Abstract
Objective:
Sleep disordered breathing (SDB) is associated with cardiovascular disease such as hypertension and left ventricular hypertrophy in middle-aged patients; however, this association is not well described in the elderly. The aim of this study was to evaluate the impact of unrecognized SDB on cardiac function and remodeling in a population-based sample of healthy elderly without cardiac disease.
Methodology:
A total of 405 healthy elderly (age ≥ 65 years) were examined by echocardiography and respiratory polygraphy. According to the apnea-hypopnea index (AHI), subjects were stratified in four categories: snorers (AHI < 5), mild (AHI: 5–15), moderate (AHI: 15–30), and severe (AHI > 30) cases.
Results:
Comparative analysis between snorers and SDB cases revealed that left atrial (LA) diameter and surface increased according to SDB severity (p < 0.05) without differences in LA mass index. In subjects with an AHI > 30, an increase was found for LV end-diastolic and end-systolic dimension (p < 0.001), as well as for LV mass (p < 0.03) and LV index (p < 0.05). The current study showed a weak but significant correlation between altered LA and LV measurements versus AHI and hypoxemia indices (p < 0.001). In the regression analysis, AHI and hypoxemia had a minimal effect, body mass index and male gender being the most significant predictors.
Conclusions:
In a population of healthy elderly with SDB, slight changes in left atrial and ventricular measurements occur in severe cases (AHI > 30). Irrespective of the lack of a strong association between SDB and cardiac dysfunction, the presence of slight cardiac pathology in severe SDB cases might be considered.
Clinical Trial Registration:
NCT 00759304 and NCT 00766584.
Citation:
Sforza E, Sabri M, DaCosta A, Isaaz K, Barthélémy JC, Roche F. Echocardiographic findings in healthy elderly people with unrecognized sleep disordered breathing. J Clin Sleep Med 2015;11(9):975–980.
Keywords: sleep disordered breathing, hypoxemia, echocardiography, elderly, left cardiac function, obesity
Sleep disordered breathing (SDB) is a common disease in adults affecting 9% of women and 24% of men aged 30–60 years, with an even higher prevalence reported in the elderly population.1,2 Several clinical and epidemiological studies have established an association of SDB with increased incidence of hypertension, coronary heart disease, stroke, heart failure, and mortality after adjustment for cardiovascular risk factors.3,4
Animal models using intermittent hypoxemia have demonstrated that chronic exposure to hypoxia induces cardiac disturbances including an increase in left ventricular (LV) mass, as well as an interstitial fibrosis in LV myocardium.5,6 An experimental model of intermittent hypoxemia mimics the repetitive nocturnal hypoxemia occurring in SDB patients, inducing increased sympathetic nervous system activity, oxidative stress, systemic inflammation,7 elevated cardiac filling, hemodynamic overload, and increased ventricular transmural pressure. Therefore, the presence of SDB may induce right ventricular hypertrophy,8 increase in left atrial (LA) and left ventricular (LV) dimensions,9 diastolic dysfunction, and concentric LV hypertrophy (LVH). Concentric LVH is known to be independently associated with an increased risk of cardiovascular morbidity and mortality.4
BRIEF SUMMARY
Current Knowledge/Study Rationale: Middle-aged patients with sleep disordered breathing (SDB) are known to frequently suffer from left atrial and ventricular dysfunction related to the hemodynamic changes and arousals from sleep occurring during respiratory events. Limited and controversial data are available in elderly, where the coexistence of cardiac dysfunction and SDB may be explained by the effect of aging in itself. In this study, the impact of SDB on cardiac function in healthy elderly subjects is investigated.
Study Impact: The study demonstrates that in a large population of healthy elderly, a slight association between SDB versus left atrial and ventricular function existed, with subjects with an apnea-hypopnea index exceeding 30 having more significant cardiac dysfunction. Understanding the prevalence of cardiac dysfunction in elderly with severe SDB is important for timely recognition of these high-risk subjects, to whom appropriate treatment should be proposed.
