Abstract
Introduction:
The association between body fat composition as measured by dual energy x-ray absorptiometry (DEXA) scanning and pediatric sleep related breathing disorder (SRBD) is not well established. We investigated the relationship between body mass index (BMI) and DEXA parameters and their association with SRBD in obese children.
Patients and Methods:
Overnight polysomnography was performed on obese/overweight children (10–17 years) with habitual snoring. Total body fat mass (g), trunk fat mass (g), total body % fat, and trunk % fat were determined by DEXA.
Results:
Forty-one subjects were studied. Logarithm (Log) total arousal index correlated with BMI (p < 0.01, r = 0.473), total body fat mass (p < 0.05, r = 0.331), and trunk fat mass (p < 0.05, r = 0.319). Log desaturation index correlated with BMI (p < 0.05, r = 0.313), total body fat mass (p < 0.05, r = 0.375), and trunk fat mass (p < 0.05, r = 0.391), whereas obstructive apnea hypopnea index (OAHI) did not. In males 10–12 years, there was a significant correlation between Log total arousal index and obesity parameters, but not for males aged 13–17 years. BMI correlated with DEXA parameters in all subjects: total body fat mass (p < 0.001, r = 0.850); total body % fat (p < 0.01, r = 0.425); trunk fat mass (p < 0.001, r = 0.792) and trunk % fat (p < 0.05, r = 0.318) and in 10–12 year old males. This relationship was not significant in males aged 13–17 years.
Conclusions:
Total body fat mass and trunk fat mass as well as BMI correlated with total arousal index and desaturation index. BMI correlated with DEXA parameters in 10–12 year old males but not in 13–17 year old males. The value of using DEXA scanning to study the relationship between obesity and SRBD may depend on age and pubertal stage.
Citation:
Bhatia R, Lesser DJ, Oliveira FG, Tran WH, Keens TG, Khoo MC, Davidson Ward SL. Body fat composition: a predictive factor for sleep related breathing disorder in obese children. J Clin Sleep Med 2015;11(9):1039–1045.
Keywords: pediatric obesity, body mass index, body fat composition, DEXA scan, sleep related breathing disorder, pediatric obstructive sleep apnea
Sleep related breathing disorder (SRBD) is common in over-weight and obese children.1,2 In the pediatric age group, it has been associated with significant metabolic and autonomic dysfunction.3–5 In children obesity has been shown to be an independent risk factor for snoring and obstructive sleep apnea (OSA).6,7 The literature also provides evidence regarding the predictors of SRBD in obese children.8
In adults, studies have shown a strong correlation between central adiposity and OSA.9 Regional fat distribution, as represented by waist circumference, waist to hip ratio, or percent fat mass correlates with SRBD in obese children.1 Carotenuto et al. suggested that waist circumference is a better predictor of SRBD than body mass index (BMI) in obese children.10 Thus, distribution of body fat mass may be more important in predicting SRBD than BMI alone.
Dual energy x-ray absorptiometry-measured mass (DEXA) allows accurate quantification of fat and its distribution compared to BMI. None of the above pediatric studies, however, employed DEXA analysis to assess regional fat distribution. Simpson et al. reported that the obstructive apnea-hypopnea index (OAHI) was best predicted by a combination of DEXA and traditional anthropometric measurements in adults.11 In this study, we investigated the relationship between BMI and DEXA parameters as well their relationship with SRBD in obese/overweight children.
BRIEF SUMMARY
Current Knowledge/Study Rationale: In adults, studies have shown a strong correlation between body fat composition (utilizing DEXA scans) and OSA. Similar studies utilizing DEXA scans to assess body fat composition as a predictor of pediatric SRBD are lacking.
Study Impact: This study showed that total body fat mass and trunk fat mass, as measured on DEXA scanning, as well as BMI, were associated with total arousal index and desaturation index in obese children. BMI also correlated with DEXA parameters in these children. However, both relationships were influenced by age.
