Abstract
Inappropriate sexual behaviour after acquired brain injury is a severe complication. Evidence for effective treatment is not available. Electrical aversion therapy (EAT) is a behavioural therapeutic option used in persons with intellectual disabilities, which might be suitable for brain-injured individuals for whom other therapies are not effective. The effect of EAT in brain injury has not been investigated previously. A single case experimental design was used. In an ABBA (baseline-treatment-treatment-withdrawal) design the frequency of the target behaviour (ie, inappropriate sexual behaviour) in a 40-year-old man was measured daily. A total of 551 measurements were recorded. A significant reduction of the target behaviour was seen after the first treatment phase (baseline 12.18 (2.59) vs 3.15 (3.19) mean target behaviours daily); this reduction remained stable over time. We conclude that EAT was effective in this patient with inappropriate sexual behaviour due to severe brain injury. EAT can therefore be considered in therapy resistant inappropriate sexual behaviour in brain-injured patients.
Background
Brain injury can lead to deficits in different areas of functioning, such as physical, cognitive, emotional, behavioural and social functioning.1 Especially severe behavioural consequences can have a negative impact on the patients’ life and social reintegration.2 Inappropriate sexual behaviour is such a severe behavioural consequence of an acquired brain injury.3
Simpson et al4 reported in a retrospective study, a prevalence of 6.5% inappropriate sexual behaviour in patients with acquired brain injury. Bezeau et al5 showed that 70% of professionals working with patients with traumatic brain injury reported sexual touching as a common problem where 20% reported sexual force as commonly used by their patients. Prevalence numbers differ substantially due to different populations and variation in the definition of inappropriate sexual behaviour.6 For example, Simpson et al4 classified rape and exhibitionism as ‘sexually aberrant behaviours’ while Britton7 listed it as ‘hyper sexuality’.
In this paper, we use the definition proffered by Johnson et al8 based on a detailed literature review. This states that inappropriate sexual behaviour embraces “a verbal or physical act of an explicit, or perceived, sexual nature, which is unacceptable within the social context in which it is carried out”.
Several forms of behavioural treatment have been suggested for the management of inappropriate sexual behaviour. Bezeau et al5 provide an overview of behavioural approaches primarily based on case studies and expert opinions.
Some case studies7 9 report positive effects of pharmacological treatment (ie, medroxyprogesterone acetate).
In the present case, behavioural and drug treatment was first offered, but did not lead to a reduction of the inappropriate sexual behaviour. Electrical aversion therapy (EAT) was then chosen as a possible therapeutic option based on the positive findings with severe behavioural disorders in people with intellectual disabilities.10 EAT offers a clearly determined coincidence between behaviour and aversive consequences which is especially important in people who are cognitively impaired.10 To our knowledge, EAT has not been studied in patients with brain injury.
The aim of this study was therefore to evaluate the effectiveness of EAT in the management of inappropriate sexual behaviour after severe brain injury in a single-case experimental design (SCED) study.
Case presentation
Study design
An ABBA, SCED was used. In a 5-week baseline phase (A1), frequency of target behaviour (ie, sexual inappropriate behaviour as defined by Johnson) was recorded daily. Supervision by the mother and medication did not change during this phase. In phase B1 (13 months), EAT was applied daily. The number of delivered electrical pulses was recorded daily, which was considered to be equal to the occurrence of the target behaviour. The intervention continued and after a measurement interval of 10 months, in phase B2 (3 months) again there were daily recordings of the number of pulses. Finally in phase A2 (2 months), the withdrawal phase, target behaviour frequency was recorded daily once again. The total amounted to over 500 measurements carried out by the mother.
The multiple treatment and baseline phases and the high number of measurements enhanced control of extraneous, potentially confounding factors. Identification of the target behaviour and four experimental phases (baseline, 2×treatment and withdrawal) allowed controlled examination of treatment effects over measurements.
