radiation exposure, non–real-time imaging, or indeterminate sleep status are limitations of the above measurements.1 Drug-induced sleep endoscopy (DISE) has been used for decades to directly examine the upper airways in sedative-induced sleep and improve treatment outcomes by acting as an adjuvant tool to assess surgical or nonsurgical treatment options.2–4
BRIEF SUMMARY
Current Knowledge/Study Rationale: Drug-induced sleep endos-copy (DISE) were performed under different sedative conditions and might affect upper airway obstructions. We aimed to evaluate the affects of sedation depth and obstructive sleep apnea severity on different upper airway obstruction patterns.
Study Impact: Under DISE, sedation depth affected velopharynx and oropharynx obstruction severity, oropharynx obstruction pattern, tongue base obstruction, epiglottis anteroposterior prolapse and folding, and arytenoid prolapse; however, OSA severity was associated with velopharynx and oropharynx obstruction severity as well as arytenoid prolapse. Bispectral index guided DISE offers an objective and reproducible method to evaluate upper airway collapsibility.
Various sedation methods have been used to allow for the performance of DISE. Roblin introduced target-controlled infusion (TCI) of propofol to DISE to allow for better control of the sedation level.5 TCI of propofol uses computer-based pharmacokinetic and pharmacodynamic models to predict and achieve a target effect site (brain) concentration of propofol. However, patients may lose consciousness at variable effect site concentrations (Ce), with a higher Ce being associated with higher upper airway critical closing pressure.6,7 Most DISE procedures are started shortly after the patients lose consciousness. A recent study revealed that when using TCI propofol infusion, the hypnotic effect takes several minutes to become steady even though loss of consciousness has been reached, which may be because the myorelaxant properties of the anesthetic are not temporally synchronized. This suggests that the upper airway collapsibility would be different at different time courses even after reaching the Ce of loss of consciousness.8
The bispectral index (BIS) translates a patient's electroencephalogram (EEG) signals into scaled numbers from 0 (EEG silence) to 100 (fully awake) to reflect levels of consciousness and sedation depth.9 It has been widely applied during general anesthesia and procedures requiring consciousness sedation to avoid oversedation, reduce adverse effects, and prevent awareness during anesthesia.10,11 Loss of consciousness has been reported to occur at BIS values between 60 and 80.12,13 Lately, several DISE studies have also investigated BIS during propofol sedation to evaluate sedation levels.14 DISE has been shown to exhibit different upper airway obstruction conditions compared to awake nasoendoscopy.15 Recently, Hong et al.16 also revealed that different BIS levels had different degrees of upper airway narrowing by repeated DISE in the same patient but different BIS level.
The aim of the current study was to evaluate upper airway collapse patterns, to test the effects of sedative depth on upper airway collapses, and thus narrow the sedation range of performing DISE.
METHODS
Patient Selection and Preparation
Patients with mild to severe OSA who did not adhere to CPAP, i.e., less than 4 h a day or 4 days a week,17 or sought alternative treatments were enrolled as the study group from August 2008 to February 2011. The exclusion criteria included: American Society of Anesthesiologists physical status classification ≥ 3; allergy to propofol, xylocaine, or food (eggs, beans, milk); congestive heart failure; moderate to severe chronic obstructive pulmonary disease or uncontrolled asthma; a history of head injury, seizures, or cerebrovascular disease; and age younger than 18 y. Snoring patients receiving sedative bronchoscopy due to chronic cough were enrolled as the normal control group if they had an apnea-hypopnea index (AHI) less than 5. This study was approved by the Chang Gung Memorial Hospital Institutional Review Board. Written informed consent was obtained from all patients.
All patients underwent overnight polysomnography (PSG) (Alice 5, Philips Respironics, MA, USA), scored according to the American Academy of Sleep Medicine 2003 guidelines, and DISE within 1 mo of the PSG. Physical examinations including modified Mallampati score (grade 1–4), tonsil enlargement (grade 1–4), macroglossia, uvula elongation or widening (> 1.5 cm in length or > 1 cm in width), retrognathia, overjet, and neck circumference were recorded.18 DISE was performed in the bronchoscopy suite after a 6-h fasting period, with a 5.3 mm flexible bronchoscope (BF-P240; Olympus; Tokyo, Japan) by an experienced pulmonologist who was blind to the sedative level. Topical, nasal xylocaine jelly was applied to one nostril for lubrication and analgesia, and 0.5 mL 2% xylocaine was injected intravenously to avoid the discomfort of the propofol injections. Patients underwent the entire endoscopy procedure positioned supine, lying flat. Awake upper airway patterns were evaluated first before the injection of propofol. The endoscope was then held with the tip in the velopharynx during the sedation induction to avoid further irritation.
DISE With BIS Monitoring
The patients received continuous oxygen (2 L/min), pulse oximetry, electrocardiography, and blood pressure monitoring throughout the procedure. Patients received intravenous propofol for induction (all by YL Lo), with an intermittent bolus technique. The level of sedation was monitored with an A-2000 BIS monitor (Version 3.11, Aspect Medical Systems, Inc., Newton, MA, USA), with a smoothing time of 15 sec. After administration of 0.5 mg/kg intravenous propofol via a syringe pump (Injectomat Agilia, Fresenius Kabi, France), another 10–20 mg was administered repeatedly every 30 sec until the patient started snoring or until velopharynx obstruction had been identified to ensure the patient had reached loss of consciousness. Continuous propofol infusion was then given with the pump for maintenance with the initial rate set at 6 mg/kg/h and then adjusted up and down by 1–2 mg/kg/h every 30 sec to maintain the BIS level between 65 and 75 (light sedation). DISE was performed (by YL Ni) after the BIS level had remained steady between 65 and 75 for at least 90 sec (DISE-LS). If the BIS level went out of range, the bronchoscope was held within the airway until another steady state was achieved after adjustments of the dose of propofol. The propofol dose was later titrated until the BIS level was between 50 and 60 (deep sedation). DISE was performed again after a 90-sec steady-state condition to evaluate upper airway collapse under deeper sedation (DISE-DS). All cases also received mandible advancement maneuver at deep sedation to evaluate the response of upper airway collapse.