METHODS
Subjects
Forty-one obese/overweight (32 males, 38 obese and 3 overweight) otherwise healthy subjects (10–17 years old) with habitual snoring were recruited from patients referred to the Children's Hospital Los Angeles Sleep Laboratory for symptoms or signs of SRBD. Habitual snoring was defined ≥ 3 nights of snoring per week as per parent report. Exclusion criteria included a history of cardiac disease, lung disease other than mild intermittent asthma, type 2 diabetes mellitus, systemic hypertension requiring the use of antihypertensive medications, or use of stimulant medications for attention deficit hyperactivity disorder. In addition, subjects using positive airway pressure therapy or supplemental oxygen were excluded. To adjust for age and gender we further classified males into 2 groups: 10–12 years and 13–17 years of age. The number of female subjects was insufficient for an age group analysis. Age was considered a surrogate for puberty. The Children's Hospital Los Angeles institutional review board approved the study, and written informed consent/assent was obtained from all subjects and their parents as appropriate.
Obesity Parameters
Each subject's weight was measured to the nearest 0.1 kg with a digital scale. Height was measured to the nearest centimeter with a wall-mounted stadiometer. BMI was calculated as weight/height2 (kg/m2), compared with standard reference values and expressed as a percentile for age/gender.12 Overweight was defined as a BMI ≥ 85th percentile but < 95th percentile for children of the same age and sex. Obesity was defined as a BMI ≥ 95th percentile for children of the same age and sex. Total body fat mass (g), trunk fat mass (g; abdominal and thoracic fat combined), total body % fat, and trunk % fat were determined from DEXA scanning using a Hologic QDR-4500 Delphi W scanner (Hologic Inc., Bedford, MA).
Polysomnography
Overnight polysomnography was performed on all subjects within 6 months of the DEXA scanning. Surface electrodes were connected to subjects to monitor electrocardiogram, chin electromyogram, electroencephalogram, and electro-oculogram. Respiratory inductance plethysmography of the chest and abdomen and pulse oximetry were recorded. Expired carbon dioxide tension was measured continuously with a sampling catheter placed on the nose and mouth. A nasal-oral thermistor was used to evaluate combined airflow. Studies were scored by 2 investigators arriving at consensus. Both investigators were blinded to the measures of adiposity. Raw data were scored according to criteria established by the American Academy of Sleep Medicine.13,14 OAHI was calculated based on the combined number of obstructive apneas, mixed apneas, and hypopneas per hour of sleep. The total arousal index (spontaneous, movement, or respiratory), a measure of sleep fragmentation, was calculated based on the total number of arousals per hour of sleep. Awakenings were also included in the arousal index. The desaturation index was calculated based on the total number of episodes of oxygen desaturation (≥ 3% drop in peripheral capillary oxy-hemoglobin saturation as measured by pulse oximetry; SpO2 from baseline) per hour of sleep. The lowest value of SpO2 (SpO2 nadir) was also recorded. Any drop in SpO2 caused by artifact was eliminated based on loss of a robust pulse wave form.
Statistical Analysis
All polysomnographic and obesity parameters were first checked for normality. The parameters that were not normally distributed were logarithm (Log) transformed to normalize the distribution. The variables were summarized as mean plus or minus the standard deviation (SD) with the range disclosed. Each polysomnographic index was tested for correlation (Pearson product moment correlation coefficient) with every obesity parameter. Obesity parameters, DEXA and BMI were also correlated with each other. Initially, the analysis was calculated for all subjects. Further correlations were done in males, females, and subgroups of males based on age. A p value < 0.05 was considered to be statistically significant.
RESULTS
Forty-one subjects (32 males; age = 12.8 ± 2.0 years (mean ± SD) (age range = 10.2–17.8 years) participated. Sleep and obesity parameters are listed in Tables 1 and 2. All participants were obese with the exception of three overweight subjects. The mean BMI was 33.3 ± 7.3 kg/m2 (range 23.0–52.9). Table 1 also shows considerable variability in the DEXA parameters across subjects.
Table 1.
Obesity parameters for all the subjects (n = 41).