Case history
Q is a 40-year-old man who sustained a severe traumatic brain injury in a road traffic accident when he was 14 years old. His initial Glasgow Coma Scale score was 5/15 with 3 months of coma with a post-traumatic amnesic period of 6 months. CT scans showed a base of skull fracture and a brainstem lesion. Initially, there was right-sided hemiparesis. More information is lacking since hospital records have been destroyed.
After 2 years, he was discharged from rehabilitation to live with his mother. Cognitive impairments were severe, including slow information processing, memory impairment, attention deficits and impaired executive functioning. Two years post injury the Wechsler Adult Intelligence Scale-Revised score was 80. There were behavioural problems such as aggressiveness and inappropriate sexual behaviour. Minor motor problems remained, including balance and coordination problems.
Before injury he was reported to have a normal development with normal school attendance and with an IQ within the average range. No inappropriate sexual behaviour or other behavioural problems were reported.
Post injury he lived with his mother and three siblings and he was able to work in a protected environment. He was able to attend social activities without getting into trouble when closely supervised by his mother.
The major problem was his inappropriate aggressive and sexual behaviour. Impact on family and caregivers, and social functioning in general was profound and the behaviour caused him to be admitted to a mental health institution, for 3 years, where he often was sedated and put into seclusion. Several pharmacological (pimozide, valproic acid, carbamazepine, selective serotonin re-uptake inhibitors and propranolol) and non-pharmacological interventions (cognitive behavioural therapy, counselling sessions, coaching sessions, verbal instruction, manipulation of surroundings, instructing the mother and others close to Q) were tried but these did not reduce his inappropriate sexual behaviour.
The mother, being the principal caregiver, did not agree with the management of her son in the mental health institution and took him home, against medical advice. He was weaned of medication without medical supervision. The aggressiveness and the inappropriate sexual behaviour continued to be of major concern during the years after the accident into adult life. Close supervision by the mother partly precluded the potential damage of his behaviour. But still there were incidents, for example, he accosted his 4-year-old niece sexually.
According to the mother the aggressive behaviour became manageable through verbal correction and by teaching him to redirect his aggression away from people. This was however not applicable to his sexual behaviour.
Professional support was minimal for about 9 years.
At 15 years post trauma, following the incident with his niece, Q was referred to our outpatient neuropsychiatric clinic. He was treated with nortriptylin (100 mg once daily) and then with cyproteron (antiandrogen; 100 mg twice daily) for 2 years, which impaired his sexual function. However, the inappropriate sexual behaviour remained.
In summary, none of the behavioural and pharmacological interventions led to a reduction of the inappropriate sexual behaviour.
We followed Q up in our outpatient clinic regularly to support him and his mother.
The behaviour was only manageable under close supervision by the mother. Nevertheless incidents, possibly harmful to others, occurred.
The family could only just cope with this problem. There was a growing concern regarding what would happen if the mother would not be able to sustain the effort. No other therapeutic options were available. This justified the exploration of the EAT option.
Measurements
Target behaviour
On the basis of the definition of Johnson et al,8 Knight et al6 developed The St Andrew's Sexual Behaviour Assessment (SASBA): a standardised recording instrument for the measurement and assessment of challenging sexual behaviour in people with progressive and acquired neurological impairment. The SASBA has a good face and content validity and a good inter-rater and test−retest reliability.
In this study items from this classification were used to determine the target behaviour of Q (see appendix, web-only file).
Q showed all of the behavioural aspects listed in the appendix. The target behaviour was scored when one of the behavioural aspects occurred. The mother was asked to intervene early in the behavioural sequence to avoid unnecessary collateral damage.
Treatment
Intervention
EAT is the immediate application of an aversive electrical stimulus following un-called-for behaviour, with the aim of reducing this behaviour. As such, it is a response contingent procedure. In a meta-analysis Didden et al11 found that the response contingent procedure was superior in behaviour problems in intellectual disability, compared to other behavioural therapeutic strategies.
EAT is based on the two-factor procedure theory of Mowrer.12 Through parallel processes of operant and classical conditioning, there will be a reduction or suppression of the target behaviour by evoking avoidance conditioning. The electrical stimulus as an aversive consequence to the behaviour makes it possible to exactly link the response and the stimulus.
