Abstract
Study Objectives:
Minimally symptomatic obstructive sleep apnea (OSA) is highly prevalent, and the effects of continuous positive airway pressure (CPAP) on myocardial function in these patients are unknown. The MOSAIC randomized, controlled trial of CPAP for minimally symptomatic OSA assessed the effect of CPAP on myocardial function in a subset of patients.
Methods:
Two centers taking part in the MOSAIC trial randomized 238 patients in parallel to 6 months of CPAP (120) or standard care (118). Of these, 168 patients had echocardiograms, and 68 patients had a cardiac magnetic resonance scan (CMR). A larger group (314) from 4 centers had brain natriuretic peptide (BNP) measured.
Results:
Mean (SD) baseline oxygen desaturation index (ODI) and Epworth sleepiness score (ESS) were 13.5 (13.2), and 8.4 (4.0), respectively. CPAP significantly reduced ESS and ODI. Baseline LV ejection fraction (LVEF) was well preserved (60.4%). CPAP had no significant effect on echo-derived left atrial (LA) area (−1.0 cm2, 95% CI −2.6 to +0.6, p = 0.23) or early to late left ventricular filling velocity (E/A) ratio (−0.01, 95% CI −0.07 to +0.05, p = 0.79). There was a small change in echo-derived LV end diastolic volume (EDV) with CPAP (−5.9 mL, 95% CI −10.6 to −1.2, p = 0.015). No significant changes were detected by CMR on LV mass index (+1.1 g/m2, 95% CI −5.9 to +8.0, p = 0.76) or LVEF (+0.8%, 95% CI −1.2 to +2.8, p = 0.41). CPAP did not affect BNP levels (p = 0.16).
Conclusions:
Six months of CPAP therapy does not change cardiac functional or structural parameters measured by echocardiogram or CMR in patients with minimally symptomatic mild-to-moderate OSA.
Clinical Trial Registration:
ISRCTN 34164388 (http://isrctn.org).
Citation:
Craig S, Kylintireas I, Kohler M, Nicoll D, Bratton DJ, Nunn AJ, Leeson P, Neubauer S, Stradling JR. Effect of CPAP on cardiac function in minimally symptomatic patients with OSA: results from a subset of the MOSAIC randomized trial. J Clin Sleep Med 2015;11(9):967–973.
Keywords: obstructive sleep apnea, myocardial function, echocardiography, magnetic resonance scanning, CPAP
Obstructive sleep apnea (OSA) has the potential to be significantly detrimental to cardiac function.1 Extreme sub-atmospheric pressure swings during apneic events can transiently reduce left ventricular (LV) emptying, and lead to increased left atrial (LA) and LV volumes, as well diastolic dysfunction. Over time, the LA may irreversibly enlarge, and patients with OSA have been shown to be more likely to develop atrial fibrillation (AF) as a possible consequence.2 In addition, it has been suggested that OSA can affect the aortic root, leading to increased rates of dilatation in some populations.3–5
Patients with OSA also have associated medical conditions such as diabetes, hypertension, and upper body obesity, which are recognized risk factors for cardiac disease. Many of these patients will have impaired diastolic function as a precursor to systolic dysfunction, and identifying this is important to prevent further functional decline. This is quantified by measuring mitral blood flow (E/A ratio and deceleration times) and LA size (diameter or area) by echocardiography. In addition, LV mass (measured by echocardiogram) has been shown to be higher in these patients6,7; it may not be independently associated with OSA and may be more directly related to obesity or hypertension.8
BRIEF SUMMARY
Current Knowledge/Study Rationale: The effect of CPAP therapy on cardiac function in patients with minimally symptomatic OSA is unknown. We used three methods (echocardiography, cardiac MR and BNP measurements) to assess cardiac function at baseline and after six months in patients randomized to CPAP or standard care.
Study Impact: There was no improvement in cardiac function in any of the three measurements although baseline cardiac dysfunction was mild. This would suggest that conventional cardiovascular reduction methods such as antihypertensive therapy or smoking cessation would be more beneficial in this group of patients.
Although echocardiograms are considered the gold standard for detecting diastolic dysfunction, cardiac magnetic resonance scanning (CMR) is emerging as a more accurate way of detecting small structural changes over short time periods, especially in the obese population where echocardiogram images can be poor. A recent uncontrolled study of 47 patients with severe OSA, assessed using both echocardiographic and CMR measures of LA, LV, LVM, and diastolic function over a one-year period on CPAP therapy found improvements in these measures as early as six months from baseline.9
However echocardiograms and CMR are labor intensive, and other methods of detecting cardiac dysfunction have been sought in the form of cardiac biomarkers. The most reliable is brain natriuretic peptide (BNP), which is secreted by the ventricles in response to an increase in ventricular volume and pressure overload. These have been shown to be increased in proportion to the level of ventricular dysfunction and LV mass.10 In addition, patients with central sleep apnea (CSA) or mixed OSA/CSA have been shown to have higher levels of BNP than patients with heart failure alone.11 These levels were reduced with noninvasive ventilation or CPAP, even if structural changes were not seen.12 Our patients did not have heart failure or CSA but we were interested in observing the possible change in BNP in patients with minimally symptomatic OSA, and whether this could act as an early, less invasive test of improvement in cardiac function with CPAP treatment.
