Abstract
The authors report of an 8-year-old girl with non-mosaic Patau syndrome. The median life expectancy of Patau syndrome is 7–10 days, and 90% die in the first year of life. Survival is often attributed to mosaicism and the severity of associated malformations. We delineate the developing phenotype and review the literature discussing potential contributory factors to longevity.
Background
Trisomy 13 was first described in 1960.1 It is one of the least common autosomal trisomies among live births with an estimated prevalence of 1:12 000–1:29 000 in newborns.2 3 It is a heterogenous disorder encompassing multiple malformations including central nervous system, cardiac and urogenital anomalies. Characteristic dysmorphic features include microphthalmia or anophthalmia, cleft lip and palate, and polydactyly.1 4 Median survival time for patients with trisomy 13 is between 7 and 10 days and it is reported that between 86% and 91% of live-born patients with Patau syndrome do not survive beyond 1 year of life.5 Survival beyond the first year has been associated with mosaicism.6 There are only eight published cases of patients with full trisomy 13 over the age of 5 years.
Here we report of an 8-year-old girl (101 months) of Somalian origin diagnosed with full trisomy 13. She is currently the third longest surviving reported case of full trisomy 13 in the UK and the eighth longest surviving worldwide.
Case presentation
This girl is the third-born child of non-consanguineous Somalian parents. At the time of birth, the mother was 27 and the father 29 years old. There was no history of miscarriages and their five other children are healthy.
A 20-week anomaly scan showed fetal cardiac disproportion. Subsequent sonography revealed normal fetal growth, bilateral duplex kidneys and calcification within the skin, heart, liver and placenta. Amniocentesis and further investigation was declined.
The girl was born at 41 weeks gestation by normal vaginal delivery following spontaneous rupture of membranes. Apgar scores were 8 at 1 and 5 min, respectively. Birthweight was 2433 g (0.4th centile) and head circumference 32 cm (0.4th centile).
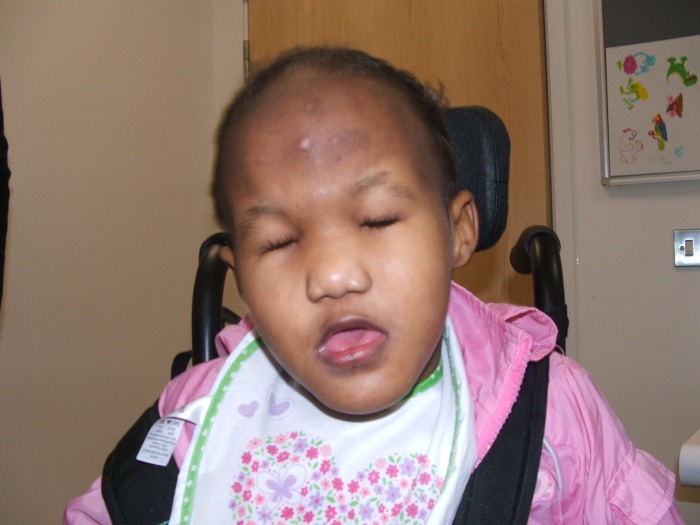
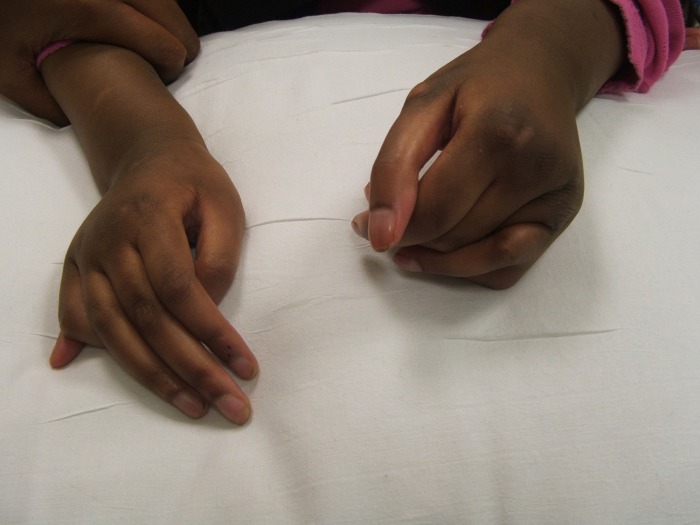
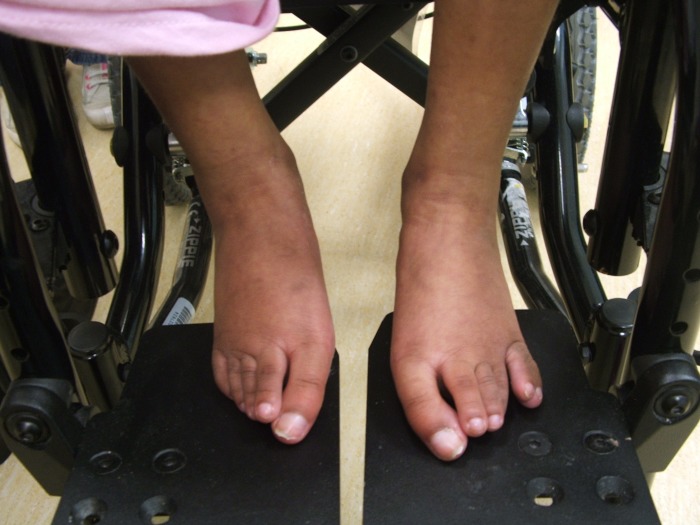
