Abstract
Background
Binge-like alcohol exposure in neonatal rats during the brain growth spurt causes deficits in adult neurogenesis in the hippocampal dentate gyrus (DG). Previous data from our lab demonstrated that twelve days of voluntary wheel-running (WR) beginning on postnatal day (PD) 30 significantly increased the number of newly-generated cells evident in the DG on PD42 in both alcohol-exposed and control rats, but 30 days later a sustained beneficial effect of WR was evident only in control rats. This study tested the hypothesis that housing rats in environmental complexity (EC) following WR would promote survival of the newly-generated cells stimulated by WR, particularly in alcohol-exposed rats.
Methods
On PD4-9, pups were intubated with alcohol in a binge-like manner (5.25g/kg/day), sham-intubated, or reared normally. In Experiment 1, animals were either assigned to WR during PD30-42 or were socially housed (SH). On PD42, animals were injected with bromodeoxyuridine (BrdU; 200mg/kg) and perfused two hours later to confirm the WR-induced stimulation of proliferation. In Experiment 2, all animals received WR on PD30-42 and were injected with BrdU on the last full day of WR. On PD42, animals were randomly assigned either to EC (WR/EC) or SH (WR/SH) for 30 days and subsequently perfused and brains were processed for immunohistochemical staining to identify BrdU+, Ki67+ and BrdU+/NeuN+ labeled cells in DG.
Results
In Exp. 1, WR exposure significantly increased the number of proliferating cells in all three postnatal conditions. In Exp. 2, the alcohol-exposed rats given WR/SH had significantly fewer BrdU+ cells compared to control rats given WR/SH. However, WR/EC experience significantly increased the number of surviving BrdU+ cells in both the alcohol-exposed and sham-intubated groups compared to WR/SH rats of the same neonatal treatment. Approximately 80% of the surviving BrdU+ cells in the DG across the conditions were co-labeled with NeuN.
Conclusions
WR followed by EC could provide a behavioral model for developing interventions in humans to ameliorate hippocampal-dependent impairments associated with fetal alcohol spectrum disorders.
Keywords: hippocampal neurogenesis, fetal alcohol spectrum disorders, rehabilitation, environmental enrichment, neuroplasticity
Introduction
Alcohol exposure during pregnancy can lead to the development of fetal alcohol spectrum disorders (FASD), in which damage to the central nervous system (CNS) and the resulting neurobehavioral deficits are significant and enduring consequences. Fetal alcohol syndrome (FAS) is diagnosed by the presence of facial dysmorphology, growth retardation and CNS damage. It typically reflects the most severely affected children in the spectrum. In the US, the estimated prevalence of FAS is approximately 1 of every 1000 live births (Sampson et al., 1997), but the prevalence of alcohol-related neurodevelopmental disorder is likely much higher, given that FASD cases without physical abnormalities are often not diagnosed; in fact, this number may be up to three times larger (Sampson et al., 1997; Morleo et al., 2011).
The hippocampal formation is a brain region affected by prenatal alcohol exposure (Norman et al., 2009) as evidenced by functional impairments in hippocampal-dependent learning and memory (Johnson & Goodlett, 2002; Hamilton et al., 2003). One potential mechanism by which prenatal alcohol exposure may interfere with hippocampal structure and function into adulthood is through altering the continuous generation of neurons in this area. Adult neurogenesis (AN) has been consistently found in two brain regions in many species: the subventricular zone of lateral ventricles and the subgranular zone in the dentate gyrus (DG) of the hippocampus (for review, see Emsley et al., 2005). The rate of AN is influenced by many factors including hormone levels, age, stress, drug treatment, environment and exercise (Tanapat et al., 1999; Crews et al., 2004; Olson et al., 2006; Van Praag et al., 1999; Holmes et al., 2004; Mirochnic et al., 2009). AN in the DG has been suggested to play an important role in hippocampal-dependent learning and memory and its disruption has been associated with deficits in this behavior (for review see Shors et al, 2001)
Alcohol exposure at various time points over the life span can affect cell proliferation and neurogenesis in the adult brain. For example, binge-like alcohol exposure in adolescent and adult rats transiently decreased AN (Nixon and Crews, 2002). Whether enduring changes in AN can result from alcohol exposure during early development is less well studied; and the few reported effects of prenatal alcohol exposure in rat models vary from no changes (Choi et al., 2005) to significant decreases in AN (Redila et al., 2006). Alcohol exposure during the brain growth spurt, which occurs during the third trimester in humans but spans the first two postnatal weeks in rodents (Dobbing & Sands, 1979) is known to be detrimental for adult brain plasticity (reviewed in Hannigan et al, 2007). Four studies have used postnatal rodent models of alcohol exposure during the third trimester equivalent and assessed effects on subsequent hippocampal AN. Ieraci and Herrera (2007) showed that administration of a single injection of 20% ethanol (5g/kg) in mice on postnatal day (PD) 7 decreased AN in the dorsal hippocampal DG when evaluated months later, on PD147. Postnatal alcohol appeared to negatively influence the pool of progenitor cells in the DG, which led to the decrease in AN. A previous study in our lab (Klintsova et al., 2007) also demonstrated that alcohol exposure on PD4-9 (5.25g/kg/day via gavage) reduced the ability of newly generated hippocampal cells to survive and decreased cumulative AN in 50- and 80-day-old rats.
