Abstract
Study Objectives:
Obstructive sleep apnea (OSA) can be severe and present in higher numbers during rapid eye movement (REM) than nonrapid eye movement (NREM) sleep; however, OSA occurs in NREM sleep and can be predominant. In general, ventilation decreases an average 10% to 15% during transition from wakefulness to sleep, and there is variability in just how much ventilation decreases. As dynamic changes in ventilation contribute to irregular breathing and breathing during NREM sleep is mainly under chemical control, our hypothesis is that patients with a more pronounced reduction in ventilation during the transition from wakefulness to NREM sleep will have NREM- predominant rather than REM-predominant OSA.
Methods:
A retrospective analysis of 451 consecutive patients (apnea-hypopnea index [AHI] > 5) undergoing diagnostic polysomnography was performed, and breath-to-breath analysis of the respiratory cycle duration, tidal volume, and estimated minute ventilation before and after sleep onset were examined. Values were calculated using respiratory inductance plethysmography. The correlation between the percent change in estimated minute ventilation during wake-sleep transitions and the percentage of apnea-hypopneas in NREM sleep (%AHI in NREM; defined as (AHI-NREM) / [(AHI-NREM) + (AHI-REM)] × 100) was the primary outcome.
Results:
The decrease in estimated minute ventilation during wake-sleep transitions was 15.0 ± 16.6% (mean ± standard deviation), due to a decrease in relative tidal volume. This decrease in estimated minute ventilation was significantly correlated with %AHI in NREM (r = −0.222, p < 0.01).
Conclusions:
A greater dynamic reduction in ventilation back and forth from wakefulness to sleep contributes to the NREM predominant OSA phenotype via induced ventilatory instability.
Citation:
Yamauchi M, Fujita Y, Kumamoto M, Yoshikawa M, Ohnishi Y, Nakano H, Strohl KP, Kimura H. Nonrapid eye movement-predominant obstructive sleep apnea: detection and mechanism. J Clin Sleep Med 2015;11(9):987–993.
Keywords: NREM-predominant OSA, obstructive sleep apnea, respiratory physiology during sleep
Rapid eye movement (REM)-predominant obstructive sleep apnea (OSA) is a phenotype recognized and described in females as a function of age and obesity.1,2 In general, upper airway obstruction and hypopnea is in REM sleep when upper airway muscle tonus is decreased as compared to nonrapid eye movement (NREM) sleep. However, OSA occurring predominantly in NREM sleep or equally in a numeric manner in both REM and NREM sleep is also encountered in clinical practice; neither prevalence nor mechanisms driving the relative distribution of REM and NREM events have been considered in any detail. In NREM sleep, detailed physiologic studies detect dynamic changes in ventilatory control during state transitions, which are considered to induce irregular breathing, and lead to obstructive apnea and/or hypopnea.3–7 Detailed studies in REM sleep are absent, leaving an opportunity for the clinical polysomnogram to inform not only REM-predominant OSA but the possibility of NREM predominant OSA as well.
Ventilation decreases an average of 10% to 15% during the transition from wakefulness to sleep due mainly to a decrease in tidal volume; however, the literature reports substantial variability among individuals.8–11 Thus, during the transition from wakefulness to sleep the individual who might have a greater decrease in ventilation would likely have a greater increase in PaCO2, all other things being equal. Although this might be an innate trait, the result will be variations in chemosensitivity and ventilation in the initiation of sleep across a population.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea (OSA) that is predominant during REM sleep is well recognized as a result of muscle atonia; however, NREM-predominant OSA is encountered and its mechanisms are unexplored.
Study Impact: The phenotype of NREM-predominant OSA is present in 17.9% of patients and is correlated with the decrease in estimated minute ventilation during transition from wakefulness to sleep. In clinical practice, although REM-predominant OSA represents the state-related decline in muscle activation, NREM-predominant OSA has different implications related to the dynamics of respiratory control.
The breathing patterning during NREM sleep is composed of mainly chemical feedback control, whereas nonchemical or behavioral factors affecting respiratory drive and muscle tone contribute to breathing patterns during REM sleep. Thus, the individual who has a greater decrease in ventilation in the transitions to NREM sleep might show irregular breathing, especially in the presence of arousals both at sleep onset and during NREM sleep. This phenomenon could cause an apparent high gain in the system, because when arousal occurs, breathing would increase further and fall more with subsequent sleep, producing variations in breathing patterns over time. In other words, the magnitude of decrease in ventilation with sleep may be a factor in determining subsequent breathing patterns and abnormalities such as apneas and hypopneas at sleep onset.
Accordingly, the hypothesis for this study is that patients who have a greater reduction in ventilation during the transition from wakefulness to sleep have a tendency for a disproportionate expression of apnea-hypopnea predominantly in NREM sleep.