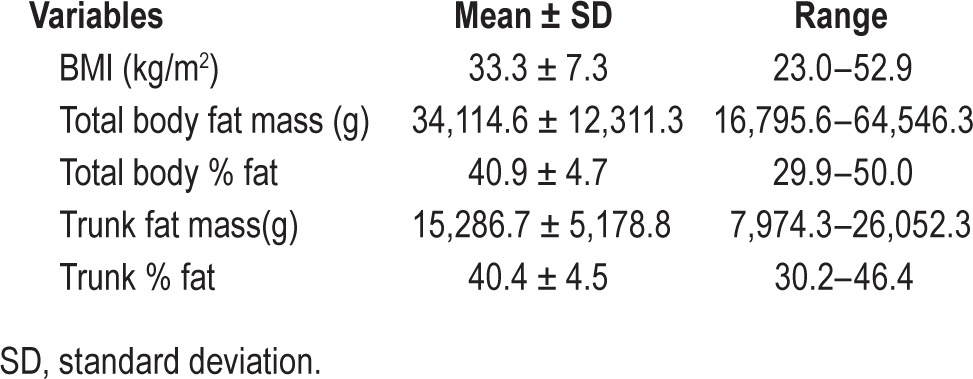
Table 2.
Polysomnography variables for all subjects (n = 41).
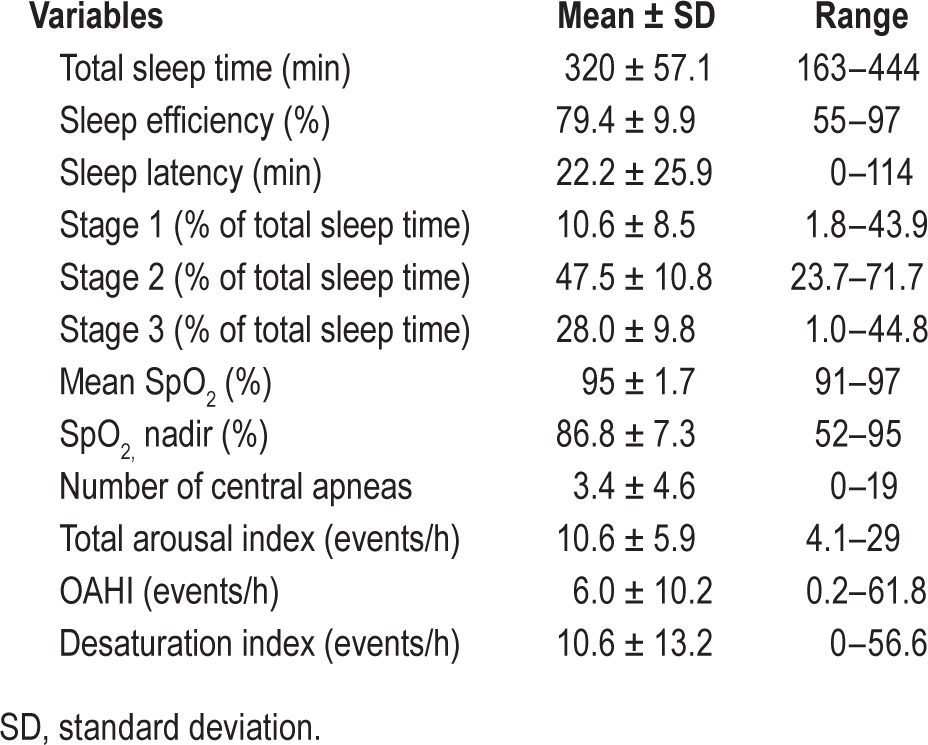
Polysomnography results (Table 2) revealed a mean OAHI of 6.0 ± 10.2 events/h with the range of 0.2–61.8 underscoring a wide variability in severity across the subjects. The mean desaturation index was 10.6 ± 13.2 events/h, ranging from 0 to 56.6. The mean total arousal index was 10.6 ± 5.9 events/h, ranging from 4.1 to 29.