Clinical studies performed in middle-aged SDB patients have demonstrated an association between severity of SDB and alterations of LV diastolic and systolic function,10–11 reversible by continuous positive airway pressure therapy.12 Although many clinical studies stress the link between SDB versus myocardial mass and function, the data are conflicting, probably due to a limited sample size as well as the analysis being restricted to middle-aged patients and severe SDB cases. Moreover, the relationship between SDB severity (i.e., AHI value), and LVH remains controversial because of three important factors: age, obesity,13 and hypertension,14 which are confounding factors that might affect LV mass and function. If the effect of age is to be considered, there is only one cross-sectional study that has examined an extensive senior population12; however, subjects with previous cardiac disease were included. In the study by Chami et al., the authors examined 2,058 participants of the Sleep Heart Health Study (aged 65 ± 12 y) by echocardiography. It was found that only subjects with an AHI ≥ 30 and significant nocturnal hypoxemia showed an increased risk of LVH.14
The principal objective of the current study was to examine the association between structural and functional cardiac changes in a population-based sample of healthy elderly with unrecognized obstructive SDB, as well as the clinical and polygraphic factors implicated in this association.
METHODS
Participants
Participants were recruited from a population-based cohort of 1,011 volunteers aged > 65 years, residing in the city of Saint-Etienne (France), and enrolled in the PROOF survey (PROgnostic indicator OF cardiovascular and cerebrovascular events).15 This is an ongoing study on the prognostic value of autonomic nervous system activity indica tors for cerebrovascular and cardiovascular morbidity and mortality. An ancillary study (the SYNAPSE study) addressing the association between SDB (assessed by home-based polygraphy) and cardiovascular consequences, during a 7-year follow-up period, was proposed to the members of the PROOF cohort. The criteria for inclusion in the SYNAPSE study were the absence of previous myocardial infarction or stroke, heart failure, insulin-dependant diabetes mellitus, atrial fibrillation, anti-arrhythmic treatment, and/or other severe medical disease. From the selected cohort of 826 subjects, volunteers accepting echocardiography (n = 480) were examined. Subsequent to exclusion of subjects with ineligible echocardiographic results (n = 45) and/or an ambulatory respiratory recording period < 5 h (n = 30), complete datasets were available for 405 subjects aged 68.8 ± 0.9 years, following completion of clinical, polygraphic, and instrumental cardiovascular assessments. The selected group of 405 subjects did not differ from the original sample of 826 subjects in terms of age, gender, or polygraphic data.
The PROOF and the SYNAPSE studies were approved by the University Hospital and the local ethics committee (CCPRB Rhone-Alpes Loire). The National Committee for Information and Liberty (CNIL) granted consent for data collection. All subjects provided written consent prior to participation in the study.
Clinical Assessment
Clinical evaluation was assessed by a standard interview, which included history of cardiac and cerebrovascular disease, hypertension, diabetes, as well as respiratory, neurological, and psychiatric disorders. Current smoking patterns, habitual alcohol consumption and current medication use were analyzed, the latter focusing on antihypertensive, anti-dyslipidemic, and hypoglycemic treatment. The body mass index (BMI), calculated as weight (kg)/height squared (m2) was measured.
Blood Pressure Assessment
Diurnal systolic (SBP) and diastolic (DBP) blood pressure were measured by a physician using a standard mercury sphygmomanometer with cuff placement on the right arm while the subject was in supine position following a 5-min period of rest. Ambulatory blood pressure monitoring (ABPM) was assessed over a 24-h period by an ambulatory recording device using the auscultatory method (Diasys Integra, Novacor, Rueil-Malmaison, France); measurements were taken at 15-min intervals during the day and 30-min intervals during the night, with cuff placement on the non-dominant arm. The diurnal and nocturnal periods were defined on the basis of the reported time when participants were lying down with the lights switched off. Average values of SBP and DBP were calculated for a 24-h period as well as for the diurnal and nocturnal phase. Subjects were defined as normotensive if a history of hypertension (HT) and antihypertensive treatment was not reported and did not demonstrate a diurnal mean SBP ≥ 135 mm Hg or a mean DBP ≥85 mm Hg during 24-h ambulatory blood pressure monitoring.