DISE Recordings
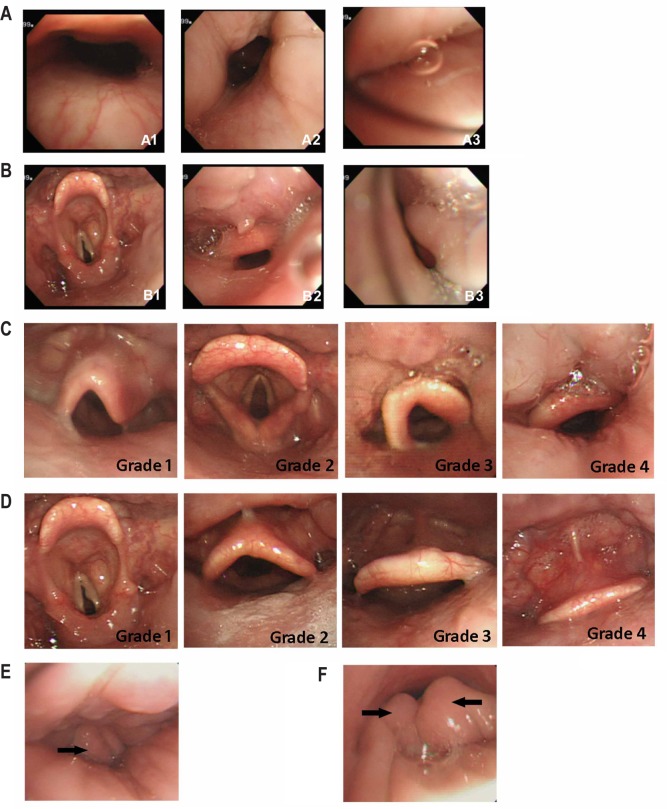
DISE findings were recorded according to four anatomic levels: velopharynx, oropharynx, hypopharynx, and larynx. The narrowest end inspiratory condition among five consecutive breaths was recorded when the BIS was steadily within the desired level. Chin lift was done when persistent complete obstruction occurred in all five breaths or desaturation occurred with SaO2 < 90%. The severity of velopharynx obstructions followed the VOTE classification (velum/oropharynx/tongue base/epiglottis), and were recorded as patent/partial/complete obstruction (Table 1, Figure 1A).19 The oropharynx, including the tonsils, tongue, and lateral pharyngeal wall, were also evaluated as having patent/partial/complete obstruction (Table 1, Figure 1B). When the lumen was less than 70% of the expira-tory status, partial obstruction was defined. Complete obstruction was recorded when no lumen could be seen. When partial or complete obstruction was noted, the obstruction pattern was classified into circumferential, decreased anteroposterior diameter, or decreased lateral diameter types. The position of the tongue base was recorded separately in four grades (Table 1, Figure 1C). Tongue base obstruction was identified when the tongue base pushed the epiglottis backward and caused lumen obstruction (grade 4). Hypopharynx obstruction was identified when the lateral pharyngeal walls constricted and caused luminal narrowing at the hypopharyngeal level. The characteristics of the larynx were evaluated in three aspects: anteroposterior prolapse of the epiglottis (Table 1, Figure 1D); lateral folding of the epiglottis (Table 1, Figure 1E); and arytenoid prolapse (Table 1, Figure 1F). When the patients had epiglottis prolapse, epiglottis anteroposterior prolapse that touched the posterior pharyngeal wall (grade 4), or epiglottis folding, they were defined as having larynx obstruction. Multiple-level obstruction was defined when two or more levels were obstructed.
Table 1.
Parameters recorded during drug-induced endoscopy and the definitions.
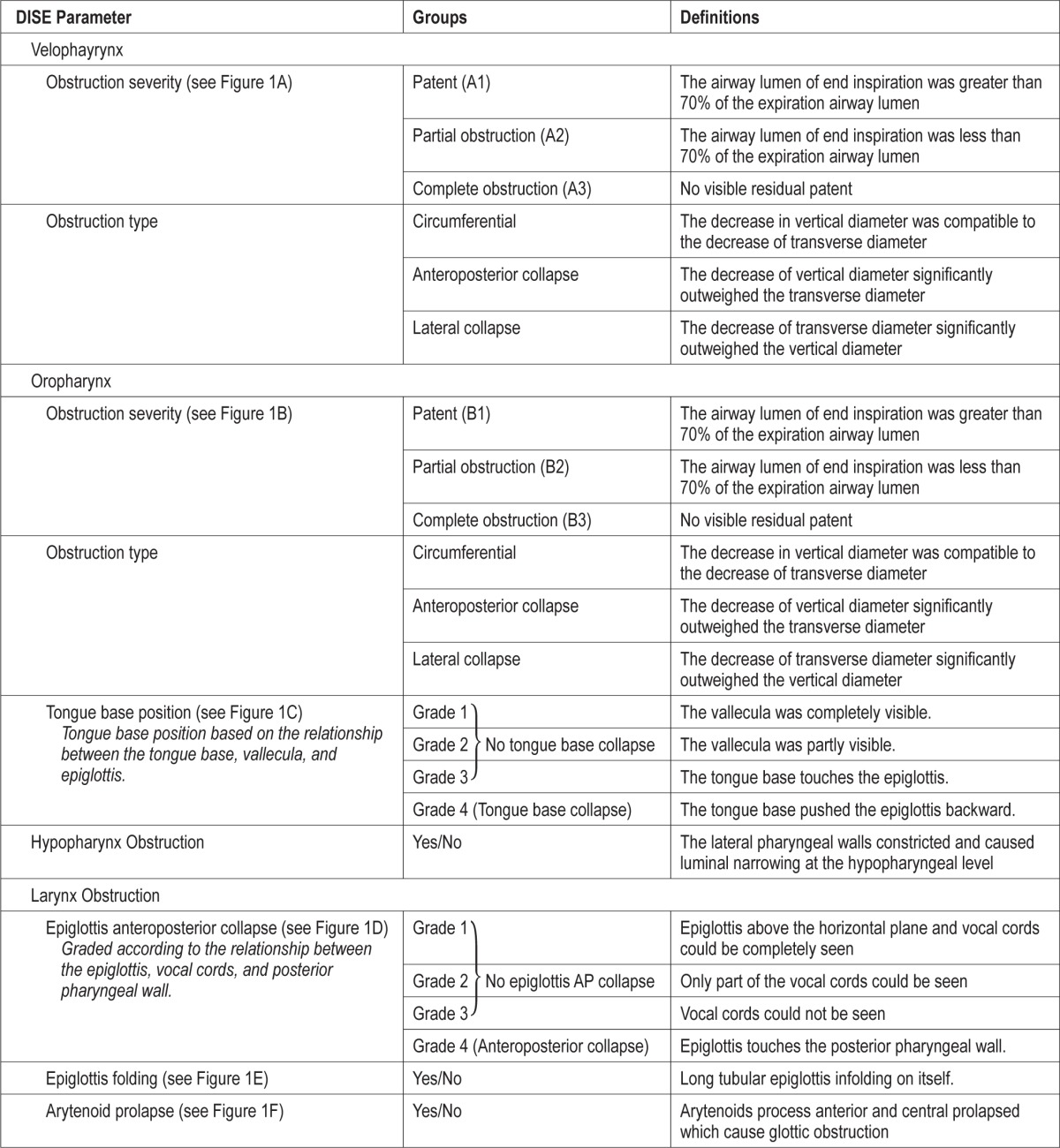
Figure 1. Visual representations of the parameters recorded during drug-induced endoscopy.