EAT is mostly applied in intellectually disabled or autistic individuals who show severe self-injuring behaviour10 and in some cases of aggressive behaviour13 and vomiting.14
Wijesinghe15 described in four case reports the treatment of deviant sexual behaviour (pederasty, indecent exposure) by EAT. In these patients no mental retardation or brain injury was involved. EAT was reported to be effective.
The use of EAT is only applicable when a patient is motivated to submit to the sessions of treatment, either by intrinsic motivation or a promised reward.
A number of negative side effects including increases in aggression, escape behaviour and negative emotional responses are mentioned.16–18 Duker and Seijs16 reported screaming and extreme anxiety in the first session followed by relaxation in the second session in some subjects. One subject refrained from behaving in any way in the first session, but was able to move again after he was reassured.
Van Oorsouw et al,17 however, failed to find negative side effects in a study with nine participants. Moreover, Matson and Taras18 reviewed 56 applied studies and reported that 96% of the side effects were positive (ie, increased social behaviour, increased activity levels and increased eye contact).
The EAT device consisted of an electrode applied to Q's right thigh, a receiver attached to his belt and connected to the electrode. A transmitter (remote controlled shocker, type HSP, Schoutissen Electronics, The Netherlands) was carried by the mother. The Dutch health authorities have approved the apparatus.
Procedure
Three 4-h sessions were arranged with Q and his mother. First EAT was introduced to Q and his mother as a treatment option and explained. Target behaviour was operationally defined in an interactive process with the mother. Subsequently, the mother underwent instruction and in vivo training in the use of the EAT device, by two experienced and qualified behavioural therapists, and leaders in the research on EAT. This included a demonstration of the device and application of the aversive stimulus to the psychiatrist involved, the mother and Q. The applied, standardised dose was not high enough to cause pain, but high enough to be unpleasant. Two and 4 weeks later, the behavioural therapists visited Q and his mother at home. They participated in the activities of the family for a day and instructed the mother in the use of the device. At the end of this process a session was planned to discuss the experience and to decide whether EAT would be started. In this session Q and his mother both gave their consent for treatment and measurement of target behaviour fully informed. The incentive for Q to consent was the prospect of being able to live on his own, depending on his ability to control his inappropriate sexual behaviour. Both Q and his mother consented to publication of these results; medical ethics approval was not necessary as the intervention and accompanying measurements were part of regular healthcare in this case.
The EAT device was installed after the baseline phase. The mother applied continuous supervision when Q was not at home. In the treatment phase he was attached to the EAT device, when among people. As soon as Q displayed the target behaviour his mother would press the button on the transmitter and a pulse would go through the electrode causing an unpleasant tickling sensation in Q's leg. The pulse is about 40 mA and is applied by alternating current of 30 Hz during less than 1 s.
Analyses
The number of target behaviours per day was presented graphically and visual analysis was conducted. Next, descriptive analyses were performed to summarise the raw data. T-tests were used to test the differences between the four phases of the experimental design. α was set at 0.05. SPSS V.15.0 for Windows was used.