Ambulatory Respiratory Monitoring
All subjects underwent unattended ambulatory respiratory monitoring using a polygraphic system (HypnoPTT, Tyco Healthcare, Puritan Bennett, Overland Park, Kansas, USA). The parameters examined included sound emission, electrocardiogram, pulse transit time, airflow measured by nasal pressure, respiratory effort and body position. Oxygen saturation (SpO2) was measured by pulse oximetry. Recordings were considered fit for analysis if monitoring duration was ≥ 5 h. All studies were visually validated and manually scored for respiratory events and nocturnal SpO2 according to standard criteria by a single scorer (FR). Intrascorer reliability was 89%. According to the Chicago criteria, hypopnea was defined as ≥ 50% reduction in airflow from the baseline value lasting ≥ 10 sec by itself or associated with ≥ 3% oxygen desaturation.19 Apnea was defined as the absence of airflow > 10 sec on nasal cannula. The absence of ribcage movements associated with apnea defined the event as central, while a progressive increase in pulse transit time and respiratory efforts allowed definition of the episode as obstructive. To minimize potential overestimation of sleep duration, subjects completed a “sleep diary” to mark begin- and endpoints for analysis mirroring lights-off and lights-on. The apnea-hypopnea index (AHI) was defined as the ratio of the number of episodes of obstructive apnea or hypopnea per hour of reported sleeping time. The indices of nocturnal hypoxemia were as follows: mean SpO2; percentage of recording time spent with SpO2 < 90%; minimum SpO2 value recorded during sleep (minimum SpO2) and oxygen desaturation index (ODI), i.e., the number of episodes of oxyhemoglobin desaturation per hour of reported sleep time during which blood oxygen level fell by ≥ 3%. In accordance with a previous study,12 subjects were stratified in 4 groups: no-SDB cases (AHI < 5), mild (AHI: 5–15), moderate (AHI: 15–30), and severe SDB (AHI > 30) cases with ≥ 85% of respiratory events scored as obstructive.
Echocardiography
In accordance with the guidelines of the American Society of Echocardiography,16 a standard M-mode, 2-dimensional, Doppler echocardiography (Vivid 7, Philips Medical System Cleveland, OH, USA) was performed on all participants. Parameters were measured from 3 cardiac cycles. All studies were performed by an investigator blinded to the SDB status of the subject. The LV end-diastolic and end-systolic dimension and LA diameter were measured. LV mass was calculated by the validated formula described by Devereux et al.,17 normalized for body surface area and additionally expressed as LV mass index in g/m2. Using previously published thresholds, LVH was defined as an LV mass index > 48 g/m2 for men and > 44 g/m2 for women.18 The LV ejection fraction was measured as a LV systolic function parameter with the normal value set at > 55%. Left atrial (LA) mass index, surface, and diameter were also calculated.
Pulsed Doppler measurements of LV diastolic inflow were obtained under 2-dimensional echo-guidance. The ratio of early peak filling velocity (E) to atrial peak velocity (A) (i.e., E/A ratio) was calculated based on mitral inflow velocities determined by Doppler echocardiography. Pulmonary systolic arterial pressure was derived with peak velocity of the tricuspid regurgitation jet by continuous-wave Doppler together with systolic right atrial pressure.
Statistical Analyses
Study population characteristics are reported as means ± standard deviation for continuous variables, and counts and percentages for categorical variables. Differences between groups were examined with the χ2 test for categorical variables, the Kruskal-Wallis test for skewed continuous variables, and ANOVA for normally distributed continuous variables. Pearson correlation coefficients were calculated to assess the relation between LV and LA mass and function, as well as the various clinical and polygraphic parameters. Multiple regression analysis was used wherein the dependent variables were LV and LA disturbances, while the independent variables were gender, hypertension, AHI, and ODI.
Data were analyzed using the Statistical Package for the Social Sciences version 17 for Windows (SPSS Inc., Chicago, IL, USA) and Stata release 11 (Stat Corp, College Station, TX, USA). All reported p values are two-tailed, with the threshold of statistical significance set at p < 0.05.