We have used data from the MOSAIC randomized controlled trial13 to investigate whether treating minimally symptomatic OSA with CPAP for six months can improve aspects of cardiac function.
METHODS
The MOSAIC trial (Multicentre Obstructive Sleep Apnoea Interventional Cardiovascular trial) was conducted between May 2006 and February 2010: 9 centers from the UK and 1 from Canada participated. Patients with minimally symptomatic OSA were randomized to either 6 months of CPAP therapy or standard care. The primary outcomes were Epworth Sleepiness Scale (ESS) score and the Pocock Cardiovascular Risk Score; the results of the trial and its full description have been published.13 In brief, it showed that CPAP treatment was associated with cost-effective reduction in daytime symptoms but did not reduce calculated cardiovascular risk.
In 2 centers (Oxford Centre for Respiratory Medicine and the Royal Berkshire Hospital, Reading, UK) echocardiograms were performed at baseline and after the 6-month intervention. Cardiac magnetic resonance scans (CMR) were also performed in Oxford between March 2007 and December 2008. The trial was approved by the Oxford research ethics committee (RECNo: 05/Q1604/159) and registered (ISRCTN 34164388).
Patients
Patients referred to sleep clinics from general practitioners and hospital specialists, usually due to snoring, witnessed apneas, or daytime sleepiness, were assessed for eligibility and a screening log was kept. All patients were diagnosed with OSA using overnight respiratory polygraphic equipment, as standard in the participating centers. Patients were considered to be eligible for the study if they were aged between 45 and 75 years, had proven OSA on the diagnostic sleep study, with a severity defined as > 7.5 oxygen desaturations > 4% per hour (oxygen desaturation index, ODI), and insufficient daytime symptoms associated with OSA to warrant CPAP therapy. This decision followed a detailed discussion between physician and patient about the evidence for possible benefits of CPAP, versus the potentially lifelong usage of a physical therapy every night. Excluded were patients with ventila-tory failure, Cheyne-Stokes breathing, previous exposure to CPAP, systolic blood pressure (BP) > 180 mm Hg or diastolic BP > 110 mm Hg on 3 successive measurements during the eligibility assessment, a heavy goods vehicle driver's licence, previous sleep-related accident, or a disability precluding either informed consent or compliance with the protocol. All patients who gave informed consent did so in accordance with Good Clinical Practice standards. In addition, to ensure technical uniformity of the ODI across centers, an additional domiciliary overnight pulse oximetry recording (Konica-Minolta Inc, Japan) was performed in all patients at baseline and at 6 months. This was used as the trial ODI value which could therefore be different from the entry ODI.
Echocardiograms
All patients taking part in this trial were assessed at baseline and at 6 months with 2D transthoracic echocardiography (Siemens G60) as defined by the British Society of Echocardiography.14 Images were saved for assessment offline with Philips Xcelera digital analysis software at a later date.
Measurements of LA diameter, LA area, aortic root diameter and area, early-to-late left ventricular filling velocity (E/A) ratio, mitral deceleration time, Doppler slope, LV volumes, and ejection fraction (EF) were taken. E/A ratio and mitral deceleration time were measured with pulse wave mode at mitral valve tips. LA area was measured by tracing the inner edge of the LA image in 4-chamber apical view at end systole. LA and aortic diameters were also measured in parasternal view. LV dimensions were measured by Simpson's method in systole and diastole. Images were analyzed by one member of the trial team (SC), blinded to randomization status, and confirmed by an experienced independent cardiologist unrelated to the trial (PL). Patient scans were excluded from the analysis if images were poor quality and thus inadequate to assess ejection fraction via Simpson's method.
Cardiac Magnetic Resonance (CMR)
A subset of patients in the Oxford center also had a CMR scan at baseline and 6 months. These patients were not selected according to any criteria, but according to further consent and the availability of scan time, between March 2007 and December 2008. Sixty-eight patients underwent a baseline scan, and 66 were successfully followed up (one declining repeat scan, one unable to schedule). Fifty-six patients had adequate quality data for accurate quantitative ventricular image analysis at both time points. All CMR scans were performed on a 1.5 Tesla MR system (40mT/m; Siemens Healthcare, Erlangen, Germany). Analysis was performed by a trained cardiologist (IK) blinded to randomization status.
Measurements included and reported here are LV mass (corrected for body surface area, Mosteller formula,15 LVMI), left ventricular end diastolic volume (LVEDV), left ventricular end systolic volume (LVESV), and left ventricular ejection fraction (LVEF).