Outcome and follow-up
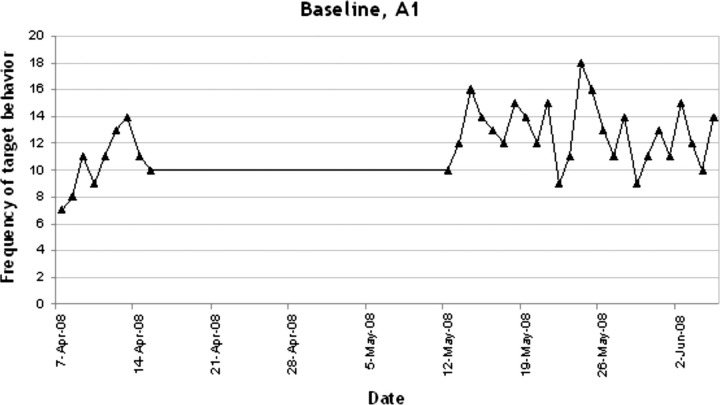
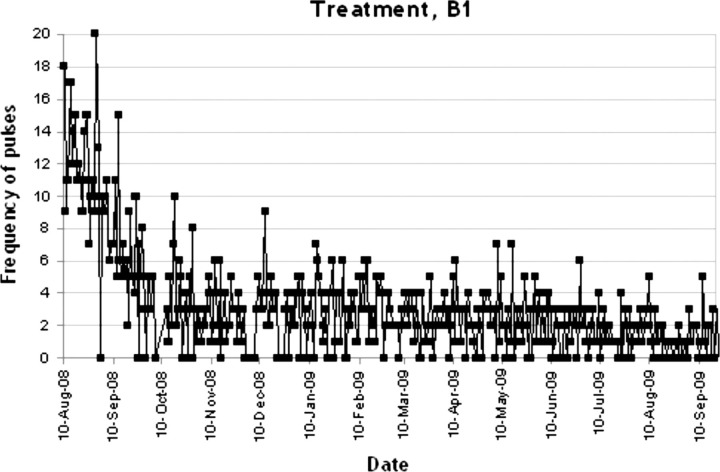
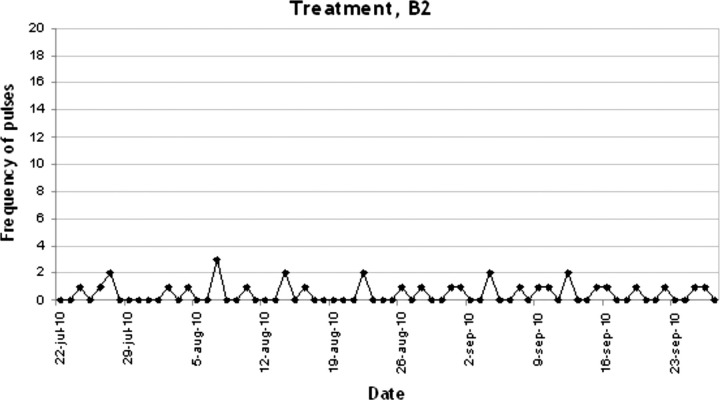
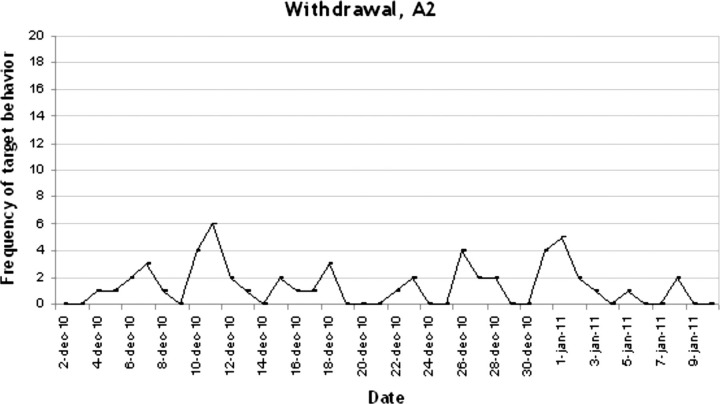
In figures 1–4 the number of target behaviours per day are shown for each of the four phases separately. In phase A1, 34 recordings were conducted with a mean target behaviour of 12.18 (2.49) per day (see figure 1). As can be seen, a stable baseline was established. In phase B1 the target behaviour was reduced dramatically to a mean of 3.15 (3.19) per day (n=409 recordings; see figure 2). This reduction further increased in the second treatment phase B2 to a mean of 0.47 (0.70) target behaviours per day (n=68; see figure 3). As can be seen in figure 4, in the withdrawal phase A2 a non-significant increase in target behaviour was shown (mean 1.35 (1.56); n=40).
Figure 1.
