Abstract
Study Objectives:
To determine prevalence of depressive symptoms in obstructive sleep apnea (OSA) and the impact of OSA treatment on depression scores.
Methods:
Consecutive new patients referred for investigation of suspected OSA were approached. Consenting patients completed a patient health questionnaire (PHQ-9) for depressive symptoms when attending for laboratory polysomnography. Those with moderate/severe (apneahypopnea index [AHI] ≥ 15 events/h) and/or symptomatic mild OSA (AHI 5–14.99 events/h) were offered continuous positive airway pressure (CPAP) therapy. PHQ-9 was repeated after 3 months of CPAP with compliance recorded. Of a maximum PHQ-9 score of 27, a cut point ≥ 10 (PHQ-9 ≥ 10) was used to indicate presence of clinically significant depressive symptoms.
Results:
A total of 426 participants (243 males) were recruited. Mean ± standard deviation body mass index (BMI) was 32.1 ± 7.1 kg/m2 and AHI 33.6 ± 28.9 events/h. PHQ-9 was 10.5 ± 6.1 and independently related to AHI (p < 0.001) and BMI (p < 0.001). In those without OSA, PHQ-9 ≥ 10 was more common in women, but no gender difference was evident with OSA. Of 293 patients offered CPAP, 228 were compliant (mean nightly use > 5 h) over 3 months of therapy. In them, with therapy, AHI decreased from 46.7 ± 27.4 to 6.5 ± 1.6 events/h, PHQ-9 from 11.3 ± 6.1 to 3.7 ± 2.9 and PHQ-9 ≥ 10 from 74.6% to 3.9% (p < 0.001 in each case). Magnitude of change in PHQ-9 was similar in men and women. Antidepressant use was constant throughout.
Conclusions:
Depressive symptoms are common in OSA and related to its severity. They improve markedly with CPAP, implying a relationship to untreated OSA.
Citation:
Edwards C, Mukherjee S, Simpson L, Palmer LJ, Almeida OP, Hillman DR. Depressive symptoms before and after treatment of obstructive sleep apnea in men and women. J Clin Sleep Med 2015;11(9):1029–1038.
Keywords: patient health questionnaire, depression, depressive symptoms, obstructive sleep apnea, continuous positive airway pressure
Persistent sleep loss causes symptoms that are similar to those of depression, in part because frontal lobe centers responsible for emotional modulation are sensitive to disturbed sleep.1 Consistent with this observation, obstructive sleep apnea (OSA), a condition characterized by fragmented sleep, is commonly associated with depressive symptoms.2 Conversely, depression is often associated with disturbed sleep. Further, depression and OSA are prevalent in the community and, hence, could be expected to coexist in a significant proportion of patients. The multidirectional relationships between depression, disturbed sleep, and OSA are a source of potential diagnostic confusion, which may explain why OSA, a generally under-recognized condition, is particularly under-diagnosed among people with depression.3 Failure to recognize and treat OSA in depressed patients may lead to inappropriate prescription of antidepressant therapy and/or persistence of depressive symptoms despite such therapy. Equally the presence of depressive symptoms in patients with untreated OSA could lead to misdiagnosis of depression.4
BRIEF SUMMARY
Current Knowledge/Study Rationale: Depressive symptoms are common in patients with obstructive sleep apnea.
Study Impact: This paper examines the nature of this relationship and the influence on it of gender and of effective treatment of obstructive sleep apnea in a large cohort of such patients.
Previous studies of the relationship between OSA and depression have demonstrated this shared symptomatology, although the extent to which depressive symptoms are present in OSA is still unclear.3,5,6 Many previous studies have been of low power or have used a variety of instruments of varying quality to assess depressive symptoms.6 Lack of direct contact with patients and retrospective assessment of depressive symptoms rather than assessment prior to diagnosis of OSA have been limitations of some of them.7 Others have failed to specify the method used to identify OSA or have used limited methods to characterize it rather than gold standard laboratory based polysomnography (PSG).6
An additional limitation in examining the relationship between OSA and depression has been that the instruments to identify depression have been validated for the general population but not in OSA patients. Given the potential for overlap in symptoms between the two conditions, it cannot be assumed that the presence of depressive symptoms in OSA patients indicates the presence of depression independent of OSA. While this may be so in some patients, it is also possible that such symptoms are attributable to OSA rather than depression or to a depressive illness caused by untreated OSA. Defining the role of OSA in the genesis of such symptoms could be achieved by elimination of the condition followed by reassessment.8 Continuous positive airway pressure (CPAP), the first-line therapy for moderate to severe OSA, provides an opportunity to do so, as it is highly efficacious and compliance with it can be accurately monitored.9
The present study has been designed to address these issues. Its purpose was to determine: (a) the occurrence of depressive symptoms among patients presenting with OSA, and (b) the extent to which depressive symptoms improved with CPAP treatment. We hypothesized that OSA would be commonly associated with depressive symptoms and that these would decrease in severity with OSA treatment. In addition, we postulated that there may be a subset of depressive symptoms in patients with OSA that persists despite treatment, and that these may more reliably identify OSA-independent depression in this population. We also wished to determine whether there were gender differences in symptomatology and response to treatment, given the different prevalences of both depression and OSA in men and women. In developing the study we were careful to observe the distinction between depressive symptoms and depression itself, as we recognized that symptoms can be shared between OSA and depression and be a source of diagnostic confusion between them. Indeed it is the potential for such confusion that provided a major rationale for the study.