RESULTS
Table 1 shows the demographic and clinical characteristics of the study sample and recorded differences between groups. The 405 participants included in this study had an average age of 68.8 ± 1 years, with a gender distribution of women (59%) versus men (41%) and a mean BMI of 25.2 ± 3.5 kg/m2. Of the population studied, 4% had type 2 diabetes, 38% had dyslipidemia, and 42% had hypertension. The mean AHI was 23.0 ± 17.3 and the mean ODI 8.3 ± 8.6. Subjects without SDB represented 8% of the total population, while 92% displayed SDB, 32%, 33%, and 27% being, respectively, mild, moderate, and severe cases (based on AHI).
Table 1.
Clinical, anthropometric and poly-graphic data for the total group and the groups of subjects stratified according to the apnea-hypopnea index (mean ± SD).
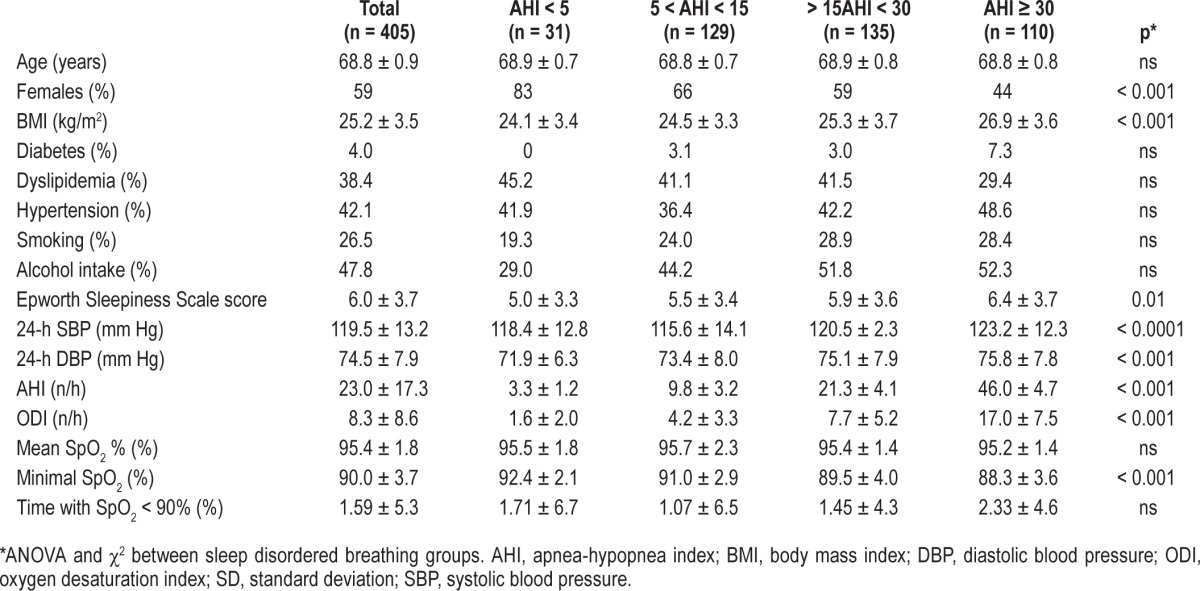
Comparative analysis between SDB groups revealed that severe cases were more frequently men, having higher 24-h blood pressure values with no differences for metabolic values, alcohol consumption, and smoking.