See Table 1 for a description of the parameters recorded during drug-induced endoscopy and the definitions.
Validation of DISE Sequences and DISE Results between Observers
Twenty patients initially received DISE under light sedation (LS1), followed by deep sedation, and then light sedation (LS2) again. Another 20 patients received deep sedation (DS1) initially, followed by light sedation, and then deep sedation (DS2). The DISE characteristics between the same sedation depth at different times were compared.
The interobserver variation regarding DISE characteristics was also evaluated in the 20 patients (40 light sedation and deep sedation fractions). YL Ni performed the DISE and TL Lin observed the procedure at the same time. Both of them rated the results independently without being informed of the sedation depth or disease severity. The agreements between the two observers were then compared. High percentages of arytenoid prolapse, epiglottis collapse, and epiglottis folding were seen in the current study. To further verify these patterns, all of the videos were reviewed. The video of each patient was trimmed into six sections to evaluate the following conditions: arytenoid prolapse (LS), epiglottis prolapse (LS), epiglottis folding (LS), arytenoid prolapse (DS), epiglottis prolapse (DS), and epiglottis folding (DS). All of the video clips were mixed and arranged in no particular order. YL Ni and YL Lo rated these results independently. The agreements regarding the three patterns between the two observers were then tested.
Statistics
All results are expressed as mean with standard deviation unless otherwise stated. Patient characteristics were analyzed using univariate analysis or the chi-square test. Differences in obstruction percentages between the severity groups were analyzed using the chi-square test. General estimation equations were used to test whether sedation depth or OSA severity had a substantial effect on each DISE finding. The validation of the 40 patients receiving different sedation depth sequences and between different observers were evaluated with kappa values and agreement percentage.20 All of the statistical analyses were performed using the Statistical Package for Social Sciences version 17 (SPSS Inc., Chicago, IL, USA). A value of p < 0.05 was considered statistically significant.
General estimation equations (SPSS 17) were used to test the effect of OSA severity and sedative depth on observed DISE patterns. The OSA severity was categorized into four groups (normal, mild, moderate, and severe) with normal subjects set as the reference. The sedative depth was categorized in to three conditions (awake, light sedation, deep sedation) with light sedation selected as the reference. DISE patterns including binary results (existence or not of an observed pattern: arytenoid pro-lapse, epiglottis folding, hypopharynx obstruction) and ordinal results (grading of the patterns: velopharynx obstruction severity, pattern, tongue base position, epiglottis anteroposterior collapse). These patterns were then tested as variables one by one. The model was binary or ordinal logistic according to the characteristics of the variable being assessed. The correlation matrix was independent. The link function was logit.
RESULTS
Eighteen snorers, 30 mild (AHI: 5–15), 30 moderate (AHI: 16–30), and 30 severe (AHI: > 30) OSA patients were enrolled in this study. Sixty-six patients with OSA (73.3%) were male. The patients with OSA had a mean age of 52.2 ± 13.5 y, body mass index of 26.1 ± 3.3 kg/m2, neck circumference of 38.6 ± 3.3 cm, and AHI of 24.2 ± 21.6 (Table 2). There were no significant differences between the snorers and patients with OSA in terms of age, body mass index, or neck circumference (Table 2). Forty-five of the patients with OSA (41.6%) experienced transient desaturation under 2 L/min oxygen support, all of whom recovered shortly after oxygen 6 L/min support or chin lift maneuver during the course of sedation. The mean procedure duration, starting from sleep induction to the end of deep sedation sleep endoscopy, was 16.2 ± 5.4 min in 68 cases, excluding the 40 cases in whom sedation sequences were tested. All of the 68 cases received mandible advancement maneuver to evaluate the upper airway response. The mean propofol dose for the procedure including mandible advancement maneuver was 249.6 ± 87.2 mg.
Table 2.
Comparison of baseline characteristics between normal subjects and patients with obstructive sleep apnea.