MATERIALS AND METHODS
Subjects
The study was approved by the Sir Charles Gairdner Research Ethics Committee. Participants were required to speak English and not to have been previously diagnosed with OSA. All participants were new patients reviewed at a sleep clinic at Sir Charles Gairdner Hospital. They were assessed by a board-certified sleep specialist and found to meet standard indications for PSG evaluation for the suspected diagnosis of OSA. All decisions regarding patient diagnosis and treatment were made by their sleep specialist: the investigators had no part in these decisions. Participants were excluded from this study if unable to complete health questionnaires meaningfully. Participants completed the Western Australian Sleep Health Study questionnaire given to all new patients attending the sleep clinic to gather information relevant to sleep disorders and general health and age, gender, use of antidepressants, and body mass index (BMI) were recorded.10
Protocol
Assessment at Baseline: Relationship between OSA and Depressive Symptoms
Each eligible patient attending the sleep clinic over a 5-month period was approached and asked to participate. Those who provided informed consent completed the patient health questionnaire (PHQ-9) to screen for depressive symptoms prior to undergoing laboratory PSG.11
Effect of Treatment of OSA on Depressive Symptoms
Those participants found to have moderate or severe OSA (apnea-hypopnea index [AHI] ≥ 15 events/h) or symptomatic mild OSA (AHI 5–14.99 events/h) were referred for a physician supervised trial of CPAP over a 3- to 6-week period, according to the standard practice of the sleep clinic. Where efficacy, compliance, and acceptance of therapy were satisfactory, the trial was extended for an additional 6–9 weeks (12 weeks in total). All CPAP compliant participants (mean use from device download ≥ 5 h/night) were reassessed for depressive symptoms with the PHQ-9 3 months after the date of commencement of this trial.12 CPAP compliance was also assessed at that time and was summarized as the mean daily usage from device download for the previous 8 to 12 weeks. Device download data was also used to determine AHI on CPAP therapy.
PHQ-9 Depression Scale (Figure 1)
Figure 1. Public health questionnaire (PHQ-9).

The PHQ-9 is a questionnaire-based depression scale that is used to assist primary care practitioners in diagnosing depression and monitoring treatment.11,13 It asks 9 questions which relate to feelings of sadness, tiredness and sleepiness or sleeping too much, little interest in doing things, thoughts of personal failures, troubles in concentration, perceived decreases in self-confidence, slow/fast speech and suicidal ideation. Each question is answered on a scale of 0 to 3, giving a range of possible scores from the sum of responses from 0 to 27. It has been suggested that a score of 0 to 4 indicates nil or minimal depression; 5 to 9 mild depression; 10 to 14 moderate depression; 15 to 19 moderately severe depression; and 20 to 27 severe depression. PHQ-9 validation studies suggest a cutoff value < 10 indicates a low likelihood of major depression in the general population.11
Polysomnography
Standard polysomnography was performed and AHI derived using the American Academy of Sleep Medicine “Chicago” scoring criteria, with hypopneas requiring either > 50% airflow reduction or a lesser airflow reduction with associated > 3% oxygen desaturation or arousal.14 The degree of hypoxemia during sleep was determined from the percentage of total sleep time spent below an arterial oxygen saturation of 90% (%TST < 90).
Analysis
The data were analyzed using the Statistical Package for Social Sciences (SPSS version 19.0). The relationship between PHQ-9 scores at baseline and age, gender, BMI, AHI, and %TST < 90 were examined by simple linear regression analysis, treating each parameter as a continuous variable. The data were then entered into a multiple linear regression model using backwards elimination of insignificant terms considering interactions with gender for each parameter to determine significant predictors of depressive symptoms.
Analysis of variance was used to examine the relationships between PHQ-9 scores and: (a) OSA severity (categorized by AHI as follows: < 5 events/h = nil; 5–14.99 events/h = mild; 15–29.99 events/h = moderate; and > 30 events/h = severe); (b) BMI (categorized according to World Health Organization guidelines as follows: < 25 kg/m2 = underweight to normal; 25–29.99 kg/m2 = overweight; and ≥ 30 kg/m2 = obese).
Pearson χ2 tests were used to examine the relationships between presence of clinically significant depressive symptoms (PHQ-9 ≥ 10) and OSA severity and BMI categories. Multiple logistic regression analysis was used to determine the independent odds of presence of clinically significant depressive symptoms with increasing OSA, BMI, and %TST < 90 (as categorical variables) and age (in years).
Paired t-tests were used to compare PHQ-9 scores before and after CPAP treatment in those who completed 3 months of treatment. The proportions of those with clinically significant depressive symptoms before and after CPAP treatment were compared using the McNemar test. The relationship between magnitude of change in PHQ-9 and hours of CPAP use was examined by linear regression analysis. These assessments were repeated in a subgroup composed of all those patients taking antidepressant medications. The sign test was used to compare responses to the component questions in the PHQ-9 score before and after CPAP therapy to determine those symptoms most responsive to control of OSA.
Numerical data were expressed as mean ± standard deviation (SD). A p value < 0.05 was considered significant.
RESULTS
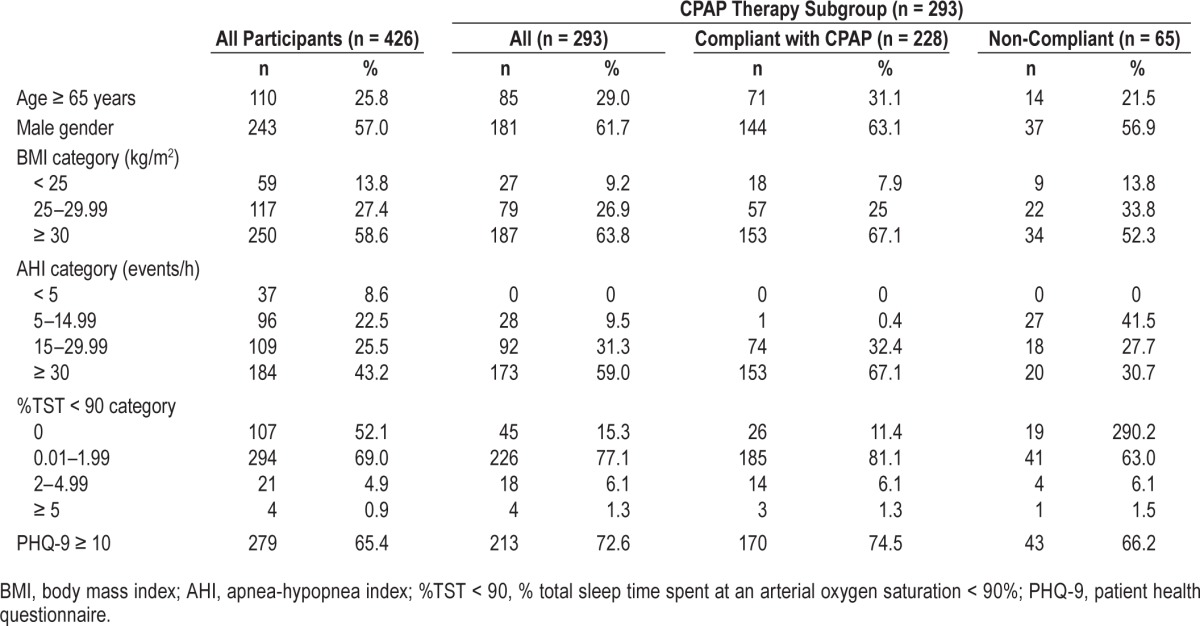
Of the 607 patients approached over the 5-month study period, 181 were excluded because they had been exposed to CPAP therapy in the past, suffered from cognitive deficits precluding meaningful completion of the PHQ-9 questionnaire, or could not speak English. The remaining 426 patients (243 men, 183 women; age 52 ± 15 years; BMI 32.1 ± 7.1 kg/m2) provided informed consent and were recruited into the study. Of these, 293 were subsequently assigned CPAP therapy. Table 1 shows the clinical characteristics (age, gender, BMI category, AHI category, %TST < 90 category, and proportion with depressive symptoms (PHQ-9 ≥ 10) of the 426 recruits at baseline, the 293 patients assigned CPAP therapy and, of those assigned CPAP, the compliant vs. non-compliant patients.