On examination, the patient was noted to have multiple dysmorphic features consistent with Patau syndrome including a prominent glabella, hypertelorism, low-set ears, microphthalmia, bilateral cataracts, cutis aplasia of the scalp, abnormal genitalia with large labia and a postaxial accessory digit on both hands and her left foot (figures 1–3). Fertilisation of in situ hybridisation analysis of a buccal smear was negative for mosaicism. Cytogenetic analysis was undertaken on cultured peripheral blood lymphocytes, which revealed an abnormal female karyotype: 47, XX, +13, non-mosaic.
Figure 1.
Photograph of the patient at 6 years and 5 months. Front profile photograph showing dysmorphic features consistent with Patau syndrome including prominent glabellae, orbital hypertelorism, low-set ears and microphthalmia.
Figure 2.
Photograph of the patient at 6 years and 5 months. Photograph reveals a postaxial accessory digit on the left foot.
Figure 3.
Photograph of the patient at 6 years and 5 months. Photograph reveals a postaxial accessory digit on both hands.
She was admitted to hospital for the first 4 weeks of life for management of feeding difficulties and jaundice. A cranial ultrasound scan revealed an abnormal corpus callosum, abnormal cortical folding and a small cerebellum. She was also noted to have bilateral duplex kidneys and was started on prophylactic antibiotics. An echocardiogram revealed a structurally normal heart.
At 10 months she developed seizures, but no abnormality was detected on EEG. At 5 years and 9 months, seizure activity became more frequent and treatment with clobazam and subsequently valproate was started with limited effect. At 6 years and 2 months, a CT scan revealed large bilateral arachnoid cysts and underlying agenesis of the corpus callosum.
The patient was unable to tolerate feeds due to a submucous cleft palate and has been receiving thickened feeds since the age of two to decrease the risk of reflux and aspiration.
On ophthalmology review aged 14 months, she was found to have almost no vision. She appeared to have limited light/dark perception, but showed no visual responses in normal room light. She generally kept her eyes closed. Examination of her hearing revealed a moderate hearing loss of ∼30/60 Db, and she was given a hearing aid which she tolerates intermittently.
Outcome and follow up
This patient has severe global developmental delay, with an overall developmental age of 3 months. She is able to move around and roll over when lying on the floor, but is unable to sit or stand without assistance. Her grasp reflex is still present and she vocalises using sounds but has no receptive or expressive language.