CPAP Intervention
Patients assigned to CPAP were instructed on the use of an auto-adjusting CPAP machine (Autoset S8, index ResMed, Abingdon, UK). Induction was by trained staff at each center who were not involved in outcome assessments or data analysis. Humidification and interface choices were not restricted and were given on an individual basis. All patients had one or more follow-up visits to download compliance data, check for residual apnea/hypopneas and leakage from the autoCPAP machine, and to make any necessary adjustments. There were routine telephone calls at 2 and 4 months, and telephone advice and replacement parts if requested by the patient. CPAP compliance over the 6-month follow-up period was determined by downloading usage data from the machine and defined as total hours used, divided by days between the set-up visit and the 6-month follow-up visit. Non-users were defined as those who admitted stopping CPAP therapy at least one month prior to their 6-month follow-up appointment. Compliance was set to 0 h/night in those non-users who had no compliance data available at 6 months, usually due to the patient having returned their machine some while before their 6-month visit.
The standard care (SC) group had an identical planned visit schedule to the CPAP group. Both groups were asked to continue on their normal medication and not given any specific advice regarding diet and exercise.
Outcomes
The primary outcomes for the main study were Epworth Sleepiness Scale (ESS) and the calculated Pocock risk score; these have been reported previously.13 The exploratory secondary outcomes based on echocardiogram reported here are: change in E/A ratio, end diastolic volumes, aortic root and LA size. CMR derivatives were: change in LV mass index, LV ejection fraction, LV end-systolic and end-diastolic volumes (LVESV, LVEDV). Change in BNP levels (NT-proBNP, LPBN assay, dimension heterogeneous immunoassay module, Siemens Healthcare Diagnostics 2008) were measured in a larger subpopulation of the MOSAIC trial, as blood samples were available from 4 centers (314 patients in total).
Additional Measurements
To characterize the cardiovascular status of the subjects the cardiovascular risk score proposed by Pocock et al.16 was calculated. This score estimates the probability of a fatal cardiovascular event within 5 years from 11 factors: age, sex, height, systolic BP, total cholesterol, creatinine, cigarette smoking (current smoker if smoked in the previous month), diabetes (either on treatment, previously diagnosed and in General Practice records, or fasting glucose ≥ 7.0 mmol/L and HbA1c > 6.5%), LV hypertrophy (Sokolov-Lyon method on ECG), and history of cerebrovascular incident and/or myocardial infarction (from General Practice records and verified from hospital case records if equivocal).
Subjective sleepiness was assessed using the Epworth Sleepiness Scale (ESS),17 which assesses the tendency to fall asleep during 8 typical daytime scenarios.
Sample Size Calculation
A sample size calculation was performed for the primary endpoints of MOSAIC13 (reduction in Epworth Sleepiness Scale score) and required 400 patients to be randomized, allowing for a 10% dropout rate. Numbers of patients recruited to the echocardiographic and CMR substudy was limited by finances, and the results are therefore exploratory.
Randomization
Randomization was carried out by telephoning the Medical Research Council Clinical Trials Unit (MRC CTU), using minimization with a random element of 80%; the minimization factors were OSA severity (ODI > or < 20/h), risk score (> or < 40), participating center, and whether having a CMR.
Data and Statistical Methods
Data were analyzed on an intention-to-treat basis using multivariable regression models with adjustment for the minimization variables, baseline value of the corresponding variable being analyzed, and baseline body mass index (BMI). Baseline and 6-month values are presented as means (SD), medians (25th and 75th percentiles), or percentages, as appropriate. All statistical analyses were performed using STATA Version 11 for Windows (Stata Corporation, TX, USA).