Table 1.
Clinical characteristics at baseline of all participants and of the subgroup assigned to CPAP therapy.
Determinants of PHQ-9 at Baseline
PHQ-9 for the 426 patients at baseline was 10.5 ± 6.1 and AHI was 33.6 ± 28.9 events/h. Linear regression showed significant associations between PHQ-9 score and BMI (p < 0.0001), AHI (p < 0.0001), and %TST < 90 (p < 0.017). The relationship between subject characteristics and baseline PHQ-9 score differed when stratified by gender. In men, AHI and %TST < 90 significantly predicted baseline PHQ-9 score. In women, BMI and AHI significantly predicted baseline PHQ-9 score. However, when these variables were analyzed by multiple linear regression analysis only AHI (β = 0.058, standard error [SE] = 0.010, p < 0.001), and BMI (β = 0.118, SE = 0.04; p < 0.005) proved to be independent predictors of PHQ-9 scores. Gender as a main effect or as an interaction term with AHI or BMI, did not have any statistical relationship with baseline PHQ-9 in a multivariate model. Similarly, %TST < 90 did not appear to explain baseline PHQ-9 score once AHI and BMI were accounted for.
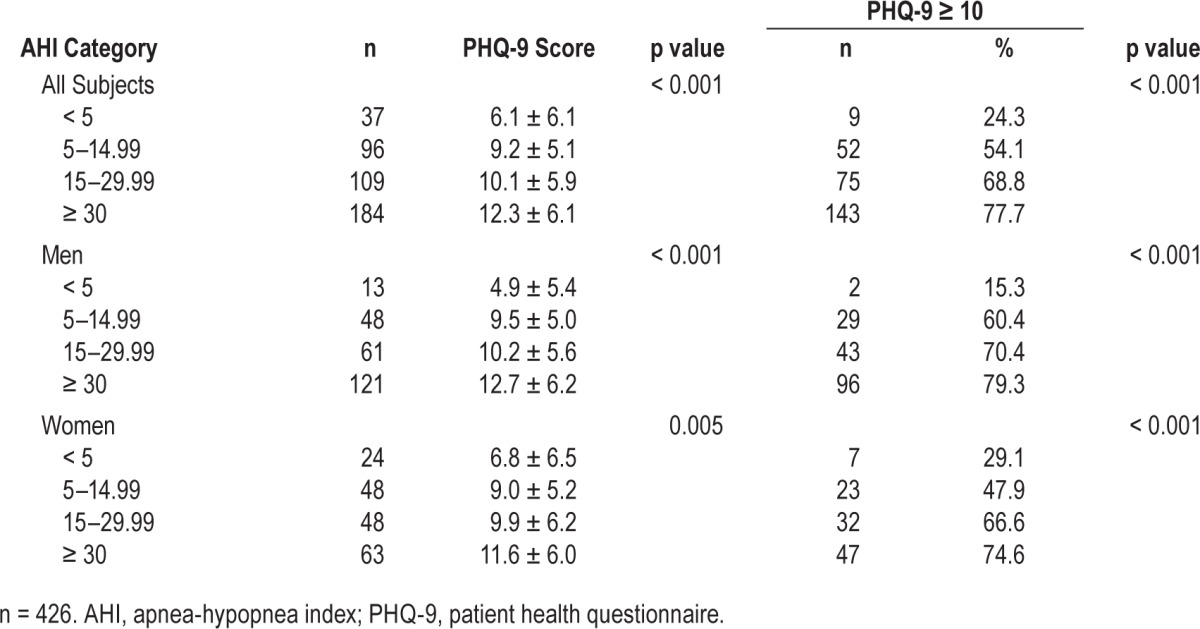
When the PHQ-9 data were examined by AHI category (Table 2), increased severity of OSA was found to be associated with increased PHQ-9 scores, and the proportion of subjects with PHQ-9 score ≥ 10 (indicative of clinically significant depressive symptoms) in both men and women (p < 0.001 in each circumstance). The PHQ scores and proportions appeared to increase progressively with increasing OSA severity category. It was notable that where OSA was absent (AHI < 5 events/h), the PHQ-9 score was greater in women than men; this was not the case where OSA was present.
Table 2.
Relationships at baseline of categorical severity of OSA to PHQ-9 score and to proportion of patients with a PHQ-9 score ≥ 10 (indicative of clinically significant depressive symptoms).
When examined by BMI category (< 25 kg/m2, 25–29.99 kg/m2, ≥ 30 kg/m2) increasing obesity was also associated with increased PHQ-9 scores and proportion of patients with clinically significant depressive symptoms in both men and women (p < 0.001 in each circumstance).