METHODS
Subjects
Using an existing database of clinical studies collected in a standard fashion from 2010 to 2011, 451 consecutive patients who had diagnostic polysomnography (PSG) and an apneahypopnea index (AHI) ≥ 5 in the Center for Sleep Disorders, Tenri City Hospital were enrolled for analysis. All patients had agreed that clinical data could be used for research and had completed written informed consent. The Human Subjects Ethics Committee of Tenri City Hospital approved the study (No. 003).
Analysis of the Respiratory Signal
The methods for extracting and analyzing respiratory data have been previously reported.12,13 In this regard, in addition to the standard PSG biocalibration, the ribcage and abdominal signals were tuned in terms of relative movement during breathing and simulated central and obstructive apneas, and by setting equivalent excursions during an isovolume maneuver. For the analysis, at least 1 min of stable respiratory signal data before and after sleep onset in the same body posture, which was not necessarily supine but most cases were in supine position, were extracted from the diagnostic PSG. These criteria were used to eliminate the confounding effects of a change in posture or relative motion of the ribcage and abdomen. During the breathing before sleep onset, wakefulness was confirmed with alpha rhythms in electroencephalography (EEG). Respiratory signals were generated by the sum of chest and abdominal signals using uncalibrated respiratory inductance plethysmography (RIP), an accepted surrogate for measures of breathing in the absence of direct flow measurements at the nose or mouth.14 Electromyography (EMG; chin and limb) was observed to detect movement; an episodically elevated EMG signal was considered to denote movement and was excluded from analysis.
Preliminary analyses addressed the reliability of a relative change in the respiratory signal over the time of interest. The sample was eight patients with > 10 min of wakefulness prior to 5 min of continuous sleep, as defined by epoch scoring, and without a change in posture. Records were examined for minute-to-minute frequency and tidal volume and values in transition from wakefulness to sleep. No significant differences (p = 0.765) were found comparing the average of 10-min segments to any 1-min segment. Thus, in the selection of studies for this study records would have up to 10 min of wakefulness before NREM sleep onset, but comparisons were made only to the first minute of NREM sleep, assured that no change in body posture could have occurred.
In the analytic phase of the study, the investigator was blinded to the patients' characteristics as well as the sleep disordered parameters of the diagnostic PSG. Breathing (at least 1 min before and 1 min after sleep onset) was analyzed breath-to-breath by determining the total duration of the respiratory cycle (Ttot), tidal volume (VT), and our surrogate of minute ventilation (60 / Ttot × VT). Percent change in Ttot, VT, and our surrogate of minute ventilation before and after sleep onset was obtained using the following formula, [(value after sleep onset) − (value before sleep onset)] / (value before sleep onset) × 100. The measurement was determined using the extracted section of respiratory signal only at the first sleep onset.
Sleep Study
Data acquisition started from 21:00 and continued until 06:00 on the following morning. PSG was performed using a polygraph system (EEG7414; Nihon Kohden, Tokyo, Japan). EEG (C3-A2, C4-A1, O1-A2, O2-A1), bilateral electrooculogram (EOG), submental EMG, electrocardiograph (ECG), and bilateral anterior tibial EMG were recorded. Airflow was monitored using an oronasal thermal sensor and/or nasal air pressure transducer. Thoracic and abdominal respiratory movements were monitored using RIP (Respitrace; Ambulatory Monitoring Inc., Ardsley, NY, USA). Oxyhemoglobin saturation and pulse rate were monitored using pulse oximetry with a finger probe (OLV-3100; Nihon Kohden, Tokyo, Japan). All the signals were digitized and stored on a personal computer. Apneas were defined as an episode of complete airflow cessation measured from the thermal sensor lasting more than 10 sec. Hypopnea was defined by ≥ 50% reduction in amplitude of the RIP sum signal or nasal pressure signal lasting more than 10 sec with ≥ 3% oxygen desaturation and/or arousal. Arousal index was scored according to the rules of the American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events, 2007.15
AHI was calculated as the number of apnea and hypopnea events per hour of sleep. AHI-NREM was calculated as the number of apnea-hypopnea events per hour of NREM sleep and AHI-REM was calculated as the number of apnea-hypopnea events per hour of REM sleep. A value of %AHI in NREM, defined as (AHI-NREM) / [(AHI-NREM)+(AHI-REM)] ×100, was calculated for each patient in order to (1) represent the relative expression of OSA in NREM and REM sleep and (2) calculate a value that would be more likely to represent the influence of chemical drive. For example, when the value of %AHI in NREM is closer to 100%, it means that a patient expresses NREM-predominant OSA, whereas the patients whose value of %AHI in NREM is closer to 0% would mean REM-predominant OSA.