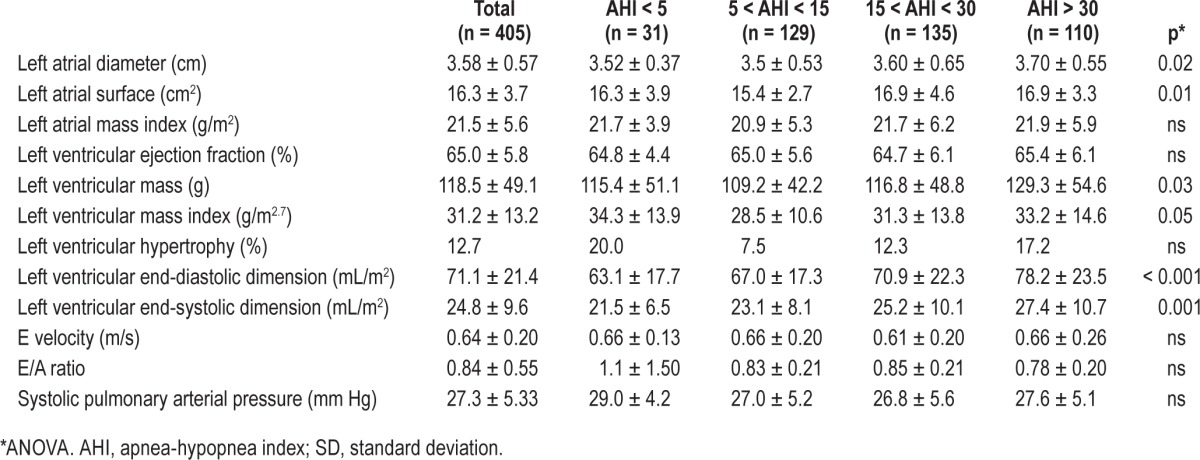
Table 2 shows the echocardiographic measurements for the 4 SDB groups. Comparative analysis revealed a tendency to progressive increase of LA diameter (p = 0.02) and surface (p = 0.01) according to the AHI severity, with no differences in LA mass index. LV end-diastolic and end-systolic dimension (p < 0.001) were significantly increased in subjects with AHI > 30, as well as LV mass (p = 0.03) and LV index (p = 0.05). No differences were found in the other cardiac measurements, in particular the prevalence of LV hypertrophy.
Table 2.
Echocardiographic data for the four groups of subjects stratified according to the apnea-hypopnea index (mean ± SD).
The Pearson correlation analysis showed that LV end-diastolic dimension (r = 0.258, p < 0.001), LV end-systolic dimension (r = 0.205, p < 0.001), LA diameter (r = 0.152, p = 0.003), and LA surface (r = 0.156, p = 0.006) were correlated with AHI. In regard to ODI, we found an association with LA diameter (r = 0.233, p < 0.001) and surface (r = 0.197, p < 0.001), LA volume index (r = 0.138, p < 0.001), LV end-diastolic dimension (r = 0.158, p = 0.002), and LV end-systolic dimension (r = 0.135, p = 0.007). No relationship was found between AHI, ODI, and LV mass index.
Multivariate regression analyses adjusted for age, gender, BMI, hypertension, AHI, and ODI were performed to assess the contribution of these variables on LA and LV measurements. For the LV end-diastolic dimension, BMI (p < 0.0001), gender (p < 0.0001), and AHI (p = 0.002) explained 3.7% of the recorded variance. Similar results were obtained for the LV end-systolic dimension, with the BMI (p < 0.001), gender (p < 0.001), and AHI (p = 0.05) explaining the 2.6% in variance. For the LA dimension and LA surface, BMI (p < 0.0001), and gender (p < 0.0001) were the only factors affecting the measurements without significant contribution of AHI and indices of nocturnal hypoxemia.
DISCUSSION
This study aims to investigate the impact of unrecognized SDB on cardiac function and remodeling in healthy elderly without preexisting cardiac disease, as well as the factors implicated in these modifications. Firstly, we found that in healthy SDB subjects, the most important remodeling was present in LV dimensions, with a slight effect on LA structure and dimension, as well as on LV mass. Secondly, as previously described, the presence of severe SDB appears to have a greater cardiac dysfunctional effect on the left ventricular dimension. Finally, despite a weak but significant association between AHI, ODI, and the above cardiac changes, the most important factors explaining the cardiac LV and LA modifications were BMI and gender, with a minor contribution of AHI. The above findings suggest that in healthy elderly, specifically overweight men with severe AHI, the presence of SDB affects cardiac structure and function. Therefore, this predisposed group of SDB elderly might be considered at a higher risk for vascular consequences and require appropriate treatment.