The third study of effects of early postnatal alcohol exposure on AN showed that voluntary exercise [wheel running (WR)] in adolescence (PD30-42) can induce comparable increases in cell proliferation and neurogenesis in alcohol-exposed and control rats, but the long-term survival of those newly generated cells in alcohol-exposed rats was compromised (Helfer et al., 2009). Those findings suggest that rats given postnatal binge alcohol exposure show the same exercise-induced increase in adult cytogenesis and neurogenesis in the hippocampal DG as was previously reported for normally-reared rodents (van Praag et al, 1999), but are deficient in the promotion of survival of those newly generated neurons. This stands in contrast to the findings by Redila et al. (2006) that WR significantly increased both cell proliferation and survival in the hippocampal DG in adult rats exposed prenatally to alcohol. Further, a fourth study also investigated the impact of voluntary WR on cell proliferation and neurogenesis during adolescence (PD23-48) using a rat model in which administration of alcohol occurred throughout gestation and during early postnatal life (PD1-10) (Boehme et al., 2011). In contrast to the results of Helfer et al., 2009 (with alcohol exposure only during the 3rd trimester equivalent), Boehme et al. found that the three-trimester exposure altered cell proliferation during adolescence and increased early neuronal maturation but had no effect on cell survival in young adult female rats. Moreover, WR enhanced cell proliferation, neuronal maturation and cell survival in all groups (alcohol-exposed, pair fed and ad libitum controls). There were a number of experimental differences between these two studies, including the alcohol administration period, the strain and sex of the animals and the duration and timing of the WR experience, so it is not certain what factor(s) may account for the different outcomes between the two studies in the long-term survival of newly generated neurons.
In following up our efforts to develop a behavioral intervention that promotes hippocampal AN in the rat model of binge alcohol exposure during the 3rd-trimester equivalent, we hypothesized that providing exposure to environmental complexity (EC) immediately following a period of voluntary WR exercise during adolescence would promote the survival of the increased number of newly generated neurons induced by WR. EC is a combination of complex inanimate and social stimulations (Rosenzweig et al., 1978). The complexity differences in EC relative to standard social housing (SH) derives from multiple behavioral variables such as the amount of social interactions between rodents, enhanced exploratory behavior and exposure to novelty (Gelfo et al., 2009). EC is known for its powerful effects on brain plasticity: previous studies have demonstrated that exposure to EC produces increased brain and weight size, increased number of dendritic bifurcations, increased dendritic length, increased spine density and enhanced cell survival (van Praag et al, 2000; Gelfo et al., 2009; Darmopil et al., 2009). The current study tested the hypothesis that exposure to EC following adolescent WR would ameliorate the deficits in long-term survival of newly generated cells in the alcohol-exposed rats demonstrated in our previous study (Helfer et al., 2009), by increasing the long-term survival of newly generated cells relative to animals in SH after WR, and that this survival-promoting effect would be more prominent for the alcohol-exposed rats.