The Definition of NREM- or REM-Predominant OSA
REM-predominant OSA has been defined in previous studies as AHI-REM/AHI-NREM > 2.1,2,16 However, in the current study, we adopted the aforementioned formula to avoid division by zero, which is in case AHI-NREM is zero. REM-predominant OSA defined by AHI-REM/AHI-NREM > 2 would be equal to %AHI in NREM ≤ 33.3% in our formula. Thus, in the same manner, we defined NREM-predominant OSA as %AHI in NREM ≥ 66.7%, and REM- predominant OSA as %AHI in NREM ≤ 33.3%.
Statistical Analysis
The difference in % change in our surrogate of minute ventilation between the groups of NREM-predominant OSA, REM-predominant OSA, and others was detected by one-way analysis of variance (ANOVA). When the ANOVA was significant, differences between individual groups was sought using t tests of the estimated marginal means (simple main effects) with adjustment for multiple comparisons using the Bonferroni correction. To investigate correlations between the % change in our surrogate of minute ventilation and %AHI in NREM, we performed Spearman correlation analysis. To determine whether % change in our surrogate of minute ventilation was independently associated with %AHI in NREM, multiple regression analysis was performed with %AHI in NREM as the dependent variable. Parameters showed significant univariate association with %AHI in NREM by Spearman correlation analysis were included as independent variables in the regression analysis. The occurrence of apnea-hypopnea as well as sleep stages usually varies with sleeping body position. Thus, analyses were additionally performed according to each body position including supine and lateral position. Data are presented as mean ± standard deviation (SD) in case values that were normally distributed and median (interquartile range) when there was a skewed distribution. Statistical analysis was done with SPSS version 21.0 for Windows software (SPSS, Chicago, IL, USA).
RESULTS
Exclusions and Final Cohort
Nineteen subjects were excluded from the analyses because the REM sleep period was less than 15 min, generally considered too short for NREM/REM analyses2,16; 108 patients were excluded because of inadequate data, i.e. (1) less than 1 min of stable respiratory signal during wakefulness or (2) less than 1 min of stable respiratory signal just after sleep onset. The final analytic sample consisted of 324 patients with 53 women. The age, body mass index (BMI), and AHI of the enrolled 324 patients were 52.3 ± 12.4 y, 25.7 (23.2–28.5) kg/m2, and 24.2 (14.5–38.9), respectively.
Change from Wakefulness to Sleep