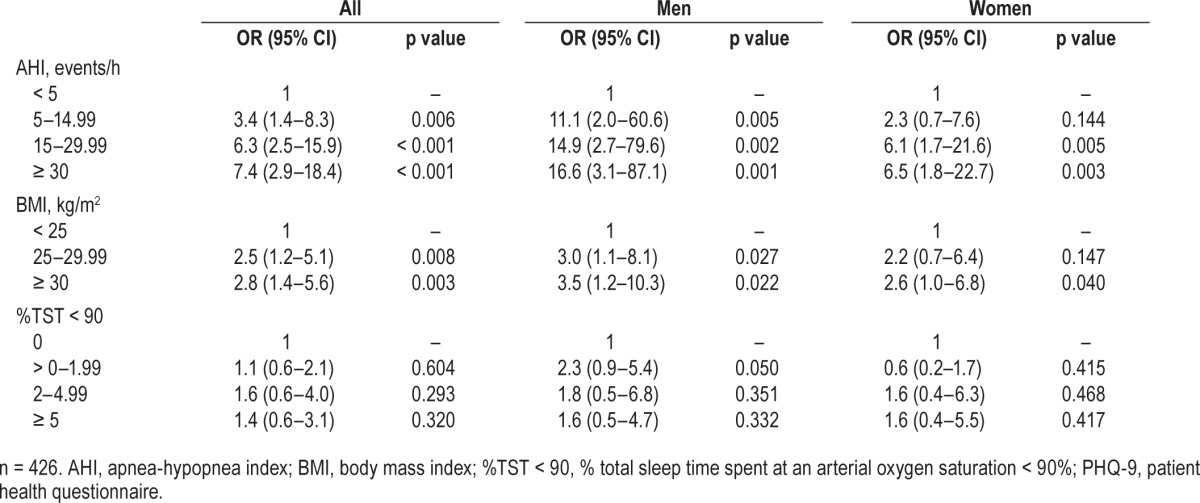
Univariate analysis of baseline data demonstrated that increases in categorical AHI, BMI, and %TST < 90 were each associated with an increase in clinically significant depressive symptoms. However multivariate logistic regression analysis showed that while AHI and BMI were independent determinants of clinically significant depressive symptoms, %TST < 90 was not (Table 3). The analysis showed a greater effect of AHI category in men than women: relative to the AHI < 5 events/h reference category, men had higher odds of depressive symptoms with all severities of OSA, while women only had greater odds of depressive symptoms where AHI was in the moderate to severe range (AHI ≥ 15 events/h). While both overweight (BMI 25–30 kg/m2) and obese (BMI > 30 kg/m2) categories increase odds of clinically significant depressive symptoms in men, only obesity increased the odds in women. In neither men nor women did age or %TST < 90 have an impact on the odds of depressive symptoms once the model was adjusted for AHI and BMI.
Table 3.
Relationships at baseline of categorical AHI, BMI, and TST < 90 to a PHQ-9 score ≥ 10 (indicative of clinically significant depressive symptoms).
Effect of Treatment of Moderate/Severe OSA on Depressive Symptoms
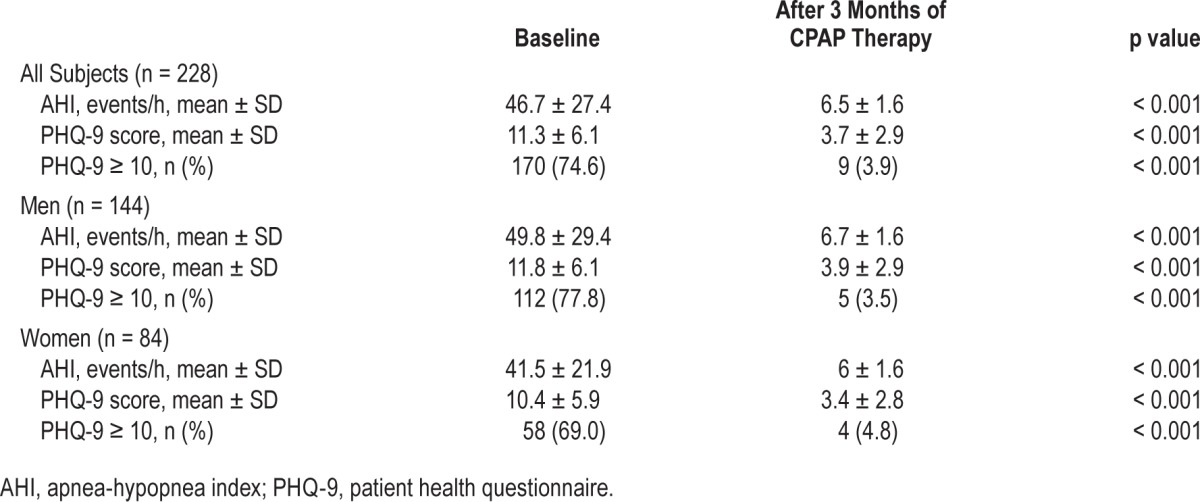
Two hundred ninety-three participants with OSA (AHI ≥ 5 events/h) were prescribed CPAP therapy following PSG, of whom 228 (144 males and 84 females) complied with 3 months of treatment. The remaining 65 participants were non-compliant (mean nightly use < 5 h) or were unable to complete the study assessments. Compliance increased with increasing AHI category, with 153 of the 173 patients with an AHI ≥ 30 events/h compliant at 3 months (Table 1). All the 228 CPAP compliant patients completed a repeat PHQ-9 assessment 3 months after initiating CPAP therapy (mean nightly use 6.9 ± 0.82 h, range 5–8 h). After 3 months of treatment, there was a substantial decrease in the mean AHI, PHQ-9 score and the proportion of individuals with clinically significant depressive symptoms (Table 4). Clinically significant depressive symptoms failed to remit in 9 individuals (5 men and 4 women). Total PHQ-9 score remained unchanged in 18 individuals, and one individual reported a PHQ-9 score post-CPAP therapy that was one point higher than at baseline; however, these patients generally had low scores (mean PHQ-9 score = 1.2, range 0–10).
Table 4.
AHI, PHQ-9 score, and PHQ-9 category at baseline and after 3 months of therapy in patients compliant with CPAP.
Of the 228 CPAP compliant patients, 51.8% were previously prescribed and used antidepressant drug therapy, and antidepressant use remained unchanged over the course of the study. Analysis of these 118 patients demonstrated a similar decline in PHQ-9 scores with therapy (from 12.0 ± 6.1 to 3.7 ± 2.8) compared with the complete patient group shown in Table 4.