Sleep Parameters vs. Obesity Parameters
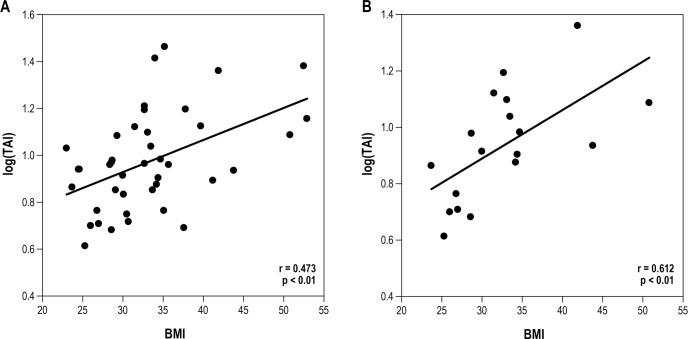
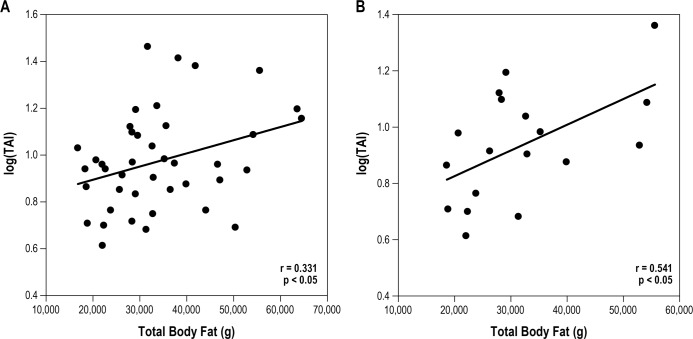
Pearson correlation of the entire group revealed a signifi-cant correlation between Log total arousal index and the obesity parameters as measured by BMI, total body fat mass, and trunk fat mass (Table 3, Figures 1A and 2A). Log desaturation index also correlated with BMI, total body fat mass, and trunk fat mass, whereas Log OAHI did not show a significant correlation. When separated by age and gender, we found that in 10- to 12-year-old males, the correlation between Log total arousal index and obesity parameters as observed for all subjects remained (Table 3, Figures 1B and 2B). However, there was no significant relationship found in younger males when Log desaturation index was correlated with obesity parameters. Log OAHI did correlate with BMI in 10–12 year old males. In contrast to these younger males, there was no significant relationship found between Log total arousal index and obesity parameters in the older males (13–17 years). In addition, Log OAHI correlated with trunk % fat. Our study failed to identify a significant relationship between sleep measurements and obesity parameters in 9 female participants.
Table 3.
Relationship (correlation coefficient, r) between sleep and obesity parameters in all subjects, males and additional subsets of younger and older males.
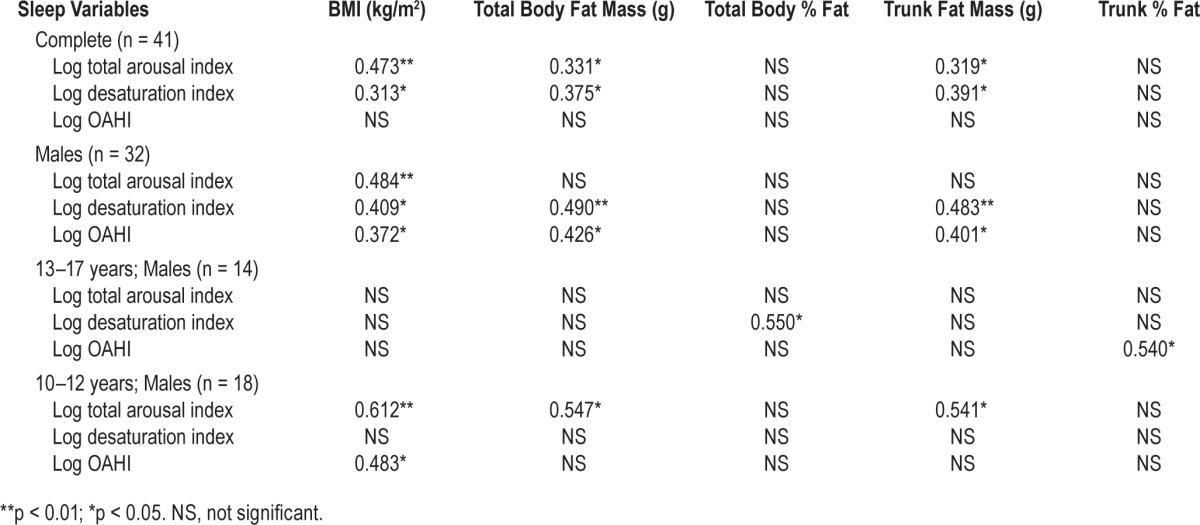
Figure 1. Correlation (correlation coefficient, r) between sleep fragmentation (Log total arousal index; TAI) and BMI (kg/m2).
All subjects (A), 10- to 12-year-old males (B). Subjects are represented as filled circles.
Figure 2. Correlation (correlation coefficient, r) between sleep fragmentation (Log total arousal index; TAI) and total body fat mass (g).