DISE Findings
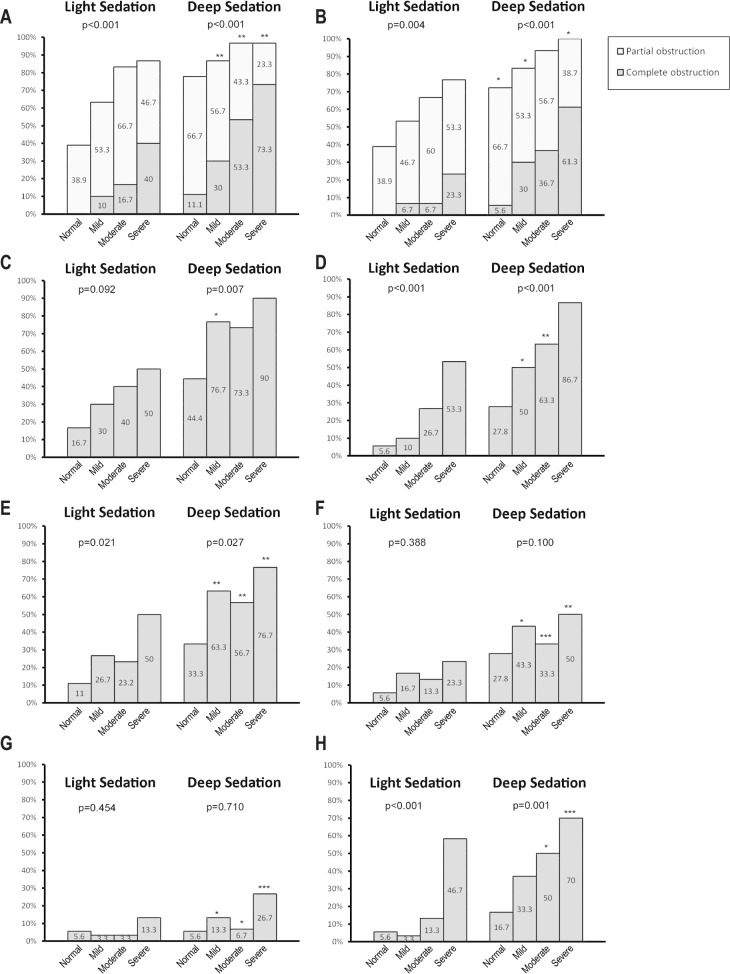
For the patients with OSA, 77.8% had velopharynx obstructions and 63.3% had oropharynx obstructions under light sedation. More patients had velopharynx (93.3%, p < 0.001) and oropharynx obstructions (92.2%, p = 0.001) under deep sedation. The obstructive severity was greater under deep sedation (complete obstruction in 52.2% at the velopharynx and 43.3% at the oropharynx) compared to that under light sedation (complete obstruction in 22.2% at the velopharynx, p < 0.001; and 10% at the oropharynx, p < 0.001). Forty percent of the OSA cases had tongue base obstruction (grade 4 tongue base) under light sedation, and 80% of them experienced tongue base obstruction under deep sedation (p = 0.001). Hypopharynx and larynx obstructions were noted in 30% and 33.3% of all patients with OSA, respectively, under light sedation, and in 66.7% and 65% of the patients, respectively, under deep sedation (hypopharynx: p < 0.001, larynx: p = 0.012). Detailed obstruction patterns regarding different subgroups are illustrated in Figure 2. In addition, the percentage of multiple level obstructions among the patients with OSA increased from 34.4% under light sedation to 74.4% under deep sedation (p < 0.001). In patients with larynx obstructions, 53.3% were epiglottis anteroposterior prolapse, 20% were epiglottis folding, and 63.3% were arytenoid prolapse under light sedation. More patients had concentric type velopharynx and oropharynx obstructions and arytenoid prolapse under deep sedation.
Figure 2. Obstruction percentage of different drug-induced sleep endoscopy (DISE) parameters in four different disease severity groups (normal, mild, moderate, and severe obstructive sleep apnea).
The percentage of velopharynx obstruction (A) and oropharynx obstruction (B) of DISE under two different sedation depths. The existence of tongue base obstruction (C) and hypopharynx obstruction (D) under two DISE sedation depths. Tongue base obstruction was defined when cases had a tongue base drop that pushed the epiglottis backward. The percentage of larynx obstruction (E) and epiglottis prolapsed that touched the posterior pharyngeal wall (F) under two DISE sedation depths. The occurrence of epiglottis folding (G) and arytenoid prolapse (H) under different DISE sedation depths. p = the difference in obstruction percentages between different OSA severities; *p < 0.5, **p < 0.05, ***p < 0.01: the difference in obstruction percentages between different sedation depths in same OSA severity entity.
Effect of Sedation Depth and Disease Severity on DISE Findings
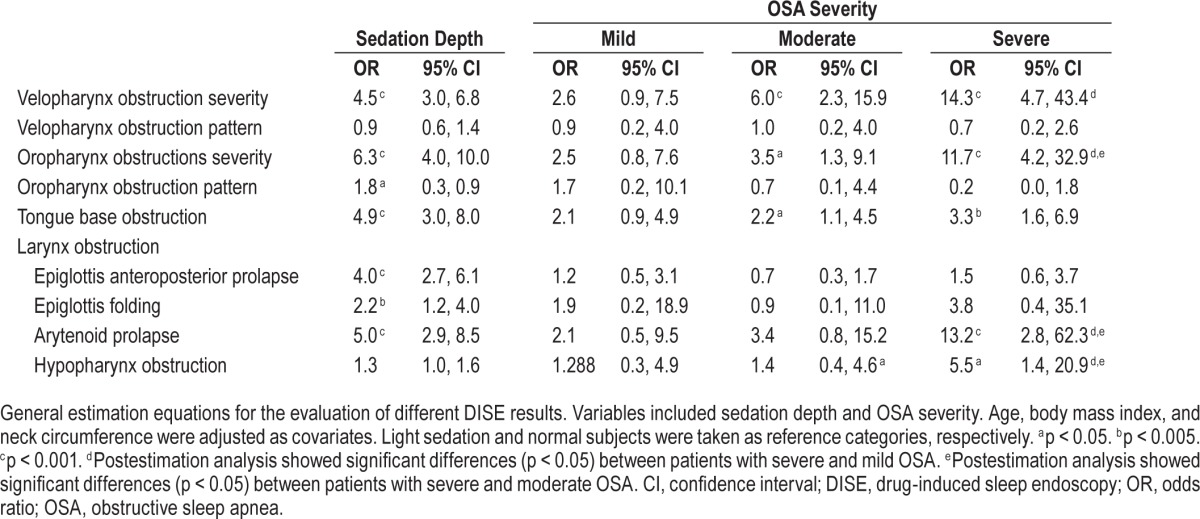
Sedation depth affected most DISE findings, including the severity of velopharynx and oropharynx obstructions, oropharynx obstruction pattern, tongue base obstruction, epiglottis anteroposterior prolapse, epiglottis folding, and arytenoid prolapse, with odds ratios (OR) ranging from 1.8 to 6.3 (Table 3). However, sedation depth did not affect the velopharynx obstruction pattern or hypopharynx obstruction. OSA severity had strong effects on velopharynx obstruction severity (OR 14.3), oropharynx obstruction severity (OR 11.7), and arytenoid prolapse (OR 13.2). However, disease severity did not affect velopharynx or oropharynx obstruction patterns, epiglottis anteroposterior prolapse, or epiglottis folding.