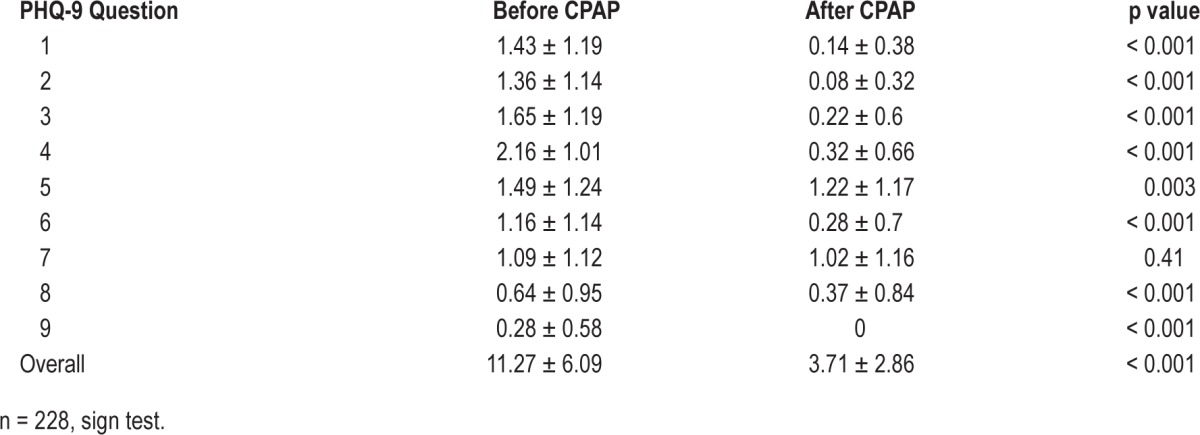
Effect of Treatment of Moderate/Severe OSA on PHQ-9 by Individual Questions
Table 5 demonstrates that scores for individual questions (Figure 1) significantly decreased in all except question 7, which related to difficulty with concentration. Of the remainder, question 5, relating to appetite, showed least change. Before CPAP, 18.3% of patients had positive responses to question 9 (thoughts of being better off dead or of self-harm), while none did after treatment. Of the 170 patients with a PHQ-9 score ≥ 10 at baseline who were compliant with CPAP at 3 months, 161 had a reduction in PHQ-9 to < 10. Comparison of them to the 9 patients whose PHQ-9 remained ≥ 10 did not suggest differences with respect to age, BMI, AHI, or PHQ-9 at baseline. Examination of differences in individual depressive symptoms between these groups was not possible due to the small number of patients in the persistently high PHQ-9 score group.
Table 5.
Effect of CPAP treatment of OSA on overall PHQ-9 score and component questions in all subjects completing 3 months of CPAP use.
DISCUSSION
The major findings of the study were that: (a) depressive symptoms are common among patients referred for investigation of OSA; (b) in patients without OSA (AHI < 5 events/h) the proportion of women with clinically significant depressive symptoms is higher than that of men but this difference is not evident when OSA is present; (c) depressive symptoms are directly correlated with the severity of OSA; (d) symptoms of depression, including suicidal ideation, are relieved by effective treatment of OSA with CPAP therapy; and (e) these beneficial effects of CPAP are seen to a similar degree in men and women and are independent of the use of antidepressants. It is important, and fundamental to the study, to note the distinction between depressive symptoms and depression itself. As our findings suggest, the shared symptomatology of OSA and depression create the potential for diagnostic confusion between these conditions. Where such symptoms are present consideration of the differential diagnosis suggested by them is required with further investigation before diagnosis is finalized.
Depressive Symptoms are Common among People with OSA
Previous investigations have suggested an association between OSA and depression based on shared symptoms.5,7 This study provides further evidence for this association, suggesting substantial potential for diagnostic confusion between the entities. It improves on previous studies by: investigating a relatively large number of participants; characterizing OSA presence and severity using gold standard laboratory polysomnography; assessing depressive symptoms prospectively using a well validated screening tool; accounting for potential age and obesity confounding; assessing the influence of gender on this relationship; and by determining the efficacy of CPAP treatment on depressive symptomatology using an objective method (device recorded hours of use) to assess compliance.
Depression is highly prevalent worldwide and a leading cause of disability and loss of quality of life. A large proportion of depression sufferers fail to respond fully to standard antidepressant therapy making it imperative to identify and rectify, where possible, factors contributing to this debilitating condition.15 It may be that the overlap between depressive symptoms and OSA is due to chance, as both conditions are common in the community. Both depression and OSA are frequently associated with disturbed sleep, fatigue, and irritability. However, the relatively uniform impact of untreated OSA across the range of depressive symptoms represented in the PHQ-9 questionnaire, does raise the possibility that disturbed sleep from OSA could play a pathogenetic role in the occurrence of depression. Frontal lobe centers responsible for emotional modulation are sensitive to disturbed sleep and it is possible that OSA-related sleep fragmentation and/or hypoxemia could disturb cerebral neurochemical/neurophysiological function resulting in depression.16 While the relatively prompt response to CPAP treatment might suggest relief of shared symptoms rather than of OSA-induced depression, the possibility of a pathogenetic role of OSA in depression remains. Sleep fragmentation appears a more likely mechanism underpinning the association between OSA and depressive symptoms than does upper airway obstruction-related hypoxemia, as our analysis demonstrates that when AHI, an index of fragmentation, is taken into account %TST < 90, an index of hypoxemia, ceases to be an independent predictor of PHQ-9 score in the untreated state.
Regardless of mechanisms, the shared symptomatology between these common conditions is such that diagnostic confusion is likely to arise. Approximately 8.7% of Australians and a similar proportion of Americans use antidepressant medications.17,18 It was notable that a far higher proportion (51.8%) of our patients were on antidepressant therapy at baseline, consistent with their elevated PHQ-9 scores. It was also notable that with CPAP treatment the proportion of patients with symptoms of clinically significant depression decreased to below these proportions of people in the general community on antidepressant therapy.
As with depression, OSA is a highly prevalent condition, affecting approximately 7% of adults to a moderate or severe degree.19 At present, the possibility of OSA is not widely considered in depression management. Indeed, OSA remains a generally under-diagnosed and under-recognized condition, possibly at least partly because symptoms are wrongly attributed to depression. Consistent with this notion, OSA seems to be particularly under-diagnosed in people with depression.20 Failure to recognize and treat OSA may lead to persistence of depressive symptoms and/or to unnecessary use of antidepressant therapy.
While this study examines depressive symptoms among OSA sufferers, the findings that these symptoms occur commonly in association with OSA and are so responsive to CPAP therapy suggest there is a case for systematic screening of depressed populations for OSA. The need for OSA screening may be even greater among people with depression who fail to respond standard antidepressant treatment.