RESULTS
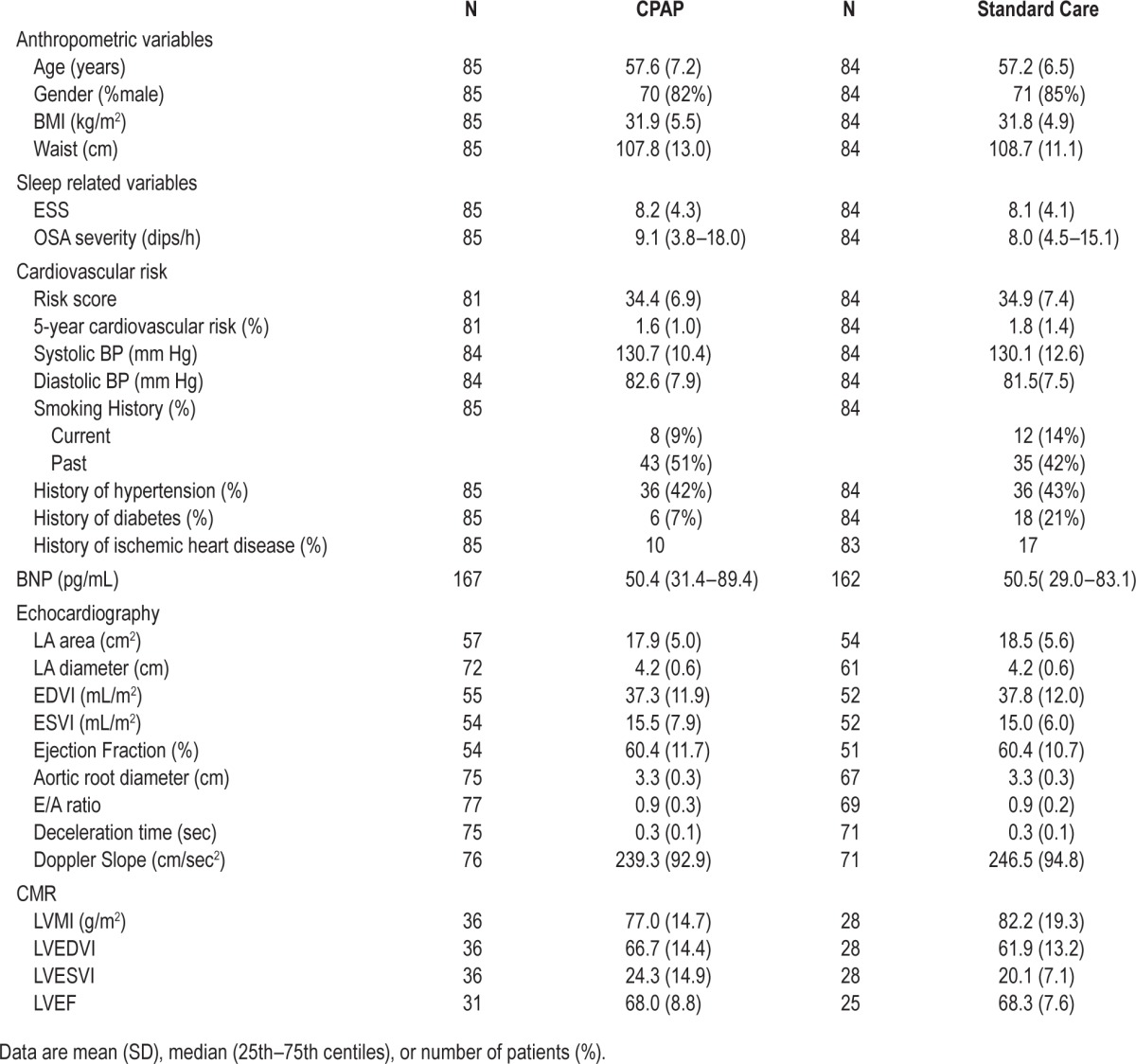
Figures 1 and 2 show the flow diagrams for the echocardiogram and CMR groups. Table 1 shows the baseline characteristics of the randomized groups, and prevalence of known cardiovascular risks such as hypertension, previous or current smoking, diabetes, and ischemic heart disease. They were minimally symptomatic (mean [SD] ESS for whole group 8.4 [4.0]) and on average had mild-to-moderate OSA on sleep study criteria (ODI 13.5 [13.2]). Baseline E/A ratio was low in the study population (0.9 [0.2]) indicating, on average, early diastolic dysfunction. The 2 groups were broadly similar with respect to their characteristics.
Figure 1. Trial profile of patients undergoing echocardiography.
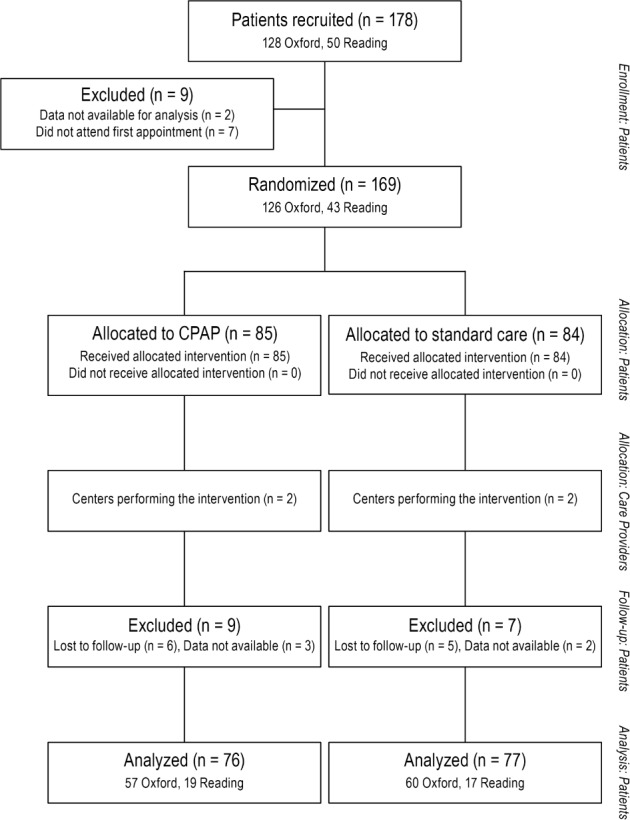
Figure 2. Trial profile of patients undergoing cardiac magnetic resonance scanning.
