Abstract
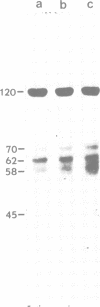
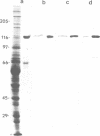
In the present work we have studied the subunit composition of kinesin, the microtubule-activated, mechanochemical ATPase, isolated from bovine brain. Polypeptides with mol. wts of 120 and 62 kd are the major components of the kinesin preparation. These polypeptides could not be separated by electrophoresis under nondenaturing conditions or by FPLC on a MonoQ column, and are therefore assumed to form a tight complex. As shown by immunoblotting with polyclonal and monoclonal antibodies to the 120-kd polypeptide and by one-dimensional peptide mapping, the 62-kd polypeptide does not appear to be a proteolytic product of the 120-kd component. Densitometric scanning of polyacrylamide-SDS gels shows that these polypeptides are present in a complex in a 1:1 molar ratio. The mol. wt of native kinesin was studied by sedimentation equilibrium and was found to be 386 +/- 14 kd. A comparison of the mol. wts of individual polypeptides with the mol. wt of the intact molecule indicates that the native molecule contains two 120-kd subunits and two 62-kd subunits.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos L. A. Kinesin from pig brain studied by electron microscopy. J Cell Sci. 1987 Feb;87(Pt 1):105–111. doi: 10.1242/jcs.87.1.105. [DOI] [PubMed] [Google Scholar]
- Chernyak V. Y., Magretova N. N. An "all-speed" autocalibration method for sedimentation equilibrium in dilute homogeneous and multicomponent solutions. I. Theory, a computation scheme, and verification by computer-simulated experiments. Anal Biochem. 1982 Jun;123(1):101–109. doi: 10.1016/0003-2697(82)90629-7. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Gorovsky M. A., Carlson K., Rosenbaum J. L. Simple method for quantitive densitometry of polyacrylamide gels using fast green. Anal Biochem. 1970 Jun;35(2):359–370. doi: 10.1016/0003-2697(70)90196-x. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S. A., Gelfand V. I. Bovine brain kinesin is a microtubule-activated ATPase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8530–8534. doi: 10.1073/pnas.83.22.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Scholey J. M., Porter M. E., Grissom P. M., McIntosh J. R. Identification of kinesin in sea urchin eggs, and evidence for its localization in the mitotic spindle. Nature. 1985 Dec 5;318(6045):483–486. doi: 10.1038/318483a0. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Vale R., Schnapp B., Schroer T., Reese T. Vesicle movements and microtubule-based motors. J Cell Sci Suppl. 1986;5:181–188. doi: 10.1242/jcs.1986.supplement_5.11. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Zamyatnin A. A. Protein volume in solution. Prog Biophys Mol Biol. 1972;24:107–123. doi: 10.1016/0079-6107(72)90005-3. [DOI] [PubMed] [Google Scholar]