Our surrogate of minute ventilation decreased an average of 15% during the transition from wakefulness to sleep with great dispersion between individuals (−15.0 ± 16.6%). The decrease in our surrogate of minute ventilation tended to be greater in males than in females (−15.7 ± 16.5 versus −11.3 ± 16.8, p = 0.075, data not shown). This decrease in our surrogate of minute ventilation was mainly due to a decrease in VT (Table 1).
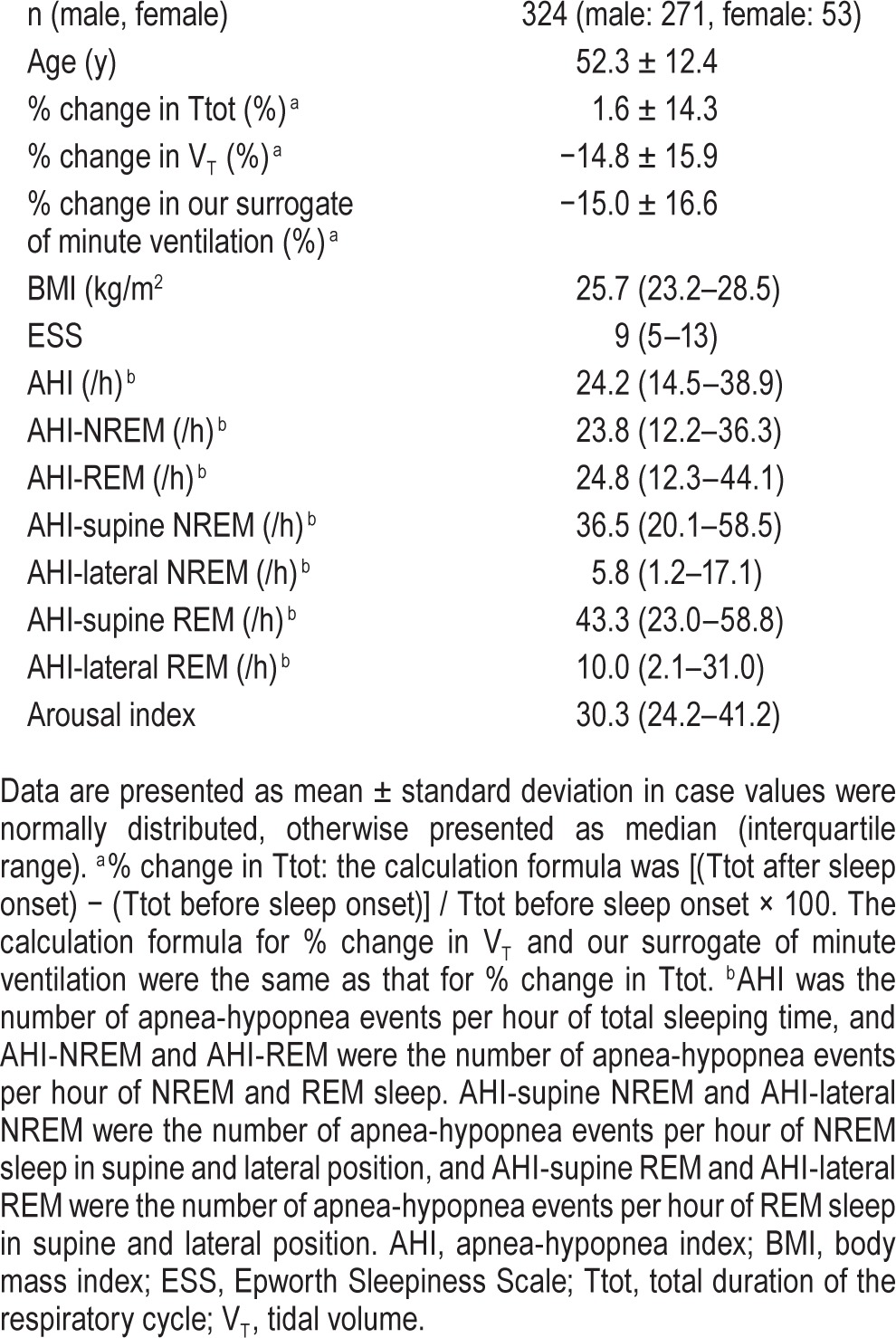
Table 1.
Patient characteristics.
Fall in our Surrogate of Minute Ventilation and State Expression of AHI
Using the aforementioned definitions of NREM-predominant OSA and REM-predominant OSA, the prevalence of NREM- and REM-predominant OSA were 17.9% and 21.3% (58 and 69 of 324 subjects, respectively). Subjects were divided into three groups based on the %AHI in NREM (Figure 1). Decreases in our surrogate of minute ventilation were greatest in NREM-predominant OSA, intermediate in nonspecific OSA, and least in REM predominant OSA; these differences were statistically significant using ANOVA analysis (F = 13.96, p < 0.01). Post hoc analysis demonstrated that % change in our surrogate of minute ventilation in NREM-predominant OSA was significantly greater than REM-predominant OSA (p < 0.01).
Figure 1. The % change in our surrogate of minute ventilation in the transition from wakefulness to sleep for three groups categorized by %AHI in NREM.

Bars represent the mean and standard deviation. The right bar represents those with REM-predominant OSA. The left bar indicates the phenotype of NREM-predominant OSA. There is a significantly different reduction in our surrogate of minute ventilation during transition from wakefulness to sleep. The calculation formula for %change in the surrogate of minute ventilation and %AHI in NREM was: [(minute ventilation after sleep onset) − (minute ventilation before sleep onset)] / (minute ventilation before sleep onset) × 100, or (AHI-NREM) / [(AHI-NREM) + (AHI-REM)] ×100. AHI, apnea-hypopnea index; NREM, nonrapid eye movement; OSA, obstructive sleep apnea; REM, rapid eye movement.
Univariate Associations
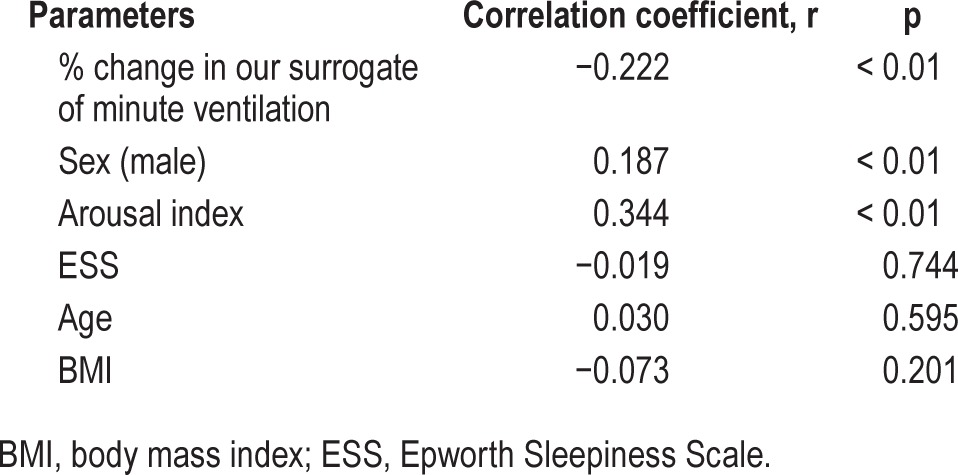
%AHI in NREM was significantly associated with % change in our surrogate of minute ventilation (r = −0.222, p < 0.01), which imply that greater decrease in ventilation during transition from wakefulness to sleep appears to lead to apnea-hypopnea more in NREM rather than in REM sleep (Figure 2A). There were also positive correlations between other parameters including sex (male) and arousal index and %AHI in NREM (r = 0.187, p < 0.01, r = 0.344, p < 0.01, respectively) (Table 2).