Depressive Symptomatology and Depression
This study used the PHQ-9 to assess depressive symptom-atology. It is a tool developed to help diagnose depression in primary care. Its diagnostic validity has been established in studies involving large numbers of patients and has been used successfully in specialized clinics.21,22 The scale is brief, easy to administer and its nine questions are directly based on the DSM-IV criteria for a depressive episode. As Kroenke and colleagues point out, the quantitative nature of the score allows it to be used to both establish the diagnosis of depression and grade depressive symptom severity.11 Care was taken to minimize reporting bias by using word “well-being” rather than “depression” when first introducing the questionnaire and ensuring it was answered before any discussion of OSA with the sleep physician. Tellingly, one of the 9 questions in the PHQ-9 questionnaire asks about suicidal ideation, and this elicited a positive response prior to treatment in 41 of 228 patients who went on to successfully use CPAP therapy and in none of them at the 3-month follow up.
While the PHQ-9 has not been formally validated as a tool to diagnose depression in sleep clinic populations, it has been used in a substantial number of OSA-related studies.20,23–30 In the present paper we have been careful to refer to depressive symptoms, not depression, recognizing that it may be the symptoms that are confused between the conditions. The scoring system used (PHQ-9) is a widely validated method of diagnosing depression, seeking out as it does the key depressive symptoms, being based directly on the nine diagnostic criteria for major depressive disorder in the DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition).31 The exclusive focus of PHQ-9 on these symptoms appears to be an advantage in terms of its utility in detecting depression.11 Our findings demonstrate that these widely accepted depressive symptoms are highly prevalent in OSA, providing the potential for OSA to be misdiagnosed in any setting, including the primary care setting for which the PHQ-9 was originally devised and in which setting this simple tool has been validated to diagnose depression. Both depression and OSA are common problems in primary care. The high proportion of patients on antidepressants at enrolment in the present study (51.8% [vs 8.7% in the general community]) suggests that depression was diagnosed before the possibility of OSA was considered.
We used a PHQ-9 cut point of ≥ 10 to indicate clinically significant depressive symptoms, as scores of < 10 are seldom associated with major depression.11 It is important to note that depressive symptoms do not necessarily equate to depression. This may be particularly so in the context of OSA, given the potential symptomatic overlap between the conditions. Regardless, our findings suggest that where depressive symptoms are present the possibility of OSA, a widely prevalent disorder, should be considered.
Depressive Symptoms are Equally Prevalent among Women and Men where OSA is Present
Comparing the results between genders, where there is no OSA (AHI < 5 events/h) mean PHQ-9 scores were lower among men than women, a finding that is consistent with a greater female prevalence of depression in the general population.32 However, when OSA was present, this gender imbalance was no longer evident. Indeed, multivariate analysis shows that the effect of AHI category is greater in men than women. Women only have greater odds of clinically significant depressive symptoms when AHI is in the moderate range; however, men with OSA have high odds of such symptoms regardless the AHI category. Only obesity (BMI ≥ 30 kg/m2) increases odds of clinically significant depressive symptoms in women, whereas in men both overweight and obesity increase the odds. In both men and women neither age nor %TST < 90 have an impact on odds of clinically significant depressive symptoms once the model is adjusted for AHI and BMI. This overriding effect of OSA on the gender differences in depressive symptoms that are present without OSA underlines the apparently powerful effect it has on occurrence of these symptoms that our other findings also suggest.
Depressive Symptoms are Independently Related to the Severity of OSA
The relationship between PHQ-9 and AHI was independent of BMI. As our findings show, obesity is also independently associated with depressive symptoms. The association between obesity and OSA is well known hence it is essential that it is taken into account in determining the relationship between OSA and depression.33
It was also evident that while there was a univariate relationship between %TST < 90 and PHQ-9 scores, this association was no longer apparent on multivariate analysis when AHI was taken into account. Again, this suggests that the sleep disruption and fragmentation that AHI represents is of greater importance in the generation of depressive symptoms than the hypoxemia that can accompany these obstructive events.
Logistic regression analysis (Table 3) demonstrates that the odds of having clinically significant depressive symptoms increases progressively with increasing categorical severity of OSA, relative to those with without OSA (AHI < 5 events/h).
Depressive Symptoms in OSA Patients are Relieved by CPAP Therapy
The symptomatic response to effective treatment of OSA is an important in helping determine the role of untreated OSA in symptom genesis. A recent meta-analysis of the effect of treatment of OSA on depressive symptoms in 19 randomized controlled trails demonstrates an overall improvement, although there was significant heterogeneity between the trials in their designs and outcomes.34 Many of them involved low numbers of patients, short follow-up periods, poor CPAP compliance, or failure to include patients with severe OSA. None of them involved the present study's combination of large numbers of patients, lengthy follow-up periods, optimization of CPAP compliance, and inclusion of severe OSA. While future studies addressing these issues are called for, it is difficult to envisage ethical approval for randomized controlled trials which require withholding effective therapy for severe OSA for lengthy periods.35,36
PHQ-9 has previously been demonstrated to be a responsive and reliable measure of depression treatment outcomes.37 Our study demonstrates a substantial effect on this metric of depressive symptoms in this large patient group, where OSA of all severities was studied with CPAP compliance optimized and sustained over a lengthy (3-month) period. This effect was seen in both men and women regardless of whether antidepressants were prescribed or not. Only those achieving a minimum mean nightly use of 5 hours of CPAP therapy at 3 months were reevaluated for depressive symptoms.
Such efficacy emphasizes the importance of screening people with depressive symptoms for OSA so that, if present, it can be identified and effectively treated. At a minimum, such patients should be asked about common OSA symptoms including habitual snoring, witnessed apneas, disrupted sleep, and excessive daytime sleepiness.
Examining the response to therapy by individual questions from the PHQ-9 score demonstrated a significant decrease in scores for each question, apart from Question 7, (“do you have trouble concentrating?”). It seems unlikely that this symptom is an indicator of depression that is independent of OSA, as neurocognitive deficits are often associated with OSA and appear to improve with CPAP therapy.38 The narrow range of scores for the individual questions provide limited room for improvement and so may be subject to floor effects.