All subjects (A), 10–12 year old males (B). Subjects are represented as filled circles.
BMI vs. DEXA
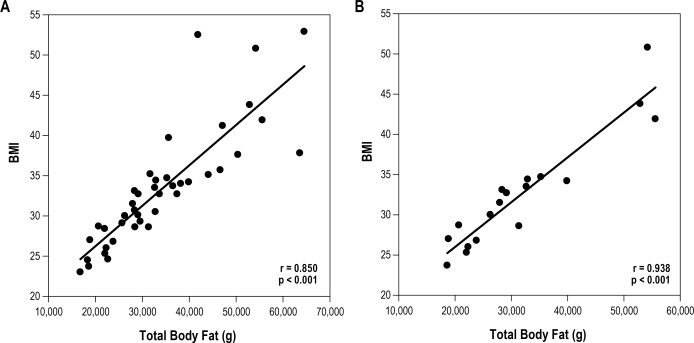
As expected, the study sample demonstrated a significant correlation between BMI and DEXA parameters (Table 4, Figure 3A). However, this correlation varied across age and gender. In 10- to 12-year-old males, the association between BMI and DEXA was significant (Table 4, Figure 3B). This statistically significant relationship was absent in older males aged 13–17 years. Females did demonstrate a significant relationship between BMI and total body fat mass and trunk fat mass despite low numbers (n = 9).
Table 4.
Relationship (correlation coefficient, r) between BMI and DEXA parameters in all subjects and in subsets.
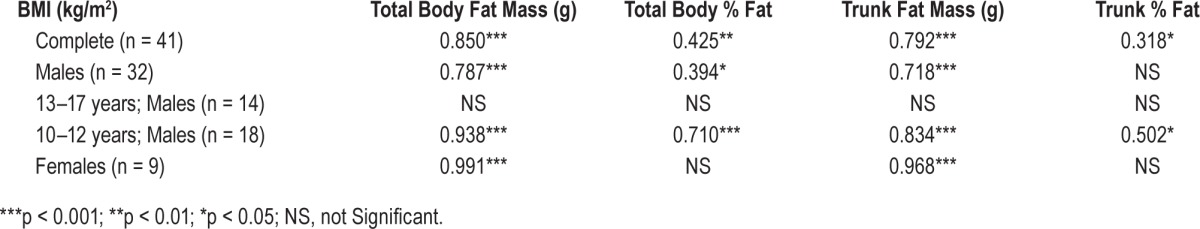
Figure 3. Correlation (correlation coefficient, r) between BMI (kg/m2) and total body fat mass (g).
All subjects (A), 10- to 12-year-old males (B). Subjects are represented as filled circles.
DISCUSSION
SRBD imparts significant metabolic and autonomic implications for obese children.4,5,15,16 This study investigated whether DEXA scans could be used to show that a sensitive measure of fat mass and distribution of fat correlates with SRBD in obese/overweight pediatric patients. We found that total body fat mass and trunk fat mass as measured on DEXA scanning, as well as BMI, were associated with total arousal index and desaturation index. This relationship varied when the cohort was split according to age. BMI correlated with DEXA parameters in younger (10–12 years) but not in the older males (13–17 years).
Sleep Parameters vs. Obesity Parameters
The significant correlation between Log total arousal index and total body fat mass or trunk fat mass suggests increased sleep fragmentation occurs with increasing levels of total body fat mass and distribution of fat. Interestingly, subgroup analysis showed that in younger males this was consistent but absent in older males. It may be that hormonal factors associated with puberty, such as an increase in muscle mass and transition to a more adult pattern of central obesity, affects the pathophysiology of SRBD in adolescents. Sleep fragmentation has been shown to be associated with autonomic and metabolic dysfunction in obese children.17 We previously found that sleep fragmentation was associated with impaired glucose tolerance in obese adolescent Latino males.4
Desaturation index also showed a significant correlation with total body fat mass and trunk fat mass. The degree of trunk fat likely affects respiratory mechanics leading to oxy-hemoglobin desaturation. Thoracic fat affects chest wall mechanics by mass loading with reduction in lung compliance. In addition, abdominal fat decreases the functional residual capacity (a measure of pulmonary reserve) by resisting the downward movement of the diaphragm and decreasing lung volumes.18,19 These reductions in functional residual capacity and lung compliance cause reduction in oxygen reserves and ventilation/perfusion mismatching, predisposing to episodic desaturation during sleep. The correlation between Log desaturation index and total body fat mass was absent when younger and older males were analyzed separately. While this could be related to low numbers in subgroup analysis, there may be factors other than age and puberty that play a role (e.g., poor lung reserve) in affecting this relationship.