Figure 2. The associations between % change in our surrogate of minute ventilation and %AHI in NREM in different sleeping body position.
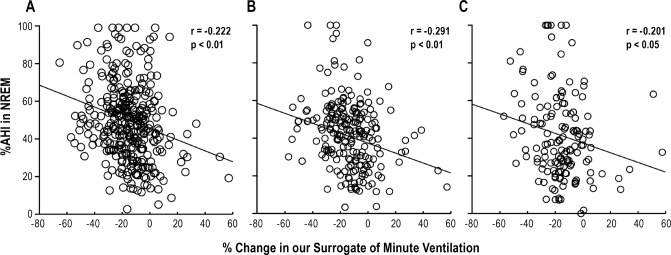
The graphs present the data in both supine and nonsupine body positions (A), in supine body position (B), and in lateral body position (C). % change in our surrogate of minute ventilation: the calculation formula was [(minute ventilation after sleep onset) − (minute ventilation before sleep onset)] / (minute ventilation before sleep onset) × 100. %AHI in NREM: (AHI-NREM) / [(AHI-NREM) + (AHI-REM)] × 100. AHI, apnea-hypopnea index; NREM, nonrapid eye movement; r, correlation coefficient of Spearman's correlation analysis; REM, rapid eye movement.
Table 2.
The univariate association between percentage of the apnea-hypopnea index in nonrapid eye movement and the parameters by Spearman correlation analysis.
Adjustment for Body Position
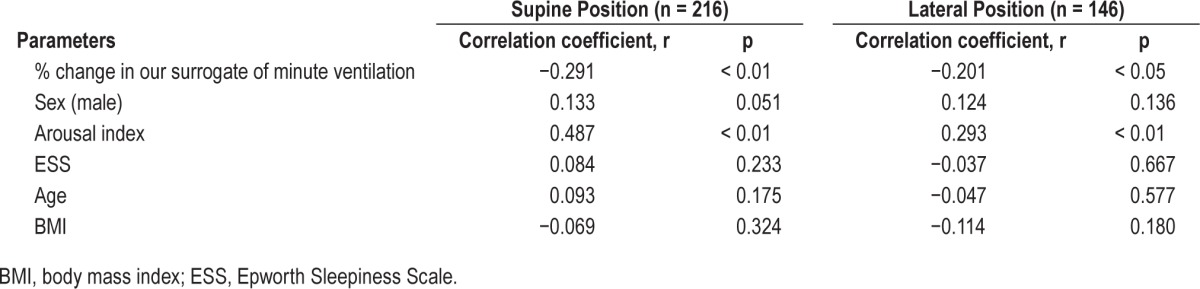
In the analysis of supine and lateral position, 216 patients (177 males and 39 females) who had more than 15 min of supine REM sleep and supine AHI > 5, and 146 patients (121 males and 25 females) who had more than 15 min of lateral REM sleep and lateral AHI > 5 were eligible for the analysis. As shown in Figures 2B and 2C, %AHI in NREM both in supine and lateral sleeping position were significantly associated with % change in our surrogate of minute ventilation (r = −0.291, p < 0.01, r = −0.201, p < 0.05, respectively). The association in the supine position was stronger than associations in the lateral or whole body positions. Moreover, different from the whole body position, sex was no longer associated with %AHI in NREM, but arousal index was still associated with %AHI in NREM both in supine and lateral sleeping position (Table 3).
Table 3.
The correlation coefficients between percentage of the apnea-hypopnea index in nonrapid eye movement in supine and in lateral sleeping position and the parameters by Spearman correlation analysis.