Table 3.
Effects of sedation and obstructive sleep apnea disease severity on different drug-induced sleep endoscopy obstruction patterns.
Reproducibility of DISE
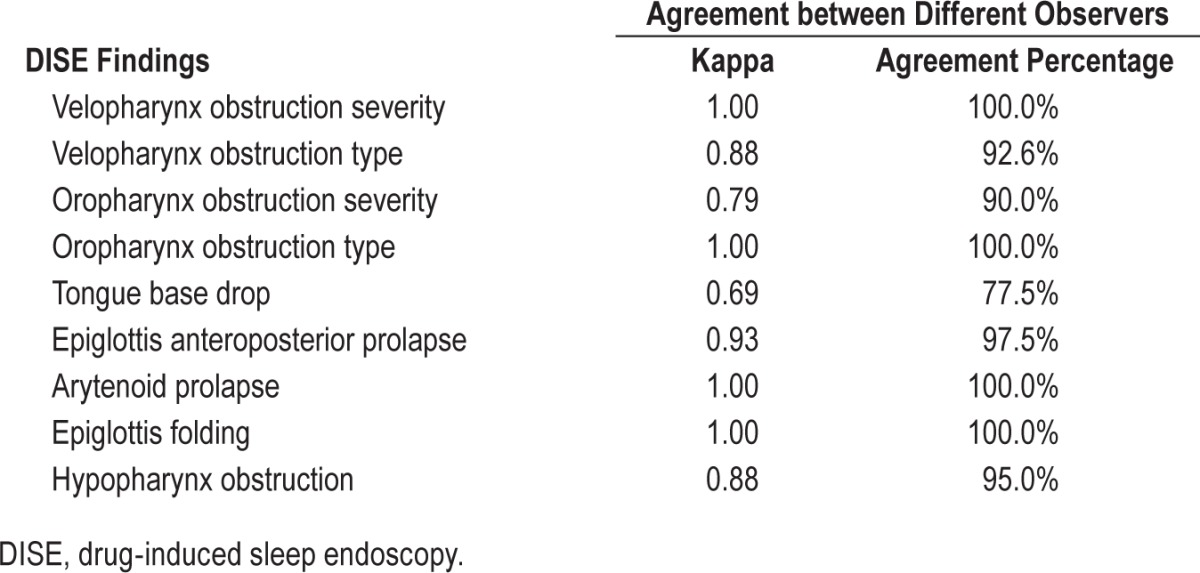
The agreements of all DISE findings between LS1 and LS2 were above 0.6, and most of them were above 0.8 (Table 4). The agreements between different observers with regard to DISE characters were mostly good to excellent (Table 5). All video clips were reviewed and the agreements between the original grade and second grade were also good to excellent (arytenoid prolapse, kappa = 0.896, agreement percentage 90.3%; epiglottis folding, kappa = 0.852, agreement percentage 86.1%; epiglottis collapse, kappa = 0.765, agreement percentage 90.7%).
Table 4.
Agreement of drug-induced sleep endoscopy findings between light sedation 1/light sedation 2 and deep sedation 1/deep sedation 2.
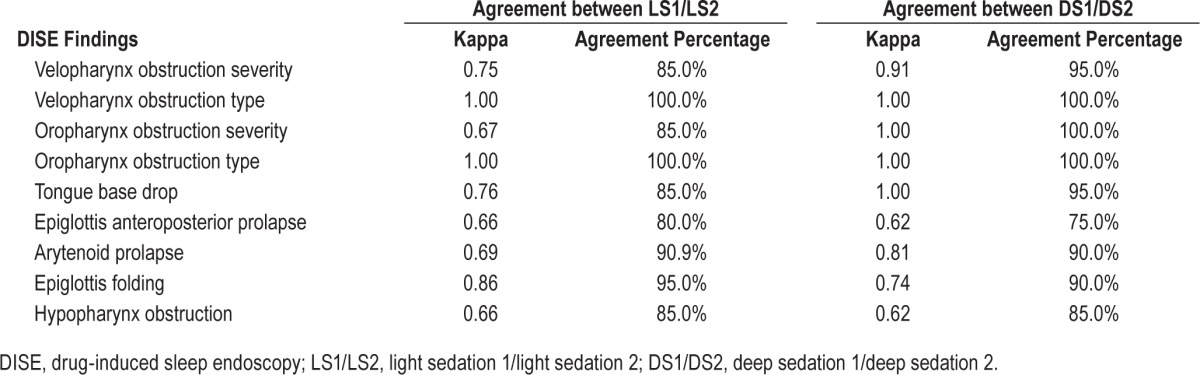
Table 5.
Agreement of drug-induced sleep endoscopy findings between different observers.
DISCUSSION
The current study revealed that 77.8%, 63.3%, and 33.4% of patients with OSA had velopharynx, oropharynx, and multiple-level obstructions when receiving DISE with the sedation depth kept at light sedation with a BIS level of 65–75, as compared to 38.9%, 38.9%, and 0% of normal patients under similar conditions. The percentages increased to 93.3%, 92.2%, and 74.4% when the sedation depth was maintained at deep sedation with a BIS level of 50–60 as compared to 77.8%, 72.1% and 33.3% of normal patients under similar conditions. Sedation depth affected most DISE results except for velopharynx obstruction pattern and hypopharynx obstruction. Meanwhile, OSA severity affected velopharynx and oropharynx obstruction severity and arytenoid prolapse more evidently. Validation of the DISE results under different time sequences and depths all had good to excellent agreement. Validation between different investigators also showed mostly good to excellent agreement.