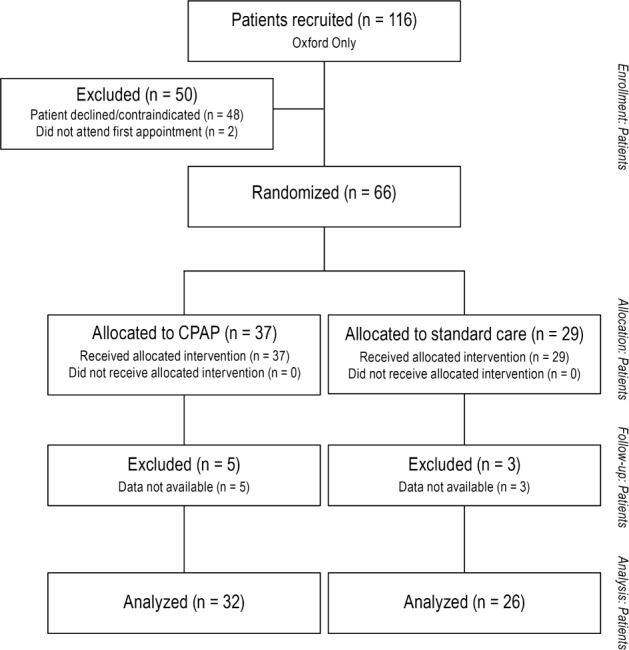
Table 1.
A comparison of baseline characteristics in CPAP vs standard care groups undergoing echocardiography, CMR and BNP measurements.
Echocardiography Outcomes
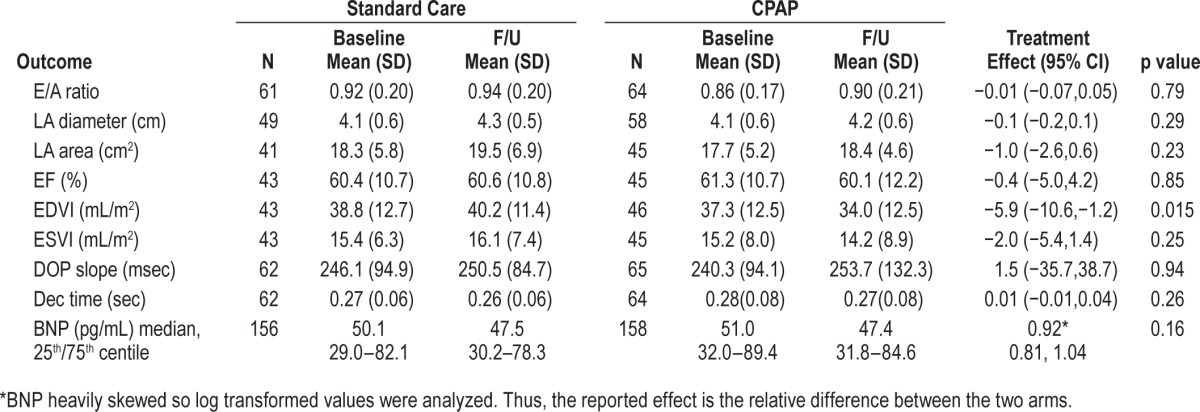
There was no significant change in E/A ratio or LA size with CPAP therapy (Table 2). Ejection fraction and end systolic volume was unchanged with CPAP, but there was a statistically significant improvement in end-diastolic volume (−5.9 mL, 95% CI −10.6 to −1.2, p = 0.015).
Table 2.
Treatment effects for echocardiography and BNP outcomes.
CMR Outcomes
There was no change in LV mass index, end-diastolic or end-systolic volumes, or LV ejection fraction with CPAP therapy (Table 3)
Table 3.
Treatment effects for CMR outcomes.

BNP
BNP levels were heavily skewed and a log transformation was used to normalize the data for modelling. Thus the treatment effects for BNP are expressed as a relative difference between the CPAP group and control intervention. There was a statistically nonsignificant 8% reduction in BNP (Table 2) in the CPAP arm relative to control (95% CI 19% reduction to 4% increase, p = 0.16).
Effect of CPAP on Systolic BP
The adjusted treatment effect on systolic BP for the main MOSAIC trial was +1.8, 95% CI 0.0 to +3.5, p = 0.049.