Multiple Regression Analyses
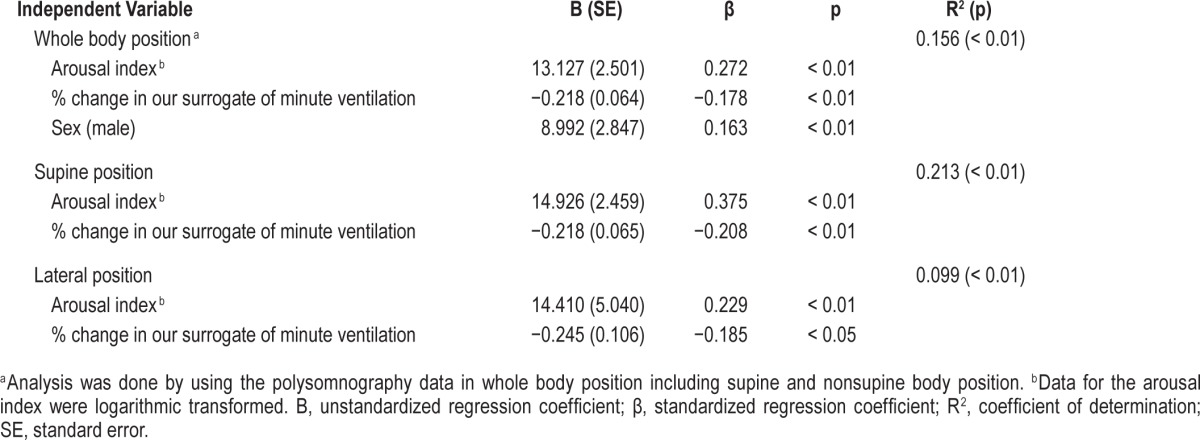
In these models, data for the arousal index underwent lateral log transformation because the values were not normally distributed. As shown in Table 4, % change in our surrogate of minute ventilation as well as arousal index and sex were independently associated with %AHI in NREM in whole body position, whereas significant independent associations between sex and % AHI in NREM were lost in supine and lateral sleeping positions. This implies that major contributors to the NREM predominance of OSA are arousal index and % change in our surrogate of minute ventilation, with a minor influence of sex.
Table 4.
Multiple regression models of percentage of the apnea-hypopnea index in nonrapid eye movement with whole, supine, and lateral sleeping position.
DISCUSSION
Our study confirmed prior reports in small numbers of subjects8–11 that our surrogate of minute ventilation falls approximately 15% during the transition from wakefulness to sleep due to a decrease in tidal volume not respiratory frequency. This largest collection of patients demonstrated a large variance across individuals. The major consequence of a large cohort is the recognition that the magnitude of change in our surrogate of minute ventilation at sleep onset associated with the predominance of apnea-hypopnea events with sleep state, i.e., NREM or REM, and especially of a new phenotype—NREM-predominant OSA. Furthermore, multiple regression analysis showed that our surrogate of minute ventilation during transition from wakefulness to sleep and arousal index independently contributed to the NREM predominant OSA.
We evaluated the change in ventilation before and after sleep onset (wakefulness and NREM sleep) using the RIP sum signal, and confirmed an approximately 15% ventilatory decrease due to the decrease in tidal volume. These findings are supported by the prior studies using more accurate measures, reporting that ventilation decreases approximately 10% to 15% in NREM sleep compared with wakefulness, attributed to a reduced metabolic rate and respiratory muscle function.8–11 Findings in current study include the existence of a dispersion of values (SD = 16.6) among subjects in the uncalibrated RIP change in ventilation during transition from wakefulness to sleep. Although some of this could represent a measurement error, subgrouping could account for effects by position, indicating that differences were not the result of randomness. Such is the value of larger databases when measurements are less precise. Neither a calibrated RIP nor by implication flow measures over the nose and mouth were necessary to identify this OSA phenotype of NREM-predominant events; however, for an individual patient such precise measures could be useful.
Prior literature on stage-specific OSA has focused on REM-predominant events. Koo et al.1,2 reported that REM-predominant OSA is more prevalent in women than in men. Our findings are consistent, showing that female sex was significantly associated with REM-predominant OSA in the univariate regression and that male sex was associated with NREM-predominant OSA. We found a prevalence of REM-predominant OSA of 21.3%, comparable to former studies,1,2,16 and a prevalence of NREM- predominant OSA of 17.9%.
Although there was a statistically significant univariate association between % AHI in NREM and % change in our surrogate of minute ventilation, this association was in the moderate to weak range, which may imply that it is not by itself sufficient to account for the NREM-predominant OSA only with the greater ventilatory decrease at sleep onset. Thus, other factors will affect the distribution of apneic events across sleep stages. In this study, arousal was identified another contributor, implying that greater dynamic changes in ventilation during back and forth between sleep and wakefulness; in another words, assumably dynamic changes in PaCO2 caused by arousal might predispose to recurrent apnea-hypopnea in NREM sleep. In fact, irregular breathing has been shown to lead to obstructive apnea-hypopnea.3–7 In this regard, especially in NREM sleep which is the phase that breathing behavior is controlled mainly by chemical control, when arousal occurs, breathing becomes irregular. Invoking the engineering term “loop gain”, this phenomenon might occur if the respiratory system responded dynamically more to the disturbance, which in this case are the changes in ventilation with sleep onset and arousals. Thus this phenotype could be considered having propensity for ventilatory instability. This idea is partially supported by the recent report by Terrill et al.17 showing that patients with NREM-predominant OSA have high loop gain.