Materials and Methods
All procedures were done in accordance with the animal use protocol approved by University of Delaware Institutional Animal Care and Use Committee. For both experiments, Long-Evans rat pups from timed pregnancies were obtained and litters were culled to eight pups on postnatal (PD) 3. Pups were then pseudorandomly assigned to their postnatal conditions. Litters contained either only suckle control animals or a combination of sham-intubated and alcohol-exposed animals. The random assignment of either sham-intubated versus alcohol-exposed animal was done on PD3. Thirty-nine animals (27 males, 12 females from five litters) were in Experiment 1 (Fig 1a) and thirty-six animals (18 males, 18 females) were used in Experiment 2 (Fig 1b). Gestational age was used as a reference for the developmental timing of all treatments, thus gestational day (GD) 0 was the day when the plug was found and GD22 was considered to be the day of birth (PD 0). During PD4-9, alcohol-exposed (AE) pups were intubated with alcohol (11.3% v/v in milk formula) in a binge-like manner (two feedings two hours apart, total of 5.25g/kg/day), sham-intubated, or reared normally (Helfer et al., 2009). On PD4 blood samples were collected from a tail clip of each AE pup 90 min after the second alcohol intubation to determine the blood alcohol concentration (BAC). BACs were assayed from the plasma of each blood sample using an Analox GL5 Alcohol Analyzer (Analox Instruments, Boston, MA).
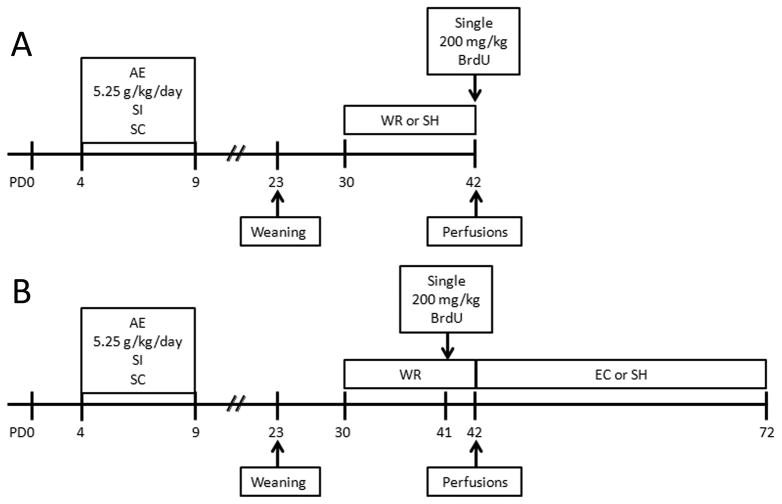
Figure 1.
Schematic timeline of experimental procedures. Rat pups were either intubated with 5.25 g/kg/day alcohol in a binge-like manner on PD4-9 (AE), sham-intubated (SI) or were left undisturbed (suckle control, SC). After completion of postnatal treatment pups remained with their mothers until weaning on PD23. In the first experiment (A), rats from all three treatment groups were divided between voluntary exercise (wheel-running, WR) and social housing conditions on PD30. WR rats had free access to the running wheel between PD30 and 42. On PD42 all rats were injected with 200 mg/kg BrdU (i.p.) and sacrificed two hours later. In the second experiment (B), rats were exposed to the same postnatal treatment but on PD30 all animals were placed in cages with free access to the running wheels (WR) for twelve days. They were injected with 200 mg/kg BrdU on PD41. On PD42 rats were further divided between two housing conditions: large environmental complexity cages (EC) or standard housing cages (SH), where they remained for thirty days, until PD72. Adult hippocampal neurogenesis was analyzed at PD72.
For Experiment 1, which assessed the effect of exercise on cell proliferation, animals of each postnatal condition were further divided into either WR (24 hours free access) or SH conditions from PD30-42 (12 days) (Fig. 1a). All rats were housed in same sex cages (2–3 animals/cage). Given that previous work in our lab has demonstrated that postnatal treatment does not significantly affect daily WR activity (Helfer et al., 2009), animals of different postnatal conditions were housed together. On PD42, at the onset of the light cycle (9:00AM) and following the cessation of the WR treatment period, rats received a single injection of bromodeoxyuridine (BrdU; 200mg/kg). All animals were anesthetized and sacrificed by transcardial perfusion two hours after the BrdU injection.