Frequency of target behaviour during baseline, A1.
Figure 2.
Frequency of pulses during treatment, B1.
Figure 3.
Frequency of pulses during treatment, B2.
Figure 4.
Frequency of target behaviour during withdrawal, A2.
In table 1 the mean (SD) number of target behaviours per day are shown per period. The differences between the baseline phase (A1) and all other phases are significant (p<0.001). There was no significant difference between the treatment phase and withdrawal. Thus, the treatment effect was sustained 2 months after withdrawal. Q reported that electrical aversion therapy was not stressful or painful for him, though aversive. Furthermore he reported no side effects.
Table 1.
Target behaviour per treatment phase: means, ranges and SD scores for each period
| Phase | N | Mean | SD | Range | Differences between phases | T |
|---|---|---|---|---|---|---|
| 1 (A1) | 34 | 12.18 | 2.49 | 7–18 | 1−2 | 16.10* |
| 2 (B1) | 409 | 3.15 | 3.19 | 0–20 | ||
| 3 (B2) | 68 | 0.47 | 0.70 | 0–3 | Phases 1−3 | 26.86* |
| 4 (A2) | 40 | 1.35 | 1.56 | 0–6 | Phases 1−4 | 21.94* |
*p<0.001.
Discussion
This first study on EAT in inappropriate sexual behaviour after brain injury was motivated by the severity, persistence and major impact of this problematic behaviour. To create evidence that it was EAT that caused the effect, we started a single case experimental design study. In this case the inappropriate sexual behaviour was significantly reduced, and because the supervision of the mother remained the same during the experiment, we think that the beneficial effect was due to the EAT.
After the experiment the closeness of supervision could be relaxed which increased the quality of life as reported by both Q and his mother.
In phase B1 there was an early reduction of target behaviour, followed by a fairly stable number of pulses for the rest of the B1 phase (see figure 2). In phase B2 there was a small further reduction. To establish the optimal duration of measurement we compared our findings with Duker and Seijs.16 They discern a general response pattern in all of their 12 subjects with a strong response initially and a relapse after several weeks. Measurements continued from 2 to 47 months but in two subjects the effect did not remain. In our study there was an early response, but no relapse occurred.
In the study of Q the principal caregiver was instructed in the use of the device. Comparing this procedure with the Duker and Seijs studies with the intellectual disabled, this is common if subjects live at home.
Q reported an increase in societal participation and a relaxation of the supervision by his mother, which he appreciated. In addition, the mother reported reduction of caregiver burden.
The large number of measurements taken enhanced internal validity.
The fact that there was a single rater provided consistency.
In future studies we recommend to include measurements of participation, quality of life and caregiver burden.
Ethical considerations were discussed throughout the process. We only proposed this potentially controversial intervention, because there were no other options after all available therapies had been tried, and the impact of the sexual inappropriate behaviour on Q and his family was profound. Over the years Q and his mother repeatedly asked for other options. The prospect of the mother getting older and what would become of Q when his mother would no longer be able to supervise his behaviour, made us decide to introduce EAT. As such, the ethical consideration in this case is analogous to compassive treatments of cancer, when every other intervention failed to show effect.
Because of the ethical issues we went through the informed consent process very painstakingly. Q was given the opportunity to withdraw from the EAT at any time and without explanation.
On the basis of the results of this SCED, EAT could be considered in brain-injured patients with inappropriate sexual behaviour resistant to other behavioural and pharmacological treatments.
Learning points.
In patients with a history of severe brain injury, showing inappropriate sexual behaviour not responding to behavioural and pharmacological treatment, electrical aversion therapy (EAT) may be effective.
Because of the ethics involved in this possibly controversial treatment a rigorous informed consent process must be performed and EAT is only justified if the impact on participation and significant others is profound and all other therapeutic options failed.
Single-case experimental design is feasible in clinical settings with limited research resources and can be used to study a new intervention.
Footnotes
Competing Interests: None.
Patient consent: Obtained.