Recently, Wellman and colleagues18,19 developed an OSA phenotyping method, whereby OSA results from four physiologic abnormalities: (1) collapsible upper airway, (2) oversensitive ventilatory control system (a high loop gain), (3) poor pharyngeal muscle response, and (4) low arousal threshold. OSA can result from abnormalities in any of these four physiologic properties. The degree to which there are abnormalities in these physiologic components is variable across individuals. Their concept of OSA phenotyping raises the opportunity for alternatives to continuous positive airway pressure (CPAP) in reducing AHI in selected patients, such as oxygen supply and/or acetazolamide lowering the loop gain and hypnotics elevating the arousal threshold.20–22 We did not directly compare our approach to measures and interventions available only in a physiologic laboratory nor have we estimated these specific phenotypes, but instead demonstrate how signals usually available in clinical polysomnography can be informative. Although the treatment effects were not examined in our current study, one might predict that those who showed greater change in ventilation and in PaCO2 back and forth between sleep and wakefulness, and showed NREM-predominant apneic events might be able to be treated effectively with oxygen supplement therapy and/or hypnotic agents. One study supports this idea that apnea-hypopneas were not well controlled with CPAP during therapeutic titration in patients with NREM-predominant OSA.23
In the current study, analyses were performed with consideration for body position because sleeping body position affects not only expression of OSA but sleep stages including REM and NREM sleep. After adjusting for body position, there was a greater association between % change in our surrogate of minute ventilation and %AHI in NREM (especially the analysis focusing on the supine sleeping position). The analysis of lateral position displayed a weaker association between % change in our surrogate of minute ventilation and %AHI in NREM. One explanation could be a lower AHI in lateral sleeping position compared with supine position and reduced sample size due to lateral AHI ≤ 5 in many cases, and decrease in ventilation at the sleep onset was measured in supine position in most cases. The presence of positional OSA might affect the difference in the associations between % change in our surrogate of minute ventilation and %AHI in NREM in different sleeping position.
There are potential limitations. First, there is the limited ability of RIP-sum signal to only semiquantitatively evaluate VT and a surrogate of minute ventilation. However, the calculation of the decrease in ventilation was done within the same body position before and just after sleep onset; extracted sections of respiratory signal before and after sleep onset were as close as possible to each other; and EMG variations in a given position were examined so as to exclude body movements. Thus, a change in position of the RIP bands is unlikely to produce random noise in the included patients, but there are limitations to applying this approach to all studies. Further, although we used an uncalibrated RIP, the collected parameters were relative change in ventilation within the individual, and analyses were made with relative change between subjects. Thus, the effect of measuring the ventilation caused by using uncalibrated RIP-sum signal could be considered small. Second, one may doubt that ventilatory decrease during transition from wakefulness to NREM sleep is comparable through the night. To clarify these issues, we performed the pilot studies. Percent changes in our surrogate of minute ventilation during the transition from wakefulness to sleep at the first sleep onset and at the midst of sleep in 10 patients were quite similar (p = 0.749, data not shown). Thus, we can secure that ventila-tory decrease at the first sleep onset represents the ventilatory decrease during back and forth from wakefulness to sleep during whole night. Third, looking at Figure 2, despite a mean 15% decrease in ventilation, it is the existence of those with a large change up or down from wakefulness to sleep that is the key finding. Detecting and confirming by different laboratories was beyond the scope of the current study and outcomes dependent on phenotype will require prospective studies in large populations. Fourth, although recent report has demonstrated that obese individuals without apnea exhibited greater pharyngeal muscle responsiveness during sleep compared with their OSA,24 we did not assess upper airway anatomy, physiology, collapsibility and/or compensation; thus, we are not able to exclude the effects of those factors on ventilatory reduction during transition from wakefulness to sleep reflecting NREM- or REM-predominant OSA phenotype. Fifth, there is concern that arousals were used in the definition of hypopnea in the current study; thus, significant correlation between %AHI in NREM and arousal index influenced our results of multiple regression. However, before entry into the multiple regression model, there was no significant correlation between the independent variables of arousal index and % change in our surrogate of minute ventilation (r = −0.090, p = 0.108), which implies both values could be entered independently to determine contribution to the %AHI in NREM. Last, an interventional study will be needed to test whether suppression of arousal, which is a trigger for irregular breathing, disproportionately improves OSA in NREM sleep.
In summary, we conclude that patients who show greater decrease in ventilation during back and forth from wakefulness to sleep, and presumably a greater dynamic change of PaCO2, exhibit unstable breathing. This unstable breathing results in events predominantly in NREM sleep, a state where breathing patterning is dominated by chemical control. In clinical practice, paying attention to the ventilatory decrease at sleep onset in the diagnostic PSG might be helpful for identifying the phenotype of NREM- predominant OSA, with implications for planning the treatment strategy.