For Experiment 2, which measured effect of environment on survival of cells born on the last day of WR, all animals were first housed in cages with 24-hour free access to WR from PD30-42. Housing conditions and grouping were identical to those described above. In order to reduce the stress of removal from the wheels, animals were injected with BrdU on the morning of the last full day of WR (PD41). Then, at 9:00AM on PD42, rats were removed from wheels and randomly assigned to either environmental complexity (WR/EC) or standard housing (WR/SH) condition for thirty more days (Fig. 1b). The EC consisted of a 30″ × 18″ × 36″ galvanized three-story steel cage (model: R-695; cagestore.com) with three ramps, two balconies, a full middle floor and a drop-in 3 ½ inch plastic pan filled with bedding. Cages were equipped with a variety of objects such as hammocks, buckets, blocks, etc. that were changed every other day. There were 6–9 same sex animals per EC cage. On PD72, animals were anesthetized and transcardially perfused.
For both experiments, animals were perfused with heparinized 0.1M phosphate-buffered saline (PBS, pH 7.2) followed by 4% paraformaldehyde in PBS (pH 7.2). Brains were stored in 4% paraformaldehyde for 2 days, then transferred to 30% sucrose in 4% paraformaldehyde. Brains were sectioned at 40μm on a cryostat and serial sections were collected maintaining order throughout the entire hippocampus so that the entire extent of the DG was collected and stored 20 °C in cyroprotectant solution. A systemati c random sampling procedure was used in selecting the sections for processing (Helfer et al., 2009; Hamilton et al., 2011).
Briefly, to analyze the number of BrdU+ and Ki67+ cells, sections were placed in the primary antibody solution (rat anti BrdU, 1:500; Accurate Chemical, OBT0030; mouse anti Ki67, 1:1000; Novacastra Laboratories, NCL-L-Ki67-MM1) diluted in blocking solution, followed by incubation in secondary antibody solution (2 hours, biotinylated anti-rat made in goat, 1:250; Vector Laboratories, BA-9400; 1 hour, biotinylated anti-mouse made in goat, 1:1000; Vector Laboratories, BA-9200, respectively) made in blocking solution. Detection of the reaction was done using nickel-enhanced diaminobenzidine as a chromagen (Hamilton et al., 2011). The precision of estimates of both the BrdU+ and Ki67+ cell number in a set of sections for each animal was expressed using coefficients of error (CE) (Gundersen et al., 1999; Slomianka and West, 2005). The stereological sampling scheme was considered adequate when the CE was less than 0.10 (Gundersen and Jensen, 1987; Gundersen et al., 1999). Actual CE ranged between 0.06 and 0.09 for the individual animals and did not differ between experimental groups.
In order to determine the number of mature neurons in the population of cells born on PD41 and survived for 30 days, double labeling with BrdU and NeuN, a mature neuronal marker, was performed as described previously (Helfer et al., 2009). Briefly, sections were incubated for 48 hours at 4°C in a cocktail of primary antibodies solution made in blocking solution (rat anti-BrdU, 1:1000; Accurate; mouse anti-NeuN, 1:500; Millipore) followed by incubation for 2 hours in secondary antibodies made in blocking solution (for Brdu, Biotin SP-conjugated anti-rat IgG, 1:1000; Jackson Laboratories; for NeuN; Cy3-conjugated anti-mouse IgG, 4μml/ml, Jackson Laboratories) and then with Cy2-conjugated streptavidin (Jackson Laboratories). Sections were mounted on slides and coverslipped using antifade media (Prolong Gold, Molecular Probes).
In order to assess cell death, sections were stained with the nucleic acid stain Hoechst 33342, trihydrochloride, trihydrate. Sections were mounted on slides and a hydrophobic ring was drawn around each section using a PAP pen. Sections were placed in PBS for 5 mins followed by application, in the dark, of Hoechst stain (10μg/ml dilution; Molecular Probes; H-21492) and then a wash in PBS (5 mins). Slides were then coverslipped using Vectashield and stored at −20°C in the dark. Analysis of apoptotic cells consisted of visual identification of fragmented or condensed nuclei and increased blue fluorescence (Zhivotovsky & Orrenius, 2001). For apoptosis analysis, every 32nd section (~4 sections/animal) through the entire extent of hippocampal DG was stained with Hoechst stain to determine the presence of the apoptotic cells.