Among the mechanisms underlying cardiac dysfunction in subjects with SDB, hypoxemia, increased sympathetic activity and acute hemodynamic changes4,11,19 associated with apnea contribute to the reported cardiac structural end functional alterations. Moreover, the elevated negative intrathoracic pressure18 due to upper airway collapse and the consequent mechanical stretching of the LA wall,19–20 the increased trans-mural cardiac pressure, and ventricular wall tension and after-load21 may explain LV remodeling and diastolic and systolic cardiac dysfunction.
Despite a trend for progressive increase in LA and ventricular dimensions in more severe SDB cases, the present study did not confirm previous data12 showing a key role of AHI and ODI in a large epidemiological sample (n = 2,058 participants) of the Sleep Heart Health Study (aged 65 ± 12 y). The different in results may be explained by methodological differences. The Chami study considered only left ventricular hypertrophy as the most important cardiac variable without discussing other cardiac measurements in a sample recruited by different centers and for which no clinical, HT, and metabolic data were available.12 Moreover, in contrast to the study sample described here, the majority of the participants (54.5%) showed an AHI < 5, with only 11% and 5%, respectively, falling in the moderate and severe groups, which represents different case characteristics. A recent paper on a group of 79 middle-aged SDB patients found no significant differences between patients with and without SDB for some cardiac parameters with a weak association between REM sleep, AHI, and LV diastolic dysfunction.25 This suggests that even in middle-aged patients, the presence of SDB does not strongly affect cardiac function.
To explain the weak association between AHI, hypoxemia severity, and cardiac morphology and function in our sample of higher average age, we can suggest a possible interference of both physiological cardiac changes occurring with age and the presence of compensatory mechanisms to prevent increased upper airway collapsibility22,23 in the elderly with SDB. It is known that the increased age-related collapsibility of the upper airways24 explains a higher percentage of SDB, the rise in apnea-hypopnea duration,25 and sometimes hypoxemia severity in the elderly. In contrast to expectations, the healthy elderly examined in this study did not show severe hypoxemia, the percentage time of SpO2 < 90% being not different between mild and severe cases. Thus, the impact of hypoxemia on cardiac function appears reduced in healthy elderly. In this study, the presence of moderate or severe levels of AHI without severe nocturnal hypoxemia is similar to those recently reported.26 This supports the hypothesis of a different phenotype in elderly SDB patients in whom compensatory mechanisms in the arousal threshold and cortical excitability prevent the pathological consequences of a chronic intermittent hypoxia.27
When the role of intrathoracic effort on the LA and LV wall stretching is considered, it is important to realize that there are mechanical changes in the elderly to prevent upper airway collapse. An experimental study on male rats28 demonstrated that chronic intermittent hypoxia affects the sternohyoid muscle structure and function in aged male rats, suggesting a structural muscle remodeling that improves hypoxic tolerance with age. In 40 patients with SDB and/or upper airway resistance syndrome and in 116 patients with mild to severe SDB, the respiratory efforts developed to counteract upper airway occlusion decreased with age, suggesting a reduced role of the mechanical thoracic effect on the left atrial and ventricular function in elderly.34,35
Strengths and Limitations
The strengths of this study include relatively large sample size, use of extensive clinical and cardiovascular assessment, and analyses performed in elderly without arrhythmia or cardiovascular disease. However, some discussion points on the methodology must be mentioned. Beyond its cross-sectional design, some measurements of cardiac function were not performed, such as septal and E/e' ratio, as well as more specific markers of LV diastolic function. Moreover, ambulatory respiratory recording during sleep was used with possible under- or overestimation of the disease severity. Despite these limitations, the strict method used to score ambulatory polygraphy and the exclusion criteria to define recordings as being available for analysis enabled an accurate estimate of SDB.