References
- 1.Fann JR, Burington B, Leonetti A, et al. Psychiatric illness following traumatic brain injury in an adult health maintenance organization population. Arch Gen Psychiatry 2004;61:53–61. [DOI] [PubMed] [Google Scholar]
- 2.Williams WH, Evans JJ. Brain injury and emotion: an overview to a special issue on biopsychosocial approaches in neurorehabilitation. Neuropsychol Rehabil 2003;13:1–11. [DOI] [PubMed] [Google Scholar]
- 3.Alagiakrishnan K, Lim D, Brahim A, et al. Sexually inappropriate behaviour in demented elderly people. Postgrad Med J 2005;81:463–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson GK, Blaszczynski A, Hodgkinson A. Sex offending as a psychosocial sequela of traumatic brain injury. J Head Trauma Rehabil 1999;14:567–80. [DOI] [PubMed] [Google Scholar]
- 5.Bezeau SC, Bogod NM, Mateer CA. Sexually intrusive behaviour following brain injury: approaches to assessment and rehabilitation. Brain Inj 2004;18:299–313. [DOI] [PubMed] [Google Scholar]
- 6.Knight C, Alderman N, Johnson C, et al. The St Andrew's Sexual Behaviour Assessment (SASBA): development of a standardised recording instrument for the measurement and assessment of challenging sexual behaviour in people with progressive and acquired neurological impairment. Neuropsychol Rehabil 2008;18:129–59. [DOI] [PubMed] [Google Scholar]
- 7.Britton KR. Medroxyprogesterone in the treatment of aggressive hypersexual behaviour in traumatic brain injury. Brain Inj 1998;12:703–7. [DOI] [PubMed] [Google Scholar]
- 8.Johnson C, Knight C, Alderman N. Challenges associated with the definition and assessment of inappropriate sexual behaviour amongst individuals with an acquired neurological impairment. Brain Inj 2006;20:687–93. [DOI] [PubMed] [Google Scholar]
- 9.Emory LE, Cole CM, Meyer WJ. Use of Depo-Provera to control sexual aggression in persons with traumatic brain injury. J Head Trauma Rehabil 1995;10:47–58. [Google Scholar]
- 10.Duker PC, Seys DM. A quasi-experimental study on the effect of electrical aversion treatment on imposed mechanical restraint for severe self-injurious behavior. Res Dev Disabil 2000;21:235–42. [DOI] [PubMed] [Google Scholar]
- 11.Didden R, Duker PC, Korzilius H. Meta-analytic study on treatment effectiveness for problem behaviours with individuals who have mental retardation. Am J Ment Retard 1997;101:387–99. [PubMed] [Google Scholar]
- 12.Hadley NH. Foundations of Aversion Therapy. Lancaster, England: MTP Press Ltd., 1985. [Google Scholar]
- 13.Foxx RM, McMorrow MJ, Bittle RG, et al. The successful treatment of a dually diagnosed deaf man's aggression with a program that included contingent electric shock. Behav Therapy 1986;17:170–86. [Google Scholar]
- 14.Wright L, Thalassinos PA. Succes with electroshock in habitual vomiting. Clin Pediatr 1973;12:594–7. [DOI] [PubMed] [Google Scholar]
- 15.Wijesinghe B. Massed aversion treatment of sexual deviance. J Behav Ther Exp Psychiat 1977;8:135–7. [Google Scholar]
- 16.Duker PC, Seijs DM. Long-term use of electrical aversion treatment with self-injurious behavior. Res Dev Disabil 1996;17:293–301. [DOI] [PubMed] [Google Scholar]
- 17.Van Oorsouw WMMJ, Israel ML, Von Heyn RE, et al. Side effects of contingent shock treatment. Res Dev Disabil 2007;29:513–23. [DOI] [PubMed] [Google Scholar]
- 18.Matson JL, Taras ME. A 20 year review of punishment and alternative methods to treat problem behaviors in developmentally delayed persons. Res Dev Disabil 1986;10:85–104. [DOI] [PubMed] [Google Scholar]