Quantification of BrdU+ and Ki67 cells was performed by an investigator blind to experimental treatments in an unbiased stereological manner using optical fractionator (Stereo Investigator, Micro Bright Field Inc., Williston, VT). Every 16th section (~7 sections/animal) beginning around Bregma −3.20 through the entire granule cell layer of hippocampal DG was analyzed. The section-sampling fraction was 1/16; the area-sampling fraction was 1 and the section thickness fraction was the ratio of the dissector height (12 μm) to the mean thickness of the sections, measured using the software at every counting frame. Slides were coded so that the experimenter was blind to treatment condition and counts were made within a known volume of the DG.
Twenty-five BrdU+ cells in DG granule cell layer per animal were analyzed in NeuN-stained tissue to assess the mature neuronal phenotype using confocal microscopy (LSM 510 confocal microscope, Zeiss, Thornwood, NY). Phenotyping of these cells was performed on the 3D digital reconstructions and orthogonal representations from a series of confocal images taken at 0.5 μm intervals. Cells were identified as colabeled if an overlap of the Cy2 and Cy3 labels was observed within a given cell in each of the xy-, xz- and yz-planes in the orthogonal view. Values of “calculated neurogenesis” were computed by multiplying the percentage of double-labeled cells by the total number of BrdU cells on PD72.
For statistical analysis, ANOVAs and post hoc (Tukey) tests were performed for measures in each experiment. The PASW statistical package was used for all analyses. The level of significance was set at p < 0.05 for all tests. The data in the text and figures is presented as mean +/− SEM.
Results
The mean peak BAC (±SEM) measured on PD4 for all alcohol-exposed animals was 321.19 ± 14.03 mg/dl. These BACs are similar to previously published studies using the same alcohol dose and delivery approach (Klintsova et al., 2007).
For both experiments, all animals continued to gain weight throughout treatments as summarized in Table 1. The influence of postnatal treatment on body weights was determined using a repeated-measures ANOVA with POSTNATAL TREATMENT (AE, SI, SC) as the between subjects factor and POSTNATAL DAY as a within subjects factor, followed by post hoc analysis (Tukey’s test). To ensure that the assumption of ANOVA concerning homogeneity of variance was not violated, body weights from the neonatal period (PD4,9) versus adolescence (PD31, 42) were analyzed separately. For neonatal body weights (both experiments), a day by postnatal treatment interaction was evident [F2, 42=14.35, p < 0.01] as was a main effect of day [F1,42=382.67, p < 0.01]. No main effect of postnatal treatment was evident. Post hoc tests revealed that this effect was due to AE animals weighing significantly more than SC animals at the onset of postnatal treatment. By PD9, AE animals had significantly lower weights compared to SI animals (p < 0.01) but not SC animals. Analysis of adolescent rats’ body weights revealed a main effect of day [F1, 42=656.01, p < 0.01] but neither a main effect of postnatal treatment nor a treatment X day interaction. On average, the numbers of wheel revolutions (±SEM) per 24-hour period (indication of voluntary exercise activity) were 4508±881 (approximately 5.0km) for animals in the proliferation study (Experiment 1) and 5293±765 (approximately 5.9km) for animals in the survival study (Experiment 2). A one-way ANOVA for EXPERIMENT reveals no significant difference (F1,17 = 0.526, p = 0.478).
Table 1.
| Males and females combined | |||
|---|---|---|---|
| Postnatal treatment | |||
| SC | SI | AE | |
| Weight (g) | |||
| PD4 | 8.4 ± 0.3b | 8.7 ± 0.2 | 9.3 ± 0.2 |
| PD9 | 14.6 ± 0.9 | 15.5 ± 0.6 | 12.8 ± 0.4c |
| PD30/31 | 84 ± 4 | 87 ± 3 | 77 ± 3 |
| PD41/42 | 146 ±8 | 148 ± 5 | 138 ± 6 |
PD, postnatal day; AE, alcohol exposed; SI, sham-intubated; SC, suckle control. The weights are reported as group means ± SEM.
SC weigh less than AE, p<0.05.
AE weigh less than SI, p<0.01.