Effect of CPAP Compliance
Overall compliance was 3.27 (2.36) h/night. Table 4 shows the effect of using CPAP for less than, or more than, four hours per night compared to control on the echocardiographic outcomes, E/A ratio and EDV and EF. There was no statistically significant difference between groups; thus there was no dose-response effect to suggest that the subset of highest compliers might have experienced improvements in cardiac function.
Table 4.
Comparison of good (> 4 h usage) versus poor (< 4 h usage) compliance with CPAP on the treatment effect of echocardiography outcomes.
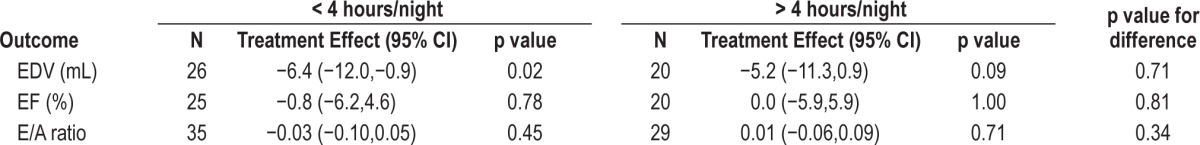
DISCUSSION
The results of the main MOSAIC randomized controlled six-month trial in minimally symptomatic and, on average, mild-to-moderate severity OSA have been reported elsewhere.13 There was a highly significant reduction in daytime symptoms (ESS), but no reduction in vascular risk, as measured by the Pocock risk score. This study on a subset of patients from the MOSAIC trial has not shown any convincing change in cardiac functional or structural parameters, measured by echocardiography or cardiac MR. This was despite E/A ratio being low in our population at baseline, indicating, on average, early diastolic dysfunction. Neither was there any suggestion that BNP levels improved. To our knowledge, this is the first RCT to include CMR measures of structural changes in patients with mild-to-moderate OSA and only minimal symptoms.
Although most of our findings were not significantly influenced by CPAP therapy, there was a small, statistically signifi-cant reduction in end-diastolic volume (EDV, measured from the echocardiogram). However, this is likely to be a chance finding, given that it was not supported by the more accurate CMR equivalent. Furthermore there was no suggestion of a dose-response effect dependent on CPAP compliance. We further explored whether there might be an effect, specifically in subgroups potentially more likely to have initial significant diastolic dysfunction, by looking for an interaction between the treatment effect and either age or baseline OSA severity; however, we found no such interaction (p = 0.4 and 0.7, respectively). Therefore we are confident that, if there is any effect of CPAP on diastolic function in this population, it is likely to be very small and clinically insignificant.
Our baseline results are similar to previous observational and case-controlled studies that have shown that patients with OSA have high rates of impaired relaxation and diastolic dysfunction. Observational studies in moderate-to-severe OSA patients have shown that diastolic function is decreased in OSA patients18 and appears to be related to OSA severity.19 Case-controlled studies, again in moderate-to-severe patients, have shown that LA size and LV diastolic function is abnormal, and in uncontrolled studies improved with six months of CPAP therapy.20,21 However, the treatment groups in these studies were small (37 and 28, respectively), not randomized, and consequently potentially affected by confounding factors in these high cardiovascular risk populations, such as changes in drug compliance during the trial.
Another non-randomized study in 47 severe OSA patients showed a response in CMR derivatives to 12 months CPAP.9 This study included patients with diabetes and a smoking history, similar to our population, but their patients had more severe OSA and were more symptomatic than in our study.
There have been few randomized controlled studies in this area, and these have been in moderate-to-severe OSA patients. A randomized, placebo-controlled, crossover study in 25 men showed that patients with severe symptomatic OSA have impaired diastolic relaxation of the LV, and this improved with 12 weeks of CPAP.22 However there was no improvement in any of the many other echo derivatives, including LA or LV dimensions. A more recent parallel RCT in 30 patients, assessed LV function and LA size by 3D echocardiography, which is more accurate than conventional echocardiography for assessing structural measurements, and found improvement in LV diastolic function, but again no change in LV or LA dimensions, after 24 weeks CPAP therapy.23
The explanation for the difference between our study and these others is likely to be due to the greater OSA severity in the latter. More severe patients will tend to have higher average overnight BP levels24 (as well as more oscillations in BP), and thus risk factors for diastolic dysfunction.
Because of the hypothesis that OSA might influence aortic dimensions and perhaps influence thoracic aneurysms,3 at the time of the cardiac echo we also took the opportunity to measure aortic root diameter. However, we were unable to show a reduction in aortic root area following 6 months of CPAP (treatment effect +0.19 cm2, 95% CI −0.28 to +0.65, p = 0.43)
The strength of our study is that the patients were randomized to parallel groups and the echocardiographer and cardiology team were blinded to the treatment allocation. Also all studies were analyzed and entered into a database kept separate from the randomization code. Patients were added prospectively and not recruited for any particular characteristic, other than having minimally symptomatic OSA. Thus some patients had hypertension, and were not excluded if they smoked or had diabetes.