CONCLUSION
In a large, population-based sample of healthy elderly we found that overweight men with severe SDB had a slight but significant increase of the left atrial and ventricular dimensions. The age-related changes in cardiac function and mechanical respiratory load might explain the weak association between cardiac dysfunction versus AHI and indices of hypoxemia. However, the cardiac changes in severe SDB subjects stress the need to diagnose this high-risk population to prevent increased morbidity and mortality, as well as to propose efficacious treament.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by a grant from the French Ministry of Health (Cellule Projet Hospitalier de Recherche Clinique National, Direction de la Recherche Clinique, CHU Saint-Etienne; Appel d'Offre 1998 and Appel d'Offre 2002), and by a grant from “L'Association de Recherche SYNAPSE” (President: Michel Segura). The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The PROOF study group thanks all the subjects included in the present study as well as M. Saint-Martin, MSc; Y. Jamon, MD; S. Bayle, MD; M. Reynaud, MD; and L. Khris, MD for their expert assistance.
ABBREVIATIONS
- A
atrial peak velocity
- AHI
apnea-hypopnea index.
- BMI
body mass index
- DBP
diastolic blood pressure
- E
early peak filling velocity
- E/A ratio
early peak filling velocity to atrial peak velocity
- LA
left atrial
- LV
left ventricular
- LVH
left ventricular hypertrophy
- ODI
oxygen desaturation index
- SBP
systolic blood pressure
- SDB
sleep disordered breathing
REFERENCES
- 1.Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult: a mini-review. Gerontology. 2010;56:181–9. doi: 10.1159/000236900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Nieto FJ, Redline S, et al. Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;62:893–90. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb DJ, Yenokyan G, Newman AB, et al. A prospective study of obstructive sleep apnea and incidental coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNicholas WT, Bonsignore MR. Sleep apnea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–78. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi T, Yoshioka T, Hasegawa K, et al. Inhalation of hydrogen gas attenuates left ventricular remodeling induced by intermittent hypoxia in mice. Am J Physiol Heart Circ Physiol. 2011;301:H1062–9. doi: 10.1152/ajpheart.00150.2011. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi T, Yamashita C, Matsumoto C, et al. Role of gp91phox-containing HADPH oxydase in left ventricular remodelling induced by intermittent hypoxic stress. Am J Physiol Heart Circ Physiol. 2008;294:H2197–203. doi: 10.1152/ajpheart.91496.2007. [DOI] [PubMed] [Google Scholar]
- 7.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation and much more. Am J Respir Crit Care Med. 2008;77:369–75. doi: 10.1164/rccm.200608-1190PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berman EJ, Di Benedetto RJ, Causey DE, et al. Right ventricular hypertrophy detected by echocardiography in patients with newly diagnosed obstructive sleep apnea. Chest. 1991;100:347–50. doi: 10.1378/chest.100.2.347. [DOI] [PubMed] [Google Scholar]
- 9.Khan A, Latif F, Hawkins B, Tawk M, Sivaram CA, Kinasewitz G. Effects of obstructive sleep apnea treatment on left atrial volume and left atrial volume index. Sleep Breath. 2008;12:141–7. doi: 10.1007/s11325-007-0142-x. [DOI] [PubMed] [Google Scholar]
- 10.Usui Y, Takata Y, Inoue Y, et al. Severe obstructive sleep apnea impairs left ventricular diastolic function in non-obese men. Sleep Med. 2013;4:155–9. doi: 10.1016/j.sleep.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Chami HA, Devereux RB, Gottdiener JS, et al. Left ventricular morphology and systolic function in sleep-disordered breathing. The Sleep Heart Health study. Circulation. 2008;117:2599–607. doi: 10.1161/CIRCULATIONAHA.107.717892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koga S, Ikeda T, Yasunaga T, Maemura K. Effect of nasal continuous positive airway pressure on left ventricular concentric hypertrophy in obstructive sleep apnea syndrome. Intern Med. 2012;51:2863–8. doi: 10.2169/internalmedicine.51.8062. [DOI] [PubMed] [Google Scholar]