Discussion
It is unknown why some individuals with full trisomy 13 can live up to their first decade and beyond, in view of the median life expectancy being 7–10 days. Curiously, race and gender appear to subtly affect survival rates, with females and Afro-Carribeans having longer life spans,5 although reasons for this remain unclear.
Only 46% of long-lived full trisomy 13 patients are reported to have cardiac defects in contrast to 50–85% of all Patau syndrome cases.6 7 Our patient did not have any cardiac defects. The apparent lower incidence of heart defects in long-lived patients with full trisomy 13 may potentially be a factor underlying their survival.
Facial clefting has only been reported in one other child with full trisomy 13 surviving beyond 5 years.6 The collective incidence of cleft lip or palate in all subtypes of trisomy 13 is between 43% and 80%.6–8 Facial clefting can be associated with other midline defects, which may adversely affect the survival of an affected individual.8 The lower incidence of cleft lip/palate in the long-lived patient population with trisomy 13 may reflect a lower incidence of other midline defects in these patients. We have fully documented the clinical features of our patient in comparison to all other full trisomy 13 reported cases over the age of 5 years old (table 1).
Table 1.
| Birth details and dysmorphic features | Current patient | Percentage of patients with full Patau syndrome over the age of five (n=8) | Frequency of clinical features in all infants with trisomy 13 |
|---|---|---|---|
| Birth time | Term | – | – |
| Birth weight | 2433 g | – | – |
| Neonatal asphyxia | + | 38–75 | – |
| Pregnancy complications | − | 38 | – |
| Brain | |||
| Microcephaly/central nervous system defects | + | 88–100 | 75 |
| Holoprosencephaly | − | 0 | 60–70 |
| Seizures | + | 62–88 | 25–50 |
| Learning disabilities | + | 100 | 100 |
| Eyes | |||
| Microphthalmia | + | 75 | 60–88 |
| Coloboma/cataracts | + | 63–75 | 33% |
| Ears | |||
| Hearing loss | + | 38–63 | 50% |
| Low-set ears | + | 75–100 | 80–92% |
| Abnormal auricles | − | 63–88 | – |
| Head and face | |||
| Scalp defect | + | 25–88 | 44 |
| Sloping forehead | + | 75–100 | 16 |
| Broad, flat nose | + | 75–100 | – |
| Cleft lip and/or palate | + | 13 | 43–80 |
| Protruding lower lip | + | 35–63 | – |
| Cardiac | |||
| Heart defects | − | 50–69 | 80–84 |
| Genitourinary | |||
| Renal malformations | + | 38–63 | 37 |
| Genital anomalies | + | 75 | 30 |
| Musculoskeletal | |||
| Polydactyly | + | 38 | 60–76 |
| Flexion deformity of fingers | + | 63–88 | 68% |
| Prominent calcaneus | + | 63–88 | 28 |
| Joint contractures | + | 38–88 | − |
| Kyphosis, scoliosis | + | 13–75 | – |
| Nutrition | |||
| Failure to thrive | + | 88–100 | 87–100 |
| Recurrent infections | + | 88–100 | – |
Learning points.
It has been speculated that aggressive medical care may play a role in the increasing life expectancy of patients with trisomy 13.9 Conversely, Rasmussen et al5 have suggested that the average life expectancy of the trisomy 13 patient population has decreased over time, and that this may be due to less aggressive medical care provided as clinicians become increasingly aware of the poor prognosis.
Some cases of mosaic trisomy 13 and trisomy 13 due to translocations have been associated with a longer average life expectancy than full trisomy 13 patients.6–17 The cases of long-lived Patau syndrome reported in the literature have largely relied on cytogenetic analysis of peripheral lymphocytes for diagnosis.18 Therefore, a theoretical possibility exists that the long-lived cases may have a tissue-limited mosaicism that remains undiagnosed. However, as in this case, further investigation is not clinically indicated.
