Abstract
We report a case of a 53-year-old man presenting with depressed alertness and severe excessive sleepiness in the setting of neurosarcoidosis. Neuroimaging demonstrated hypothalamic destruction due to sarcoidosis with a CSF hypocretin level of 0 pg/mL. The patient also experienced respiratory depression that presumably resulted from hypocretin-mediated hypothalamic dysfunction as a result of extensive diencephalic injury. This is a novel case, demonstrating both hypocretin deficiency syndrome, as well as respiratory dysfunction from destruction of hypocretin neurons and extensive destruction of key diencephalic structures secondary to the underlying neurosarcoidosis.
Citation:
May MC, Deng JC, Albores J, Zeidler M, Harper RM, Avidan AY. Hypocretin deficiency associated with narcolepsy type 1 and central hypoventilation syndrome in neurosarcoidosis of the hypothalamus. J Clin Sleep Med 2015;11(9):1063–1065.
Keywords: hypocretin, orexin, hypothalamus, neurosarcoid, narcolepsy
Human narcolepsy is currently considered to reflect dysfunction of the hypocretin system. The vast majority of patients with narcolepsy and cataplexy have low or undetectable levels of hypocretin-1 in the cerebrospinal fluid (CSF). Abnormally low hypocretin levels have also been described in cases of secondary narcolepsy due to hypothalamic damage from a variety of CNS causes, including neurosarcoidosis.1–3 In this case report, we present a patient with profound hypersomnolence in the setting of neurosarcoidosis of the diencephalon, and respiratory failure secondary to central alveolar hypoventilation.
REPORT OF CASE
A previously healthy 53-year-old-man presented with alterations in alertness and hypothalamic lesion noted on MRI. Prior to onset of symptoms, he had unexplained, unintentional weight loss, severe and pathological excessive sleepiness, subjective fevers, and night sweats. Diagnosis of neurosarcoidosis was established by hypothalamic biopsy demonstrating non-caseating granulomas and exclusion of other infectious diseases. No other organ involvement was noted on whole-body positron emission topography (PET) scan, computerized tomography of the chest, or 2D echocardiogram. Chest radiograph was unremarkable with normal lung volumes. The patient underwent treatment for sarcoidosis. Dream enactment, snoring, apneic spells, cataplexy, hallucinatory episodes and sleep paralysis were absent, but sleep fragmentation was present. Physical examination revealed a lethargic, thin man who awakened intermittently to verbal stimuli, but held a very short attention span of approximately 5-to-10 seconds, lapsing back to sleep. During the course of hospitalization, he developed acute hypercapnic respiratory failure of unclear origin.
RESULTS
Laboratory Studies
Lumbar puncture demonstrated a CSF hypocretin level of 0 pg/mL. The CSF was clear and colorless with 2 RBC and 3 WBC, without evidence for demyelination. CSF was negative for the following: coccidiomycosis, cryptococcus, JC virus, toxoplasmosis, VDRL, varicella PCR, HSV1/2 PCR, mycobacterial PCR, acid fast stain and culture, bacterial culture, viral culture, and fungal culture.
HLA DQB1*0602 was negative.
Blood gas showed respiratory acidosis with a pH of 7.29, PaCO2 = 91 mm Hg, PaO2 = 50 mm Hg, HCO3 = 42.6 mmol/L. Subsequent diaphragmatic ultrasound sniff test depicted normal bilateral diaphragmatic movement with respiration. Pulmonary function tests were deferred due to the patient's diminished mental status. Computerized tomography of the chest before and during his acute respiratory failure depicted only bibasilar atelectasis, stable micronodules without evidence of lesions, or masses along the course of the phrenic nerve. There was no evidence of infiltration of the diaphragm with sarcoid. The patient did not have evidence of lymphadenopathy, pulmonary fibrosis, or disease along the bronchovascular bundle. Phrenic EMGs depicted normal bilateral phrenic nerve compound muscle action potential (CMAPs).
Imaging Studies
Brain magnetic resonance imaging (MRI) indicated involvement of the hypothalamus (Figure 1), with both T2 and T1 post-contrast hyperintensity within that region. No brainstem involvement was detected by MRI at that time, nor were any appreciable lesions found in the locus coeruleus or raphe nuclei.
Figure 1.
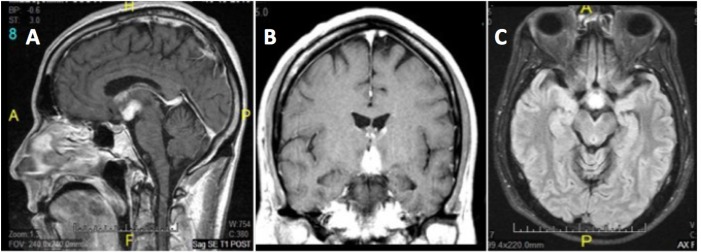
(A) Sagittal T1 post contrast. (B) Coronal T1 post contrast. (C) Axial T2 Flair. T1 and T2-weighted MRI images depicting hypothalamic hyperintensities. T2 images show hyperintensity in the anterior hypothalamus, with signal abnormalities lining the mildly expanded anterior third ventricle. No brainstem involvement appeared in MR images at that time, nor were there any appreciable lesions of the locus coeruleus or raphe nuclei.