We did not identify a significant correlation between obesity parameters and the severity of OSA when all subjects were analyzed together. The pediatric literature has shown mixed results with regard to the relationship between OSA and the severity of obesity.1,20–22 Redline et al. showed significant correlations between OAHI and BMI, although Verhurst et al. did not in a study of 91 overweight/obese subjects.1,20 In a study of 197 children age 2–18 years, Kang et al. found significantly increased OAHI in obese as well as underweight subjects compared to a normal weight group.22
Combined with our findings, these studies highlight that factors other than obesity such as puberty, genetics, respiratory control, and upper airway anatomy significantly contribute to the presence and severity of OSA in this population. For instance, Bruno et al. found a significant relationship between fat mass percentage in the neck region (measured by DEXA) and severity of OSA but no correlation between percent of abdominal fat mass and OSA.23 When upper airway structure and body fat composition were examined with magnetic resonance imaging in obese children, Arens et al. found that the size of upper airway lymphoid tissues correlated with severity of OSA, whereas abdominal visceral fat did not correlate with severity of OSA.24 Magnetic resonance imaging used to compare fat distribution in men and women also suggested a role for the pattern of fat deposition adjacent to the upper airway for the development of OSA rather than obesity per se.25 Thus, OAHI is significantly impacted by upper airway factors such as regional neck fat distribution as opposed to the overall degree of adiposity. Although our study did not identify a significant correlation between obesity parameters and OSA in the entire group, relevant findings emerged in the subgroup analysis. We identified a correlation between Log OAHI and BMI when all males, and 10- to 12-year-old males, were considered. This relationship was absent in older males (Table 3). These data underscore that BMI alone does not fully measure the degree of obesity in older adolescent males. In younger males, the severity of sleep apnea (Log OAHI) correlated with BMI while in older males the severity of sleep apnea correlated with trunk % fat but not BMI, suggesting that distribution of fat may become more relevant in post-pubertal males. We did not identify a significant relationship between sleep measurements and obesity parameters in nine female participants. This is likely due to the low numbers of female participants and should be interpreted with caution.
BMI vs. DEXA
The present study suggests that the relationship between BMI and DEXA parameters in males varies by age. In younger males BMI correlated with the DEXA parameters, suggesting BMI can be used as a proxy for body fat in younger children. In older subjects, however, a significant correlation between BMI and DEXA parameters was not demonstrated. As muscle mass increases in post-pubertal subjects, the correlation between BMI and fat diminishes. Although several investigators have examined the reliability of BMI to identify children with obesity, the associations vary considerably.26,27 BMI cannot differentiate between body fat, muscle mass, and skeletal mass.28 The literature shows that increases in BMI levels of boys during adolescence seem to result primarily from increases in fat-free mass rather than body fat.29 For instance, Demerath et al. concluded that BMI percentile changes may not accurately reflect changes in adiposity in children over time, particularly among male adolescents.29 Our findings are in agreement with the previous study conclusions that BMI may not be a reliable indicator of body fat in post-pubertal males.
Limitations of this study and their implications merit discussion. The first limitation of our study comprises an absence of a control group with similar BMI, age, and gender without history of snoring. Such a comparison would have been interesting and clinically relevant. However, our study design differed slightly from a more traditional method of selecting a pool of normal (no SRBD) subjects and comparing experimental measures to a group of subjects with SRBD. We aimed to describe how the severity of obesity varies as a function of SRBD severity. Thus, the study was not designed to compare patients with and without SRBD as defined by the presence of snoring. We were, however, able to study subjects with a broad spectrum of OAHI severity ranging from normal to more than 60 events per hour.
A lack of neck fat distribution measurements, which likely play a role in the pathophysiology of SRBD, represents another limiting factor. An additional limitation of our study is the absence of Tanner staging, which would have facilitated a more precise measure of puberty. In place of Tanner staging, we performed subgroup analyses by separating the cohort by sex and age (Table 3). We found that the results varied by age and speculate that pubertal stage may be contributing to this effect. Lastly, the limited number of subjects in the subgroups may explain the absence of correlation with SRBD and obesity parameters for some of the analyses.