While 41 of 228 patients who complied with CPAP therapy had positive responses to question 9 (“do you think you would be better dead or of self-harm?”) before treatment, none did at 3 month follow-up. However, approximately 5% of those treated with CPAP had persistent clinically significant depressive symptoms despite therapy. Persistent depression independent of OSA treatment in some individuals is to be expected given its widespread occurrence in the general community. Indeed the proportion observed in our CPAP-treated patients was less than population prevalences of depressive symptoms.39 Apart from elimination of OSA (the presence of which may influence such population prevalences) other potential influences causing this low prevalence of persisting depressive symptoms include the nonspecific effects of the care and attention that accompany the diagnostic and treatment process and a selection bias for patients with a positive outlook among those able and willing to comply with CPAP therapy. The changes were not based on changes in antidepressant prescription, which remained constant throughout the study. Of the complete patient group 52% were using antidepressants regularly prior to attending the sleep clinic. Of those who successfully undertook CPAP therapy, 51.8% were using antidepressants prior to commencement and this remained unchanged after 3 months of therapy.
Limitations
This study involved sleep clinic patients rather than patients drawn from the general population. While this may introduce limitations in generalizing findings to the community at large, it ensured that the study population was enriched with sleep apnea cases. As a result, a wide spectrum of sleep apnea severities was present among the subjects of the study, allowing concentrated study of the influence of sleep apnea on depressive symptomatology.
By choosing an OSA-rich sample, this study demonstrated that CPAP therapy ameliorated reports of depressive symptoms in patients with OSA, such that only 9 patients had persistently high scores after 3 months of treatment. However, this design limited examination of differences in symptom report between those where OSA treatment did and did not affect depressive symptomatology. Our results indicated that there were no differences between these groups with respect to report of depressive symptoms before treatment, severity of OSA, adiposity, or age, which would suggest that these factors played a minimal role in those with persistent depression after treatment for OSA.
The study used one instrument to assess depressive symptomatology. However the PHQ-9 has been widely validated and is capable of question-by-question breakdown of responses, as was performed here, to examine DSM-IV depressive symptoms criterion-by-criterion, as each of the nine questions is directly based one of the 9 DSM-IV depression criteria. Significant changes were observed with OSA treatment across a broad spectrum of these questions suggesting that the findings were not attributable to any one or two sleep-oriented depressive symptoms. Furthermore previous experience with tools for detecting depression suggests that scores are highly correlated and therefore that no one instrument is more effective than another.40,41 The strength of the relationship between OSA treatment and resolution of depressive symptomology demonstrated in the present study was of sufficient degree to suggest that the PHQ-9 and other depression scales may be inadequate for differentiating a priori between OSA related depressive symptoms and depression independent of OSA.
An additional limitation was the observational design of the study. However, a randomized controlled design would present significant difficulties for a study involving all severities of sleep apnea, large numbers of patients, and follow-up of those started on treatment over a lengthy (3-month) period. This length of follow-up is desirable, as the first 3 months of therapy is the period over which its use stabilizes and long-term compliance becomes predictable.42 Withholding effective therapy for this period in patients with severe OSA presents ethical dilemmas.35,36 Nonetheless the present study would have been enhanced by systematic follow-up of those who did not comply with CPAP at 3 months, to facilitate partitioning of CPAP treatment effects from other influences on symptom-atology. Notwithstanding these issues, the dose-effect relationship between AHI and depressive symptoms at baseline and the remission of these symptoms with OSA treatment strongly suggests OSA was responsible for them.
CONCLUSIONS
We have shown that, in this sleep clinic population, a high percentage of patients diagnosed with moderate to severe OSA also have symptoms of moderate to severe depression, and that compliance with CPAP treatment results in significant improvement in these symptoms. In the absence of OSA, clinically significant depressive symptoms were more common in women than men, but not when OSA is present. Given the widespread prevalence of OSA, the findings of this study suggest that where depressive symptoms are elicited OSA should be considered in their differential diagnosis. Where OSA is present, effective treatment of it appears likely to result in improvement of these symptoms.
DISCLOSURE STATEMENT
This was not an industry supported study. The study was supported in part by grant 1031575 from the National Health and Medical Research Council (NHMRC) of Australia. No other external funding was involved. Dr. Hillman was supported by NHMRC grant 1031575. Dr. Hillman has received research support from ResMed and has participated in speaking engagements for ResMed and Philips Healthcare. The other authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, Forth Edition
- OSA
obstructive sleep apnea
- PHQ-9
patient health questionnaire
- PSG
polysomnography
- SD
standard deviation
- %TST < 90
% total sleep time spent at an arterial oxygen saturation <90%
REFERENCES
- 1.Kamphuis J, Meerlo P, Koolhaas JM, Lancel M. Poor sleep as a potential causal factor in aggression and violence. Sleep Med. 2012;13:327–34. doi: 10.1016/j.sleep.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Ong JC, Gress JL, San Pedro-Salcedo MG, Manber R. Frequency and predictors of obstructive sleep apnea among individuals with major depressive disorder and insomnia. J Psychosom Res. 2009;67:135–41. doi: 10.1016/j.jpsychores.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosko S, Zetin M, Glen S, et al. Self-reported depressive symptomatology, mood ratings, and treatment outcome in sleep disorders patients. J Clin Psychol. 1989;45:51–60. doi: 10.1002/1097-4679(198901)45:1<51::aid-jclp2270450107>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Schroder CM, O'Hara R. Depression and obstructive sleep apnea (OSA) Ann Gen Psychiatry. 2005;4:13. doi: 10.1186/1744-859X-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas N, Young A, Roebuck T, et al. Prevalence of depression in patients referred with snoring and obstructive sleep apnoea. Intern Med J. 2013;43:630–4. doi: 10.1111/imj.12108. [DOI] [PubMed] [Google Scholar]