The less severe OSA found in our patients may introduce a limitation, if more than six months of CPAP therapy is required to produce regression of mild structural changes in this group. In our study patients were not specifically chosen to have cardiac dysfunction but reflected the relatively mild cardiac dysfunction in our trial group. This is likely to have reduced our chances of seeing an effect with CPAP therapy and the generalizability of our results to patients with more severe cardiac dysfunction. However, we also employed BNP and CMR techniques, which should be capable of detecting small, early changes, as has been shown in other settings. For instance, CMR has been used before to demonstrate a reduction in ventricular mass in a number of intervention studies in hypertensive patients.25,26
There was no placebo CPAP used in this trial, and this is a potential limitation (discussed in main MOSAIC paper), but a placebo effect is unlikely to affect these objective measurements, particularly over an intervention period of six months.
Finally, this subset study was not powered for these endpoints, so the probability of making a type II statistical error is likely to be high. Had we studied a larger population we might have found differences. The 95% CI of the treatment effects show what difference has been excluded with 95% confidence. If smaller differences are to be explored, much larger sample sizes will be required.
In conclusion, we have found that although CPAP appeared to reduce EDV measured by echocardiogram in this milder group of OSA patients, this is likely to be a chance finding, given that this was not confirmed on CMR, there was no dose response relationship with CPAP compliance, and there was no suggestion of a bigger effect size in the more elderly patients, or those with more severe OSA. Furthermore, there was no statistically significant effect on BNP levels. This suggests that long-term diastolic functional improvements with CPAP will be at best very minimal in this group of mild patients with near-normal cardiac function and unlikely to match the improvements in vascular risk seen with, for example, antihypertensives. We would suggest that, based on these findings, conventional vascular risk reduction methods are much more important in this population.
DISCLOSURE STATEMENT
Finding was provided for this paper by: The British Heart Foundation - project grant, Oxford Health Services Research Committee paid for research salaries, ResMed UK made an unrestricted charitable donation to support research work in the Oxford Sleep Unit in 1998 and 2006, and supplied the CPAP machines for this trial. Dr. Kohler was a recipient of an ERS (No. 118) and University of Zurich research fellowship. The authors have indicated no other financial conflicts of interest.
Funders of the trial had no role in study design, data collection, data analysis or interpretation, or writing of the report. Authors fulfilled the criteria for authorship, had full access to all data in the study, and had final responsibility for the decision to submit for publication. SC is the guarantor for the study.
ABBREVIATIONS
- AF
atrial fibrillation
- BMI
body mass index
- BNP
brain natriuretic peptide
- BP
blood pressure
- CI
confidence interval
- CMR
cardiac magnetic resonance
- CPAP
continuous positive airway pressure
- E/A
early to late ratio
- EDV
end diastolic volume
- EF
ejection fraction
- ESS
Epworth Sleepiness Scale
- ESV
end systolic volume
- LVM(I)
left ventricular mass (index)
- ODI
oxygen desaturation index (> 4% dips in SpO2)
- OSA
obstructive sleep apnea
- SD
standard deviation
REFERENCES
- 1.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 2.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–7. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 3.Kohler M, Blair E, Risby P, et al. The prevalence of obstructive sleep apnoea and its association with aortic dilatation in Marfan's syndrome. Thorax. 2009;64:162–6. doi: 10.1136/thx.2008.102756. [DOI] [PubMed] [Google Scholar]
- 4.Stowhas AC, Namdar M, Biaggi P, et al. The effect of simulated obstructive apnea and hypopnea on aortic diameter and BP. Chest. 2011;140:675–80. doi: 10.1378/chest.10-2799. [DOI] [PubMed] [Google Scholar]
- 5.Clarenbach CF, Camen G, Sievi NA, Wyss C, Stradling JR, Kohler M. Effect of simulated obstructive hypopnea and apnea on thoracic aortic wall transmural pressures. J Appl Physiol. 2013;115:613–7. doi: 10.1152/japplphysiol.00439.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drager LF, Bortolotto LA, Figueiredo AC, Silva BC, Krieger EM, Lorenzi-Filho G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest. 2007;131:1379–86. doi: 10.1378/chest.06-2703. [DOI] [PubMed] [Google Scholar]