Sleep Studies
Due to the autonomic and respiratory instability and hypernatremia, the patient was too unstable to be transferred to the sleep center to undergo a formal polysomnogram. Two portable sleep studies were attempted during the course of his hospitalization, but both were technically challenged.
DISCUSSION
The patient's symptoms appear to represent a central nervous system hypersomnia in the form of narcolepsy type 1 by the International Classification of Sleep Disorders 3, resulting from significant hypothalamic destruction due to neurosarcoidosis. While the majority of patients with narcolepsy type 1 are HLA-DQB1*0602 positive, with its mechanism appearing to be related to decreased numbers of hypocretin secreting cells in the posterolateral hypothalamus,4 low CSF hypocretin in patients who are negative for the HLA-DQB1*0602 allele, as in this case, are presumed to have a secondary and neurologically-mediated mechanism as a disease phenotype.4
We postulate that, in addition to the patient's severe hypersomnia, his hypoventilation and hypercapnia were also mediated by hypocretin deficiency in the absence of significant pulmonary or neuromuscular pathology to account for this finding. Hypocretin neurons contribute to multiple “drives” of breathing, including thermal drive, forebrain “arousal” influences to the musculature (which includes respiratory muscles), and, of special importance to the findings here, CO2 facilitation of breathing.5,6
The patient's prolonged hypoventilation potentially exacerbated neural injury in brainstem respiratory and autonomic areas, leading to impaired ventilation. Studies in another sleep disordered breathing condition, congenital central hypoventilation syndrome (CCHS), show significant injury to hypothalamic, ventral and dorsal medullary, periaqueductal gray, and parabrachial pontine areas.7 Although medullary and pontine injury did not appear in MRI studies of this case, it is important to note that CCHS MRI studies often do not show injury unless specialized procedures of diffusion tensor imaging or T2 relax-ometry studies are collected. Hypocretin plays many excitatory roles, among which is exciting chemoreception. Sensing of chemoreception takes place in many sites in the brain, including areas near the hypothalamus, as well as in projections to medullary and cerebellar chemoreception sites.8 A loss of hypocretin and its excitatory influence on sensing CO2 could lead to hypoventilation, and underlie the findings here.
In conclusion, while findings of narcolepsy type 1 are often encountered in patients who present with diencephalic lesions, clinicians who encounter these patients should also pay attention to respiratory function. Hypocretin deficiency is invariably associated with profound hypersomnia, and hypocretin neuronal projections to brainstem structures can mediate a pattern of central hypoventilation syndrome. For this reason, patients presenting with significant diencephalic disease should be monitored carefully, and assessed for development of respiratory failure.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Avidan has participated in speaking engagements for Merck and UCB and has consulted for Best Doctors. Dr. Zeidler has received research support from and participated in speaking engagements for Forest Pharmaceuticals and has received the loan of research equipment from ResMed. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. Emmanuel Mignot and the Stanford University Center for Narcolepsy and Related Disorders for graciously providing the CSF hypocretin assay.
REFERENCES
- 1.Aldrich MS, Naylor MW. Narcolepsy associated with lesions of the diencephalon. Neurology. 1989;39:1505–8. doi: 10.1212/wnl.39.11.1505. [DOI] [PubMed] [Google Scholar]
- 2.Servan J, Marchand F, Garma L, Seilhean D, Hauw JJ, Delattre JY. [Narcolepsy disclosing neurosarcoidosis] Rev Neurol(Paris) 1995;151:281–3. [PubMed] [Google Scholar]
- 3.Rubinstein I, Gray TA, Moldofsky H, Hoffstein V. Neurosarcoidosis associated with hypersomnolence treated with corticosteroids and brain irradiation. Chest. 1988;94:205–6. doi: 10.1378/chest.94.1.205. [DOI] [PubMed] [Google Scholar]
- 4.Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep. 1997;20:1012–20. [PubMed] [Google Scholar]
- 5.Corcoran A, Richerson G, Harris M. Modulation of respiratory activity by hypocretin-1 (orexin A) in situ and in vitro. Adv Exp Med Biol. 2010;669:109–13. doi: 10.1007/978-1-4419-5692-7_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nattie E, Li A. Respiration and autonomic regulation and orexin. Progr Brain Res. 2012;198:25–46. doi: 10.1016/B978-0-444-59489-1.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahid IZ, Rahman AA, Pilowsky PM. Orexin A in rat rostral ventrolateral medulla is pressor, sympatho-excitatory, increases barosensitivity and attenuates the somato-sympathetic reflex. Br J Pharmacol. 2012;165:2292–303. doi: 10.1111/j.1476-5381.2011.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodman T, Resnick ME, Berkowitz RD, Fennelly JF, Olivia J. Alveolar hypoventilation due to involvement of the respiratory center by obscure disease of the central nervous system. Am J Med. 1962;32:208–17. doi: 10.1016/0002-9343(62)90290-5. [DOI] [PubMed] [Google Scholar]