- 6.Harris M, Glozier N, Ratnavadivel R, Grunstein RR. Obstructive sleep apnea and depression. Sleep Med Rev. 2009;13:437–44. doi: 10.1016/j.smrv.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Vandeputte M, de Weerd A. Sleep disorders and depressive feelings: a global survey with the Beck depression scale. Sleep Med. 2003;4:343–5. doi: 10.1016/s1389-9457(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 8.Diamanti C, Manali E, Ginieri-Coccossis M, et al. Depression, physical activity, energy consumption, and quality of life in OSA patients before and after CPAP treatment. Sleep Breath. 2013;17:1159–68. doi: 10.1007/s11325-013-0815-6. [DOI] [PubMed] [Google Scholar]
- 9.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34:111–9. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson L, Hillman DR, Cooper MN, et al. High prevalence of undiagnosed obstructive sleep apnoea in the general population and methods for screening for representative controls. Sleep Breath. 2013;17:967–73. doi: 10.1007/s11325-012-0785-0. [DOI] [PubMed] [Google Scholar]
- 11.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: implications for future interventions. Ind J Med Res. 2010;131:245–58. [PMC free article] [PubMed] [Google Scholar]
- 13.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509–15. [Google Scholar]
- 14.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 15.McManus P, Mant A, Mitchell P, Britt H, Dudley J. Use of antidepressants by general practitioners and psychiatrists in Australia. Aust N Z J Psychiatry. 2003;37:184–9. doi: 10.1046/j.1440-1614.2003.01132.x. [DOI] [PubMed] [Google Scholar]
- 16.El-Ad B, Lavie P. Effect of sleep apnea on cognition and mood. Int Rev Psychiatry. 2005;17:277–82. doi: 10.1080/09540260500104508. [DOI] [PubMed] [Google Scholar]
- 17.Stephenson CP, Karanges E, McGregor IS. Trends in the utilisation of psychotropic medications in Australia from 2000 to 2011. Aust N Z J Psychiatry. 2013;47:74–87. doi: 10.1177/0004867412466595. [DOI] [PubMed] [Google Scholar]
- 18.Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67:1265–73. doi: 10.1001/archgenpsychiatry.2010.151. [DOI] [PubMed] [Google Scholar]
- 19.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 20.Ejaz SM, Khawaja IS, Bhatia S, Hurwitz TD. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8:17–25. [PMC free article] [PubMed] [Google Scholar]
- 21.Huang FY, Chung H, Kroenke K, Delucchi KL, Spitzer RL. Using the Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. J Gen Int Med. 2006;21:547–52. doi: 10.1111/j.1525-1497.2006.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spitzer RL, Williams JB, Kroenke K, Hornyak R, McMurray J. Validity and utility of the PRIME-MD patient health questionnaire in assessment of 3000 obstetric-gynecologic patients: the PRIME-MD Patient Health Questionnaire Obstetrics-Gynecology Study. Am J Obstet Gynecol. 2000;183:759–69. doi: 10.1067/mob.2000.106580. [DOI] [PubMed] [Google Scholar]
- 23.DeZee KJ, Hatzigeorgiou C, Kristo D, Jackson JL. Prevalence of and screening for mental disorders in a sleep clinic. J Clin Sleep Med. 2005;1:136–42. [PubMed] [Google Scholar]
- 24.Froese CL, Butt A, Mulgrew A, et al. Depression and sleep-related symptoms in an adult, indigenous, North American population. J Clin Sleep Med. 2008;4:356–61. [PMC free article] [PubMed] [Google Scholar]
- 25.Hayley AC, Williams LJ, Venugopal K, Kennedy GA, Berk M, Pasco JA. The relationships between insomnia, sleep apnoea and depression: findings from the American National Health and Nutrition Examination Survey, 2005-2008. Aust N Z J Psychiatry. 2015;49:156–70. doi: 10.1177/0004867414546700. [DOI] [PubMed] [Google Scholar]
- 26.MacGregor KL, Funderburk JS, Pigeon W, Maisto SA. Evaluation of the PHQ-9 Item 3 as a screen for sleep disturbance in primary care. J Gen Intern Med. 2012;27:339–44. doi: 10.1007/s11606-011-1884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCall WV, Kimball J, Boggs N, Lasater B, D'Agostino RB, Jr., Rosenquist PB. Prevalence and prediction of primary sleep disorders in a clinical trial of depressed patients with insomnia. J Clin Sleep Med. 2009;5:454–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Mulgrew AT, Ryan CF, Fleetham JA, et al. The impact of obstructive sleep apnea and daytime sleepiness on work limitation. Sleep Med. 2007;9:42–53. doi: 10.1016/j.sleep.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Su CS, Liu KT, Panjapornpon K, Andrews N, Foldvary-Schaefer N. Functional outcomes in patients with REM-related obstructive sleep apnea treated with positive airway pressure therapy. J Clin Sleep Med. 2012;8:243–47. doi: 10.5664/jcsm.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wheaton AG, Perry GS, Chapman DP, Croft JB. Sleep disordered breathing and depression among U.S. adults: National Health and Nutrition Examination Survey, 2005-2008. Sleep. 2012;35:461–7. doi: 10.5665/sleep.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American Psychiatric Association. 4th edition. Washington DC: American Psychiatric Association; 2000. Diagnostic and statistical manual of mental disorders. text revision. [Google Scholar]
- 32.Weissman MM, Olfson M. Depression in women: implications for health care research. Science. 1995;269:799–801. doi: 10.1126/science.7638596. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5:185–92. doi: 10.1513/pats.200708-137MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Povitz M, Bolo CE, Heitman SJ, Tsai WH, Wang J, James MT. Effect of treatment of obstructive sleep apnea on depressive symptoms: systematic review and meta-analysis. PLoS Med. 2014;11:e1001762. doi: 10.1371/journal.pmed.1001762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown DL, Anderson CS, Chervin RD, et al. Ethical issues in the conduct of clinical trials in obstructive sleep apnea. J Clin Sleep Med. 2011;7:103–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Bonsignore M, Battaglia S, Zito A, Lombardi C, Parati G. Sleep apnoea and systemic hypertension. Eur Respir Mon. 2010;50:150–73. [Google Scholar]
- 37.Lowe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care. 2004;42:1194–201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology. 2013;18:61–70. doi: 10.1111/j.1440-1843.2012.02255.x. [DOI] [PubMed] [Google Scholar]
- 39.Shim RS, Baltrus P, Ye J, Rust G. Prevalence, treatment, and control of depressive symptoms in the United States: results from the National Health and Nutrition Examination Survey (NHANES), 2005-2008. J Am Board Fam Med. 2011;24:33–38. doi: 10.3122/jabfm.2011.01.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulrow CD, Williams JW, Jr., Gerety MB, Ramirez G, Montiel OM, Kerber C. Case-finding instruments for depression in primary care settings. Ann Int Med. 1995;122:913–921. doi: 10.7326/0003-4819-122-12-199506150-00004. [DOI] [PubMed] [Google Scholar]
- 41.Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression. Two questions are as good as many. J Gen Intern Med. 1997;12:439–45. doi: 10.1046/j.1525-1497.1997.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–14. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]