- 7.Dursunoglu D, Dursunoglu N, Evrengul H, et al. Impact of obstructive sleep apnoea on left ventricular mass and global function. Eur Respir J. 2005;26:283–8. doi: 10.1183/09031936.05.00038804. [DOI] [PubMed] [Google Scholar]
- 8.Niroumand M, Kuperstein R, Sasson Z, Hanly PJ. Impact of obstructive sleep apnea on left ventricular mass and diastolic function. Am J Respir Crit Care Med. 2001;163:1632–6. doi: 10.1164/ajrccm.163.7.2007014. [DOI] [PubMed] [Google Scholar]
- 9.Colish J, Walker JR, Elmayergi N, et al. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012;141:674–81. doi: 10.1378/chest.11-0615. [DOI] [PubMed] [Google Scholar]
- 10.Maisel AS, Koon J, Krishnaswamy P, et al. Utility of B-natriuretic peptide as a rapid, point-of-care test for screening patients undergoing echocardiography to determine left ventricular dysfunction. Am Heart J. 2001;141:367–74. doi: 10.1067/mhj.2001.113215. [DOI] [PubMed] [Google Scholar]
- 11.Carmona-Bernal C, Quintana-Gallego E, Villa-Gil M, Sanchez-Armengol A, Martinez-Martinez A, Capote F. Brain natriuretic peptide in patients with congestive heart failure and central sleep apnea. Chest. 2005;127:1667–73. doi: 10.1378/chest.127.5.1667. [DOI] [PubMed] [Google Scholar]
- 12.Pepperell JC, Maskell NA, Jones DR, et al. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Respir Crit Care Med. 2003;168:1109–14. doi: 10.1164/rccm.200212-1476OC. [DOI] [PubMed] [Google Scholar]
- 13.Craig SE, Kohler M, Nicoll D, et al. Continuous positive airway pressure improves sleepiness but not calculated vascular risk in patients with minimally symptomatic obstructive sleep apnoea: the MOSAIC randomised controlled trial. Thorax. 2012;67:1090–6. doi: 10.1136/thoraxjnl-2012-202178. [DOI] [PubMed] [Google Scholar]
- 14.Chambers J. A Minimum dataset for a standard Adult Transthoracic Echocardiogram. From the British Society of Echocardiography Education Committee. British Society of Echocardiography. 2005 [Google Scholar]
- 15.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 16.Pocock SJ, McCormack V, Gueyffier F, Boutitie F, Fagard RH, Boissel JP. A score for predicting risk of death from cardiovascular disease in adults with raised blood pressure, based on individual patient data from randomised controlled trials. BMJ. 2001;323:75–81. doi: 10.1136/bmj.323.7304.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 18.Baguet JP, Barone-Rochette G, Levy P, et al. Left ventricular diastolic dysfunction is linked to severity of obstructive sleep apnoea. Eur Respir J. 2010;36:1323–9. doi: 10.1183/09031936.00165709. [DOI] [PubMed] [Google Scholar]
- 19.Wachter R, Luthje L, Klemmstein D, et al. Impact of obstructive sleep apnoea on diastolic function. Eur Respir J. 2013;41:376–83. doi: 10.1183/09031936.00218211. [DOI] [PubMed] [Google Scholar]
- 20.Butt M, Dwivedi G, Shantsila A, Khair OA, Lip GY. Left ventricular systolic and diastolic function in obstructive sleep apnea: impact of continuous positive airway pressure therapy. Circ Heart Fail. 2012;5:226–33. doi: 10.1161/CIRCHEARTFAILURE.111.964106. [DOI] [PubMed] [Google Scholar]
- 21.Akar BN, Ciftci B, Durmaz T, et al. Effects of continuous positive airway pressure therapy on left ventricular function assessed by tissue Doppler imaging in patients with obstructive sleep apnoea syndrome. Eur J Echocardiogr. 2009;10:376–82. doi: 10.1093/ejechocard/jen257. [DOI] [PubMed] [Google Scholar]
- 22.Arias MA, Garcia-Rio F, Alonso-Fernandez A, Mediano O, Martinez I, Villamor J. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation. 2005;112:375–83. doi: 10.1161/CIRCULATIONAHA.104.501841. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira W, Campos O, Cintra F, et al. Impact of continuous positive airway pressure treatment on left atrial volume and function in patients with obstructive sleep apnoea assessed by real-time three-dimensional echocardiography. Heart. 2009;95:1872–8. doi: 10.1136/hrt.2009.173625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–52. [PubMed] [Google Scholar]
- 25.Friedrich MG, Dahlof B, Sechtem U, Unger T, Knecht M, Dietz R. Reduction (TELMAR) as assessed by magnetic resonance imaging in patients with mild-to-moderate hypertension--a prospective, randomised, double-blind comparison of telmisartan with metoprolol over a period of six months rationale and study design. J Renin Angiotensin Aldosterone Syst. 2003;4:234–43. doi: 10.3317/jraas.2003.038. [DOI] [PubMed] [Google Scholar]
- 26.Reichek N, Devereux RB, Rocha RA, et al. Magnetic resonance imaging left ventricular mass reduction with fixed-dose angiotensin-converting enzyme inhibitor-based regimens in patients with high-risk hypertension. Hypertension. 2009;54:731–7. doi: 10.1161/HYPERTENSIONAHA.109.130641. [DOI] [PubMed] [Google Scholar]