In summary, DEXA scanning has value in the study of obesity and SRBD. We found that the relationship between obesity parameters and SRBD depends significantly on age and fat distribution in males implying that BMI may not be an accurate measure of adiposity after puberty. In younger males BMI correlates well with DEXA parameters, suggesting further studies are needed to assess the clinical utility of DEXA across varied age ranges in identifying fat distribution and its relevance in predicting SRBD. Reliable methods to predict SRBD in obese children continue to be sought. DEXA scanning and accurate calculation of fat composition can play an important role in the study of obesity and SRBD in pediatric patients.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported in part by National Institutes of Health (NIH) grants HL090451 and EB001978; University of Southern California Center for Transdisciplinary Research on Energetics and Cancer (TREC U54 CA 116848); and grant M01 RR00047 from Children's Hospital Los Angeles General Clinical Research Center, with funds provided by the National Center for Research Resources, NIH. The authors have indicated no financial conflicts of interest. The work for this study was performed at the Children's Hospital Los Angeles, Keck School of Medicine, University of Southern California, Los Angeles, CA. The preliminary results from this study were presented as a poster at SLEEP Conference, 2010.
ACKNOWLEDGMENTS
The authors thank the Children's Hospital Los Angeles General Clinical Research Center for provision of staff and funding for this project. They also acknowledge the subjects and their families for participating in this project.
Dr. Bhatia conceptualized and designed the study, interpreted the sleep studies, collected and analyzed the data, wrote and revised the manuscript, and approved the final manuscript as submitted. Dr. Lesser interpreted the sleep studies, collected and analyzed the data, reviewed the manuscript, and approved the final manuscript as submitted. Dr. Oliveira and Mr. Tran collected and analyzed the data, reviewed the manuscript, and approved the final manuscript as submitted. Dr. Keens and Dr. Khoo conceptualized and designed the study, analyzed the data, reviewed the manuscript, and approved the final manuscript as submitted. Dr. Davidson Ward conceptualized and designed the study, supervised the entire project, interpreted the sleep studies, analyzed the data, critically reviewed the manuscript, and approved the final manuscript as submitted.
ABBREVIATIONS
- BMI
body mass index
- DEXA
dual energy x-ray absorptiometry
- Log
logarithm
- OAHI
obstructive apnea hypopnea index
- OSA
obstructive sleep apnea
- SRBD
sleep related breathing disorder
- SpO2
peripheral capillary oxyhemoglobin saturation (as measured by pulse oximetry)
REFERENCES
- 1.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Arch Dis Child. 2007;92:205–8. doi: 10.1136/adc.2006.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi B, McGuire E, Loloyan S, et al. Characterizing Sleep phenotypes in overweight adolescents who snore. Sleep. 2014;37:A334. (Abstract Suppl) [Google Scholar]
- 3.Kheirandish-Gozal L, Bhattacharjee R, Gozal D. Autonomic alterations and endothelial dysfunction in pediatric obstructive sleep apnea. Sleep Med. 2010;11:714–20. doi: 10.1016/j.sleep.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Lesser DJ, Bhatia R, Tran WH, et al. Sleep fragmentation and intermittent hypoxemia are associated with decreased insulin sensitivity in obese adolescent Latino males. Pediatr Res. 2012;72:293–8. doi: 10.1038/pr.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliveira FM, Tran WH, Lesser D, et al. Autonomic and metabolic effects of OSA in childhood obesity. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:6134–7. doi: 10.1109/IEMBS.2010.5627789. [DOI] [PubMed] [Google Scholar]
- 6.Li AM, So HK, Au CT, et al. Epidemiology of obstructive sleep apnoea syndrome in Chinese children: a two-phase community study. Thorax. 2010;65:991–7. doi: 10.1136/thx.2010.134858. [DOI] [PubMed] [Google Scholar]
- 7.Shin C, Joo S, Kim J, Kim T. Prevalence and correlates of habitual snoring in high school students. Chest. 2003;124:1709–15. doi: 10.1378/chest.124.5.1709. [DOI] [PubMed] [Google Scholar]