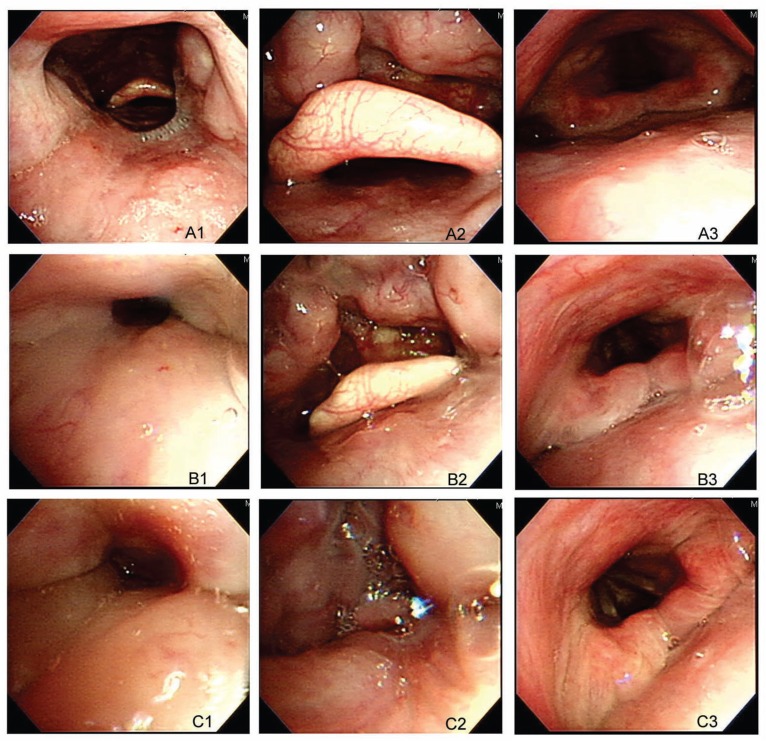
BIS has considerable individual variability, especially regarding the point of loss of consciousness.21 However, BIS level has been shown to correlate with upper airway collapsibility and is a better predictor of the hypnotic effect when compared with measured propofol concentration.6,22,23 Babar-Craig et al.14 compared the BIS values between DISE and natural sleep. Even when using propofol TCI, the BIS values, while performing DISE, were found to vary from 44 to 98.24 Hong et al.16 recently demonstrated that the upper airway narrowed with deeper sedation according to BIS level (above 70 versus below 70) in a small number of cases. The current study set a narrower sedative range to yield greater reproducibility. We also examined the effects of both sedative depth and disease severity on DISE parameters. Light sedation was performed at BIS values between 65 to 75 and when the patients snored or had velopharynx obstruction to avoid the variation of behavior control at the point of loss of consciousness. Deep sedation was defined as a BIS value of 50–60 to avoid oversedation, which may have caused an increased risk of airway collapse. The overall percentages of velopharynx and oropharynx obstructions under LS were more compatible with current imaging studies, being around 50% to 89% and 17% to 55%, respectively.25 Although the current study did not control for multiple confounding factors that affect airway collapsibility, deep sedation may have led to oversedation compared with light sedation given the overwhelming obstruction percentages (case demonstration, Figure 3).
Figure 3. Demonstration of drug-induced sleep endoscopy patterns of a 45-year-old man who had mild obstructive sleep apnea (apnea-hypopnea index 8.5).
A1, Velopharynx when awake. A2, Oropharynx when awake. A3, Hypopharynx when awake. B1, Velopharynx at LS. B2, Oropharynx at LS. B3, Hypopharynx at LS. C1, Velopharynx at DS. C2, Oropharynx at DS. C3, Hypopharynx at DS.
The current study evaluated the most severe obstruction episode to allow for compatibility between cases. However, overestimation of the severity was not frequent because complete obstruction was defined as being when no lumen could be seen, whereas the definition of partial obstruction was broader, being whenever any amount of lumen could be seen. Fluctuating upper airway collapse severity occurred during the nonrecording period of DISE when a steady state condition was not achieved. This further supports the need to narrow the sedation status. However, whether dynamic obstruction area measurements or a prolonged observation time is more compatible to the clinical entity should be further assessed. Borek et al.26 used quantitative airway analysis under DISE to evaluate sleep apnea, and this may be another method for more precise measurements of changes.
General estimation equations demonstrated that some obstruction characteristics were affected by disease severity, some by sedation depth, and some by both (Table 3). Deeper sedation and more severe disease increased upper airway collapsibility and affected both velopharynx and oropharynx obstruction severity. Epiglottis anteroposterior prolapse may be vulnerable to sedation due to a gravity effect. Further studies on position-dependent OSA cases may elucidate this issue. Epiglottis folding did not show a relationship with disease severity; however, the number of cases with this finding was limited. Whether this pattern is related to laryngomalacia is still unknown. Sedation depth alone may not trigger this phenomenon but may enhance it by increasing oropharynx and larynx collapsibility. Most other patterns were affected by both disease severity and sedation depth. Thus, narrowing the sedation depth variation by BIS may help to maintain a steady upper airway condition during DISE.
More recently, Rabelo et al.27 compared propofol-induced sleep to natural sleep in the daytime, and found no difference in AHI but significant differences in sleep macroarchitecture. They suggested that an adequate DISE performance condition under TCI propofol sedation. However, the 2-h propofol-induced sleep showed increased N3 but decreased, or no, rapid eye movement sleep, which should yield less airway collapsibility or AHI physiologically. In addition, it would be difficult, during a DISE study, to maintain a stable airway throughout a 2-h procedure. DISE studies of sedated patients dosed based on clinical responses also yields a varied range of BIS during the whole procedure.14 The current study proposed a narrowed range of sedation level, which should not require a long duration of sedation.