- 13.Libhaber CD, Norton GR, Majane OH, et al. Contribution of central and general adiposity to abnormal left ventricular diastolic function in a community sample with a high prevalence of obesity. Am J Cardiol. 2009;104:1527–33. doi: 10.1016/j.amjcard.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Gao J, Hua Q, Li J, Wang CR. Incremental effect of obstructive sleep apnea syndrome on right and left ventricular myocardial performance in newly diagnosed essential hypertensive subjects. Hypertens Res. 2009;32:176–81. doi: 10.1038/hr.2008.22. [DOI] [PubMed] [Google Scholar]
- 15.Barthélémy JC, Pichot V, Dauphinot V, et al. Autonomic nervous system activity and decline as prognostic indicators of cardiovascular and cerebrovascular events. The PROOF Study. Neuroepidemiology. 2007;29:18–28. doi: 10.1159/000108914. [DOI] [PubMed] [Google Scholar]
- 16.American Society of Echocardiography Report. Recommendation for quantification of Doppler Echocardiography: report from the Doppler Quantification Task Force of the nomenclature and standards committee of the American Society of Echocardiography J Am Soc Echocardiogr. 2005;18:1440–63. [Google Scholar]
- 17.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 18.Koshino Y, Villaraga H, Orban M, et al. Changes in left and right ventricular mechanisms during the Mueller maneuver in healthy adults. Circ Cardiovasc Imaging. 2010;10:282–9. doi: 10.1161/CIRCIMAGING.109.901561. [DOI] [PubMed] [Google Scholar]
- 19.Camen G, Clarenbach CF, Stöwhas AC, et al. The effects of simulated obstructive apnea and hypopnea on arrhythmic potential in healthy subjects. Eur J Appl Physiol. 2013;1132:489–96. doi: 10.1007/s00421-012-2457-y. [DOI] [PubMed] [Google Scholar]
- 20.Kim SM, Cho KI, Kwon JH, Lee HG, Kim TI. Impact of obstructive sleep apnea on left atrial functional and structural remodeling beyond obesity. J Cardiol. 2012;60:475–83. doi: 10.1016/j.jjcc.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Buda AJ, Pinsky MR, Ingels NB, Jr, Stinson EB, Alderman EL. Effect of intra-thoracic pressure on left ventricular performance. New Engl J Med. 1979;301:453–9. doi: 10.1056/NEJM197908303010901. [DOI] [PubMed] [Google Scholar]
- 22.Malhotra A, Huang Y, Fogel R, et al. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med. 2006;119:72e9–14. doi: 10.1016/j.amjmed.2005.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlisle T, Carthy ER, Glasser M, et al. Upper airway factors that protect against obstructive sleep apnea in healthy older males. Eur Respir J. 2014;44:685–93. doi: 10.1183/09031936.00177213. [DOI] [PubMed] [Google Scholar]
- 24.Edwards BA, Wellman A, Sands SA, Owens RL, Eckert DJ, White SP. Obstructive sleep apnea in older adults is a distinctly different physiological phenotype. Sleep. 2014;37:1227–36. doi: 10.5665/sleep.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung S, Yoon IY, Lee CH, Kim IW. Effects of age on the clinical features of men with obstructive sleep apnea syndrome. Respiration. 2009;78:23–9. doi: 10.1159/000218143. [DOI] [PubMed] [Google Scholar]
- 26.Palma JA, Fernandez S, Valencia M, Alegre M, Aerieda J, Urrestarazu E. Characterizing the phenotypes of obstructive sleep apnea: clinical, sleep and autonomic features of obstructive sleep apnea with and without hypoxia. Clin Neurophysiol. 2014;125:1783–91. doi: 10.1016/j.clinph.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 27.Joo EY, Kim YH, Koo DL, Hong SB. Altered cortical excitability in patients with untreated sleep apnea syndrome. Sleep Med. 2010;11:857–61. doi: 10.1016/j.sleep.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Skelly JR, Edge D, Schortt CM, Jones JF, Bradford A, O'Halloran KD. Respiratory control and sternohyoid muscle structure and function in aged male rats: decreased susceptibility to chronic intermittent hypoxia. Respir Physiol Neurobiol. 2012;80:175–82. doi: 10.1016/j.resp.2011.11.004. [DOI] [PubMed] [Google Scholar]