- 8.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32:731–6. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schäfer H, Pauleit D, Sudhop T, Gouni-Berthold I, Ewig S, Berthold HK. Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 2002;122:829–39. doi: 10.1378/chest.122.3.829. [DOI] [PubMed] [Google Scholar]
- 10.Carotenuto M, Bruni O, Santoro N, Del Giudice EM, Perrone L, Pascotto A. Waist circumference predicts the occurrence of sleep-disordered breathing in obese children and adolescents: a questionnaire-based study. Sleep Med. 2006;7:357–61. doi: 10.1016/j.sleep.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Simpson L, Mukherjee S, Cooper MN, et al. Sex differences in the association of regional fat distribution with the severity of obstructive sleep apnea. Sleep. 2010;33:467–74. doi: 10.1093/sleep/33.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 13.Grigg-Damberger M, Gozal D, Marcus CL, et al. The visual scoring of sleep and arousal in infants and children. J Clin Sleep Med. 2007;3:201–40. [PubMed] [Google Scholar]
- 14.Iber C, Ancoli-Israel S, Chesson AL, Jr., Quan SF for the American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st ed. [Google Scholar]
- 15.Oliveira FS, Tran WH, Bhatia R, et al. Severity of obstructive sleep apnea predicts autonomic and metabolic dysfunction in obese Hispanic boys. Sleep. 2011;34:A295. (Abstract Suppl) [Google Scholar]
- 16.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing and the metabolic syndrome in overweight and obese children and adolescents. J Pediatr. 2007;150:608–12. doi: 10.1016/j.jpeds.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira FMS, Tran WH, Bhatia R, et al. Relationships among autonomic dysfunction, obstructive sleep apnea, and insulin resistance in childhood obesity. Auton Neurosci. 2011;163:81. [Google Scholar]
- 18.Inselma LS, Milanese A, Deurloo A. Effect of obesity on pulmonary function in children. Pediatr Pulmonol. 1993;16:130–7. doi: 10.1002/ppul.1950160209. [DOI] [PubMed] [Google Scholar]
- 19.Li AM, Chan D, Wong E, Yin J, Nelson EA, Fok TF. The effects of obesity on pulmonary function. Arch Dis Child. 2003;88:361–3. doi: 10.1136/adc.88.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell RB, Garetz S, Moore RH, et al. The use of clinical parameters to predict obstructive sleep apnea syndrome severity in children: the Childhood Adenotonsillectomy (CHAT) Study Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg. 2015;141:130–6. doi: 10.1001/jamaoto.2014.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang KT, Lee PL, Weng WC, Hsu WC. Body weight status and obstructive sleep apnea in children. Int J Obes (Lond) 2012;36:920–4. doi: 10.1038/ijo.2012.5. [DOI] [PubMed] [Google Scholar]
- 23.Bruno E, Alessandrini M, Napolitano B, De Padova A, Di Daniele N, De Lorenzo A. Dual-energy X-ray absorptiometry analysis of body composition in patients affected by OSAS. Eur Arch Otorhinolaryngol. 2009;266:1285–90. doi: 10.1007/s00405-008-0844-0. [DOI] [PubMed] [Google Scholar]
- 24.Arens R, Sin S, Nandalike K, et al. Upper airway structure and body fat composition in obese children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2011;183:782–7. doi: 10.1164/rccm.201008-1249OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whittle AT, Marshall I, Mortimore IL, Wraith PK, Sellar RJ, Douglas NJ. Neck soft tissue and fat distribution: comparison between normal men and women by magnetic resonance imaging. Thorax. 1999;54:323–8. doi: 10.1136/thx.54.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics. 1997;99:804–7. doi: 10.1542/peds.99.6.804. [DOI] [PubMed] [Google Scholar]
- 27.Schaefer F, Georgi M, Wühl E, Schärer K. Body mass index and percentage fat mass in healthy German schoolchildren and adolescents. Int J Obes Relat Metab Disord. 1998;22:461–9. doi: 10.1038/sj.ijo.0800608. [DOI] [PubMed] [Google Scholar]
- 28.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–7. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 29.Demerath EW, Schubert CM, Maynard LM, et al. Do changes in body mass index percentile reflect changes in body composition in children? Data from the Fels Longitudinal Study. Pediatrics. 2006;117:e487–95. doi: 10.1542/peds.2005-0572. [DOI] [PubMed] [Google Scholar]