DISCLOSURE STATEMENT
This was not an industry supported study. This study is partly supported by Grant-in-Aid for Scientific Research (C) (25515004), Japan. Dr. Strohl is supported in part by the VA Research Service. The authors have indicated no financial conflicts of interest. Drs. Yamauchi, Yoshikawa, Strohl, and Kimura have been responsible for study concept and design, interpretation of the data. Drs. Yamauchi, Strohl, and Kimura have been responsible for drafting and revising the manuscript. Drs. Yamauchi, Fujita, Kumamoto, Ohnishi, and Nakano have been responsible for data acquisition. Drs. Yamauchi, Fujita, and Nakano have been responsible for data analysis.
ACKNOWLEDGMENTS
The authors thank Kaoru Senzaki, RPSGT, for her help with polysomnogram scoring, and Drs. Brian Koo and Reena Mehra for editorial assistance.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- EMG
electromyography
- OSA
obstructive sleep apnea
- PSG
polysomnography
- RIP
respiratory inductance plethysmography
- SD
standard deviation
- Ttot
total duration of the respiratory cycle
- VT
tidal volume
REFERENCES
- 1.Koo BB, Dostal J, Ioachimescu O, Budur K. The effects of gender and age on REM-related sleep-disordered breathing. Sleep Breath. 2008;12:259–64. doi: 10.1007/s11325-007-0161-7. [DOI] [PubMed] [Google Scholar]
- 2.Koo BB, Patel SR, Strohl K, Hoffstein V. Rapid eye movement-related sleep-disordered breathing: influence of age and gender. Chest. 2008;134:1156–61. doi: 10.1378/chest.08-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asyali MH, Berry RB, Khoo MC. Assessment of closed-loop ventilatory stability in obstructive sleep apnea. IEEE Trans Biomed Eng. 2002;49:206–16. doi: 10.1109/10.983454. [DOI] [PubMed] [Google Scholar]
- 4.Hudgel DW, Gordon EA, Thanakitcharu S, Bruce EN. Instability of ventilatory control in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;158:1142–9. doi: 10.1164/ajrccm.158.4.9712105. [DOI] [PubMed] [Google Scholar]
- 5.Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2010;181:189–93. doi: 10.1164/rccm.200810-1658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–32. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–90. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 8.Douglas NJ, White DP, Pickett CK, Weil JV, Zwillich CW. Respiration during sleep in normal man. Thorax. 1982;37:840–4. doi: 10.1136/thx.37.11.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gothe B, Altose MD, Goldman MD, Cherniack NS. Effect of quiet sleep on resting and CO2-stimulated breathing in humans. J Appl Physiol. 1981;50:724–30. doi: 10.1152/jappl.1981.50.4.724. [DOI] [PubMed] [Google Scholar]
- 10.Douglas NJ. Control of breathing during sleep. Clin Sci (Lond) 1984;67:465–71. doi: 10.1042/cs0670465. [DOI] [PubMed] [Google Scholar]
- 11.Phillipson EA. Control of breathing during sleep. Am Rev Respir Dis. 1978;118:909–39. doi: 10.1164/arrd.1978.118.5.909. [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi M, Tamaki S, Yoshikawa M, et al. Differences in breathing patterning during wakefulness in patients with mixed apnea-dominant vs obstructive-dominant sleep apnea. Chest. 2011;140:54–61. doi: 10.1378/chest.10-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamauchi M, Jacono FJ, Fujita Y, et al. Breathing irregularity during wakefulness associates with CPAP acceptance in sleep apnea. Sleep Breath. 2013;17:845–52. doi: 10.1007/s11325-012-0775-2. [DOI] [PubMed] [Google Scholar]
- 14.Redline S, Budhiraja R, Kapur V, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;3:169–200. [PubMed] [Google Scholar]
- 15.Iber C, Ancoli-Israel S, Chesson A, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st ed. [Google Scholar]
- 16.Haba-Rubio J, Janssens JP, Rochat T, Sforza E. Rapid eye movement-related disordered breathing: clinical and polysomnographic features. Chest. 2005;128:3350–7. doi: 10.1378/chest.128.5.3350. [DOI] [PubMed] [Google Scholar]
- 17.Terrill PI, Edwards BA, Nemati S, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J. 2015;45:408–18. doi: 10.1183/09031936.00062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wellman A, Edwards BA, Sands SA, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol. 2013;114:911–22. doi: 10.1152/japplphysiol.00747.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wellman A, Eckert DJ, Jordan AS, et al. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol. 2011;110:1627–37. doi: 10.1152/japplphysiol.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol. 2008;162:144–51. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards BA, Sands SA, Eckert DJ, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590:1199–211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120:505–14. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas RJ, Terzano MG, Parrino L, Weiss JW. Obstructive sleep-disordered breathing with a dominant cyclic alternating pattern--a recognizable polysomnographic variant with practical clinical implications. Sleep. 2004;27:229–34. doi: 10.1093/sleep/27.2.229. [DOI] [PubMed] [Google Scholar]
- 24.Sands SA, Eckert DJ, Jordan AS, et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med. 2014;190:930–7. doi: 10.1164/rccm.201404-0783OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