This case demonstrates the severe disability expected from a long-lived child with Patau syndrome. We believe it is paramount that the clinical findings are documented in these cases to benefit other affected individuals, and to elucidate the factors contributing to long survival in this rare disorder.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Patau K, Smith DW, Therman E, et al. Multiple congenital anomaly caused by an extra chromosome. Lancet 1960;1:790. [DOI] [PubMed] [Google Scholar]
- 2.Hook EB. Rates of 47, +13 and 46 translocation D/13 Patau syndrome in live births and comparison with rates in fetal deaths and at amniocentesis. Am J Hum Genet 1980;32:849–58. [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein H, Nielsen KG. Rates and survival of individuals with trisomy 13 and 18. Data from a 10-year period in Denmark. Clin Genet 1988;34:366–72. [DOI] [PubMed] [Google Scholar]
- 4.Taylor AI. Autosomal trisomy cases: a detailed study of 27 cases of Edward's syndrome and 27 cases of Patau's syndrome. J Med Genet 1968;5:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen SA, Wong L-YC, Yang Q, et al. Population-based analyses of mortality in trisomy 13 and trisomy 18. Pediatr 2003;111:777–84. [DOI] [PubMed] [Google Scholar]
- 6.Hsu HF, Hou JW. Variable expressivity in Patau syndrome is not all related to trisomy 13 mosaicism. Am J Med Genet A 2007;143A:1739–48. [DOI] [PubMed] [Google Scholar]
- 7.Baty BJ, Blackburn BL, Carey JC. Natural history of trisomy 18 and trisomy 13: I. Growth, physical assessment, medical histories, survival, and recurrence risk. Am J Med Genet 1994;49:175–88. [DOI] [PubMed] [Google Scholar]
- 8.Koury MJ, Corder JF, Rasmussen S. Ectopia cordis, midline defects and chromosome abnormalities: an epidemiologic perspective. Am J Med Genet 1988;30:811–17. [DOI] [PubMed] [Google Scholar]
- 9.Tunca Y, Kadandale JS, Pivnick EK. Long term survival in Patau syndrome. Clin Dysmorphol 2001;10:149–50. [DOI] [PubMed] [Google Scholar]
- 10.Cowen JM, Walker S, Harris F. Trisomy 13 and extended survival. J Med Genet 1979;16:155–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iliopoulos D, Sekerli E, Vassiliou G, et al. Patau syndrome with a long survival (146 months): a clinical report and a review of the literature. Am J Med Genet 2006;140A:92–3. [DOI] [PubMed] [Google Scholar]
- 12.Redheendran R, Neu RL, Bannerman RM. Long survival in trisomy-13-syndrome: 21 cases including prolonged survival in two patients 11 and 19 years old. Am J Med Genet 1981;8:167–72. [DOI] [PubMed] [Google Scholar]
- 13.Singh KS. Trisomy 13 (Patau's syndrome): a rare case of long survival into adulthood. J Ment Defic Res 1990;34:91–3. [DOI] [PubMed] [Google Scholar]
- 14.Zoll B, Wolf J, Lensing-Hebben D, et al. Trisomy 13 (Patau syndrome) with an 11 year survival. Clin Genet 1993;43:46–50. [DOI] [PubMed] [Google Scholar]
- 15.Jacob FD, Ramaswamy V, Kolski H. Long-term survival and late onset seizures in an adolescent with trisomy 13. Can J Neurol Sci 2010;37:694–6. [DOI] [PubMed] [Google Scholar]
- 16.de Toni T. Long-term study (7 years and 8 months) of a case of trisomy 13 with long survival. Minerva Pediatr 1983;35:951–4. [PubMed] [Google Scholar]
- 17.Magenis RE, Hecht F, Milham S., Jr Trisomy 13 (D1) syndrome: studies on parental age, sex ratio and survival. J Pediatr 1968;73:222–8. [DOI] [PubMed] [Google Scholar]
- 18.Duarte AC, Cunha E, Roth JM, et al. Cytogenetics of genetic counseling patients in Pelotas. Genet Mol Res 2004;3:303–8.. [PubMed] [Google Scholar]