An interesting finding was the high percentage of laryngeal obstruction, especially arytenoid prolapse in severe cases, although our techniques did not allow us to accurately distinguish substantial from complete collapse. Arytenoid prolapse has rarely been reported, even though Bachar et al.28 noted 34% of patients had supraglottic soft tissue collapse and anteroposterior epiglottis prolapse. There is emerging evidence for a possible relationship between laryngeal obstruction and surgical failure in OSA treatment. It has been reported that the uvulopalatopharyngoplasty response rate decreases from 5% to 40% in patients with OSA with hypopharynx or larynx involvement.29 Arytenoid prolase, which was previous reported mainly in children with laryngomalacia or after laryngotracheoplasty surgery, could cause ventilatory failure or sleep disorders.30 No history of laryngomalacia was present in our patients with OSA, and all physical findings were comparable between the patients with and without arytenoid prolapse. Only the AHI was different between these two groups. (Among all OSA subjects, 71 cases with no arytenoid prolapse under light sedation had a mean AHI of 23.3 ± 16.3. Nineteen cases with arytenoid prolapsed under light sedation had a mean AHI of 48.3 ± 25.8. p = 0.002.) All of the cases of arytenoids prolapse occurred in patients with multiple segmental obstruction. The Starling resistor model suggested that downstream obstruction might occurred when the upstream structure was collapsed.31 However, some of the arytenoid prolapse occurred when the upper stream structures were only partly obstructed. This same concept may apply to other downstream obstruction as well. Whether this implies a primary obstruction site or a secondary one due to upstream obstruction needs further clarification.
Rodriguez-Bruno et al.32 demonstrated a good test-retest reliability of DISE under propofol infusion on separate days. In the current study, the validation of the sedation sequence proved that there was no difference in DISE findings whether we performed light sedation earlier or later when BIS was used to guide a steady state of sedation level. After a steady state of sedation had been reached, the DISE yielded similar results even when performed after different durations of sedation.
There were several limitations of the study. The first is that we enrolled patients with CPAP nonadherence or who sought alternative treatment. These patients and findings may not represent all patients with OSA. In addition, although better than a bolus injection, manually controlled propofol pump infusion for sedation maintenance is still not as convenient as TCI. Whether or not BIS-controlled TCI propofol injections can be applied for DISE requires further investigations. Another limitation is that sedation status is not necessarily equal to sleep stages. The BIS algorithm analyzes EEG synchronization, burst suppression, and power spectrum frequencies. It presents a lower BIS number when synchronization is higher.33 The lower the BIS number, the deeper the sedation and the higher the upper airway collapsibility.6 Rapid eye movement sleep, which yields greater airway collapsibility, however, has mixed EEG voltage and faster frequencies that could make the BIS number higher than N3 (slow wave) sleep. Although the current study tested two different sedation depths that showed different upper airway collapsibility, whether those depths directly correspond with certain sleep stage requires further clarification. One limitation is that the BIS number was not recorded breath by breath due to the lack of synchronization of the BIS and video system. The current study cannot present the mean BIS value during the recording period. In addition, the current study used airway area changes as a measure of obstruction. This may not be completely accurate in partial lumen obstruction and arytenoid prolapse. To what extent a decrease in area reflects clinical obstruction or air flow limitation needs further confirmation by flow or pressure monitors.
In conclusion, we propose an objective and reproducible sedation method for DISE using propofol pump infusion to maintain the BIS level between 65 and 75. Sedative depth was noted to affect most upper airway obstruction patterns. Specific larynx obstruction and arytenoid prolapse patterns among nonobese male patients should be noted as they may affect treatment outcomes. Further studies such as BIS-guided propofol TCI injections for DISE, and comparing endoscopy findings during natural and drug-induced sleep are important areas for future research.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by a grant from the Chang Gung Medical Research Program (CMPRG370431). The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Dr. Ni takes the responsibility for the content of the manuscript, including the data and analysis. Drs. Lo, Ni, and TY Lin were responsible for performing drug-induced sleep endoscopy, sleep induction with propofol, and the manuscript. Dr. Wang was in charge of polysomnography interpretations. Dr. JR Lin performed the statistical analysis. Drs. Li, White, Kuo contributed to the study design and manuscript preparation.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BIS
bispectral index
- CPAP
continuous positive airway pressure
- CT
computed tomography
- DISE
drug-induced sleep endoscopy
- DS
deep sedation
- EEG
electroencephalogram
- LS
light sedation
- OR
odds ratio
- OSA
obstructive sleep apnea
- TCI
target-controlled infusion
REFERENCES
- 1.Ko MT, Su CY. Computer-assisted quantitative evaluation of obstructive sleep apnea using digitalized endoscopic imaging with Muller maneuver. Laryngoscope. 2008;118:909–14. doi: 10.1097/MLG.0b013e3181638187. [DOI] [PubMed] [Google Scholar]
- 2.Croft CB, Pringle M. Sleep nasendoscopy: a technique of assessment in snoring and obstructive sleep apnoea. Clin Otolaryngol Allied Sci. 1991;16:504–9. doi: 10.1111/j.1365-2273.1991.tb01050.x. [DOI] [PubMed] [Google Scholar]
- 3.Hewitt RJ, Dasgupta A, Singh A, Dutta C, Kotecha BT. Is sleep nasendoscopy a valuable adjunct to clinical examination in the evaluation of upper airway obstruction? Eur Arch Otorhinolaryngol. 2009;266:691–7. doi: 10.1007/s00405-008-0831-5. [DOI] [PubMed] [Google Scholar]
- 4.Kotecha BT, Hannan SA, Khalil HM, Georgalas C, Bailey P. Sleep nasendoscopy: a 10-year retrospective audit study. Eur Arch Otorhinolaryngol. 2007;264:1361–7. doi: 10.1007/s00405-007-0366-1. [DOI] [PubMed] [Google Scholar]
- 5.Roblin G, Williams AR, Whittet H. Target-controlled infusion in sleep endoscopy. Laryngoscope. 2001;111:175–6. doi: 10.1097/00005537-200101000-00031. [DOI] [PubMed] [Google Scholar]
- 6.Eastwood PR, Platt PR, Shepherd K, Maddison K, Hillman DR. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology. 2005;103:470–7. doi: 10.1097/00000542-200509000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hillman DR, Walsh JH, Maddison KJ, et al. Evolution of changes in upper airway collapsibility during slow induction of anesthesia with propofol. Anesthesiology. 2009;111:63–71. doi: 10.1097/ALN.0b013e3181a7ec68. [DOI] [PubMed] [Google Scholar]
- 8.Coppens M, Van Limmen JG, Schnider T, et al. Study of the time course of the clinical effect of propofol compared with the time course of the predicted effect-site concentration: performance of three pharmacokinetic-dynamic models. Br J Anaesth. 2010;104:452–8. doi: 10.1093/bja/aeq028. [DOI] [PubMed] [Google Scholar]
- 9.Sigl JC, Chamoun NG. An introduction to bispectral analysis for the electroencephalogram. J Clin Monit. 1994;10:392–404. doi: 10.1007/BF01618421. [DOI] [PubMed] [Google Scholar]
- 10.Lo YL, Lin TY, Fang YF, et al. Feasibility of bispectral index-guided propofol infusion for flexible bronchoscopy sedation: a randomized controlled trial. PLoS One. 2011;6:e27769. doi: 10.1371/journal.pone.0027769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myles PS, Leslie K, McNeil J, Forbes A, Chan MT. Bispectral index monitoring to prevent awareness during anaesthesia: the B-Aware randomised controlled trial. Lancet. 2004;363:1757–63. doi: 10.1016/S0140-6736(04)16300-9. [DOI] [PubMed] [Google Scholar]
- 12.Sahinovic MM, Beese U, Heeremans EH, et al. Bispectral index values and propofol concentrations at loss and return of consciousness in patients with frontal brain tumours and control patients. Br J Anaesth. 2014;112:110–7. doi: 10.1093/bja/aet342. [DOI] [PubMed] [Google Scholar]
- 13.Whitlock EL, Villafranca AJ, Lin N, et al. Relationship between bispectral index values and volatile anesthetic concentrations during the maintenance phase of anesthesia in the B-Unaware trial. Anesthesiology. 2011;115:1209–18. doi: 10.1097/ALN.0b013e3182395dcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babar-Craig H, Rajani NK, Bailey P, Kotecha BT. Validation of sleep nasendoscopy for assessment of snoring with bispectral index monitoring. Eur Arch Otorhinolaryngol. 2012;269:1277–9. doi: 10.1007/s00405-011-1798-1. [DOI] [PubMed] [Google Scholar]
- 15.Rabelo FA, Kupper DS, Sander HH, et al. A comparison of the Fujita classification of awake and drug-induced sleep endoscopy patients. Braz J Otorhinolaryngol. 2013;79:100–5. doi: 10.5935/1808-8694.20130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong SD, Dhong HJ, Kim HY, et al. Change of obstruction level during drug-induced sleep endoscopy according to sedation depth in obstructive sleep apnea. Laryngoscope. 2013;123:2896–9. doi: 10.1002/lary.24045. [DOI] [PubMed] [Google Scholar]
- 17.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schellenberg JB, Maislin G, Schwab RJ. Physical findings and the risk for obstructive sleep apnea. The importance of oropharyngeal structures. Am J Respir Crit Care Med. 2000;162:740–8. doi: 10.1164/ajrccm.162.2.9908123. [DOI] [PubMed] [Google Scholar]
- 19.Kezirian EJ, Hohenhorst W, de Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol. 2011;268:1233–6. doi: 10.1007/s00405-011-1633-8. [DOI] [PubMed] [Google Scholar]
- 20.Kottner J, Audige L, Brorson S, et al. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. J Clin Epidemiol. 2011;64:96–106. doi: 10.1016/j.jclinepi.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim AE, Taraday JK, Kharasch ED. Bispectral index monitoring during sedation with sevoflurane, midazolam, and propofol. Anesthesiology. 2001;95:1151–9. doi: 10.1097/00000542-200111000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836–47. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Triltsch AE, Nestmann G, Orawa H, et al. Bispectral index versus COMFORT score to determine the level of sedation in paediatric intensive care unit patients: a prospective study. Crit Care. 2005;9:R9–17. doi: 10.1186/cc2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Vito A, Agnoletti V, Berrettini S, et al. Drug-induced sleep endoscopy: conventional versus target controlled infusion techniques--a randomized controlled study. Eur Arch Otorhinolaryngol. 2011;268:457–62. doi: 10.1007/s00405-010-1376-y. [DOI] [PubMed] [Google Scholar]
- 25.Moon IJ, Han DH, Kim JW, et al. Sleep magnetic resonance imaging as a new diagnostic method in obstructive sleep apnea syndrome. Laryngoscope. 2010;120:2546–54. doi: 10.1002/lary.21112. [DOI] [PubMed] [Google Scholar]
- 26.Borek RC, Thaler ER, Kim C, Jackson N, Mandel JE, Schwab RJ. Quantitative airway analysis during drug-induced sleep endoscopy for evaluation of sleep apnea. Laryngoscope. 2012;122:2592–9. doi: 10.1002/lary.23553. [DOI] [PubMed] [Google Scholar]
- 27.Rabelo FA, Kupper DS, Sander HH, Fernandes RM, Valera FC. Polysomnographic evaluation of propofol-induced sleep in patients with respiratory sleep disorders and controls. Laryngoscope. 2013;123:2300–5. doi: 10.1002/lary.23664. [DOI] [PubMed] [Google Scholar]
- 28.Bachar G, Feinmesser R, Shpitzer T, Yaniv E, Nageris B, Eidelman L. Laryngeal and hypopharyngeal obstruction in sleep disordered breathing patients, evaluated by sleep endoscopy. Eur Arch Otorhinolaryngol. 2008;265:1397–402. doi: 10.1007/s00405-008-0637-5. [DOI] [PubMed] [Google Scholar]
- 29.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–77. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 30.Hart CK, Richter GT, Cotton RT, Rutter MJ. Arytenoid prolapse: a source of obstruction following laryngotracheoplasty. Otolaryngol Head Neck Surg. 2009;140:752–6. doi: 10.1016/j.otohns.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz AR, Smith PL. CrossTalk proposal: the human upper airway does behave like a Starling resistor during sleep. J Physiol. 2013;591:2229–32. doi: 10.1113/jphysiol.2012.250654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Bruno K, Goldberg AN, McCulloch CE, Kezirian EJ. Test-retest reliability of drug-induced sleep endoscopy. Otolaryngol Head Neck Surg. 2009;140:646–51. doi: 10.1016/j.otohns.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowdle TA. Depth of anesthesia monitoring. Anesthesiol Clin. 2006;24:793–822. doi: 10.1016/j.atc.2006.08.006. [DOI] [PubMed] [Google Scholar]