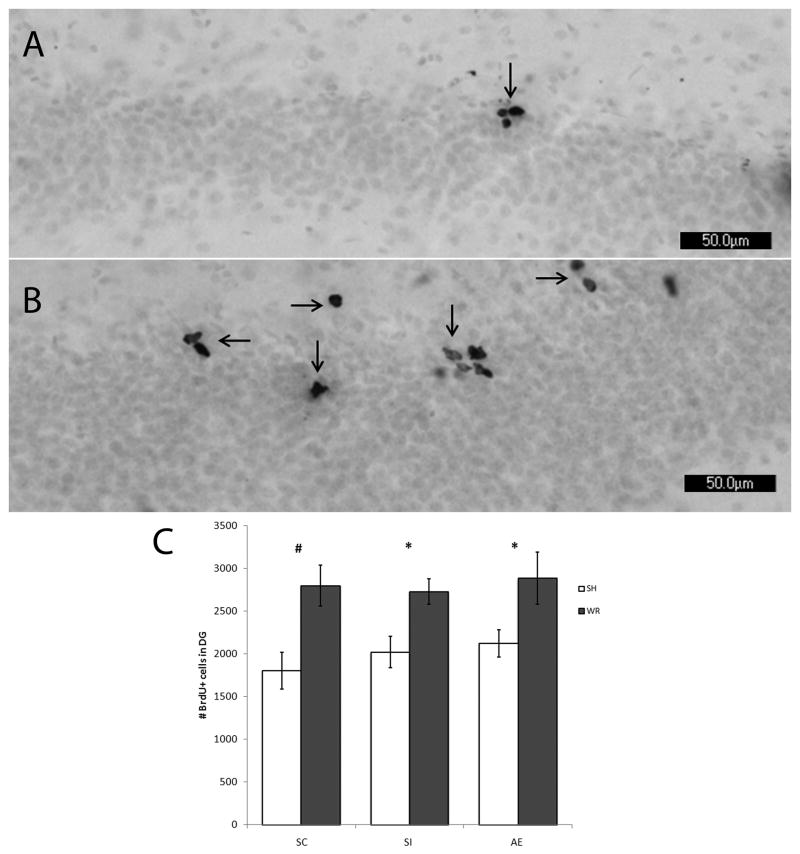
For Experiment 1, the effect of early postnatal treatment and adolescent voluntary WR exercise on the number of proliferating BrdU+ cells was assessed using DAB immunoreactivity. No difference of stain quality or color was observed between control and experimental animals. In addition, no difference in position of the cells within the DG was noted between groups (as shown in Fig. 2). Immunoreactive cells were primarily located on the interior border of the granule cell layer within the SGZ. Also shown in Fig. 2, the number of BrdU+ cells appeared to be greater in WR animals compared to SH animals. Number of BrdU+ cells was analyzed using two-way ANOVA with POSTNATAL TREATMENT (AE, SI, SC) and ADOLESCENT CONDITION (SH vs WR) as between-subjects factors followed by post hoc comparisons between SH and WR conditions within treatment group. A two-way ANOVA revealed a significant effect of adolescent condition on the number of proliferating BrdU+ cells in the DG on PD42 (F1,40=19.703, p < 0.001), but no effect of postnatal treatment (F2,40 = 0.594, p = 0.558) The POSTNATAL TREATMENT x ADOLESCENT CONDITION effect was not significant. Post-hoc comparisons (Tukey test; p < 0.05) demonstrated that 1) postnatal alcohol exposure had no significant effect on cell proliferation in hippocampal DG on PD42, as AE animals had the same number of proliferating cells compared to control animals, and 2) WR significantly increased cell proliferation in all postnatal treatment groups compared to SH animals (Fig. 2).
Figure 2.
PD42 analysis of BrdU labeling using light microscope. Images of BrdU+ labeled cells recognized by reaction with diaminobenzidine and counterstained with Pyronin Y. Images taken at 20×. (A) is taken from an AE animal exposed to SH. (B) is taken from an AE animal exposed to WR. (C) demonstrates that the number of BrdU+ cells is not significantly different in AE animals compared to controls (SI and SC) when exposed to standard housing (p = 0.558). Exposure to wheel running significantly increases the number of proliferating cells in all postnatal treatment conditions compared to standard housed littermates (p < 0.01). All values represent mean +/− SEM. * p < 0.05; # p < 0.01.
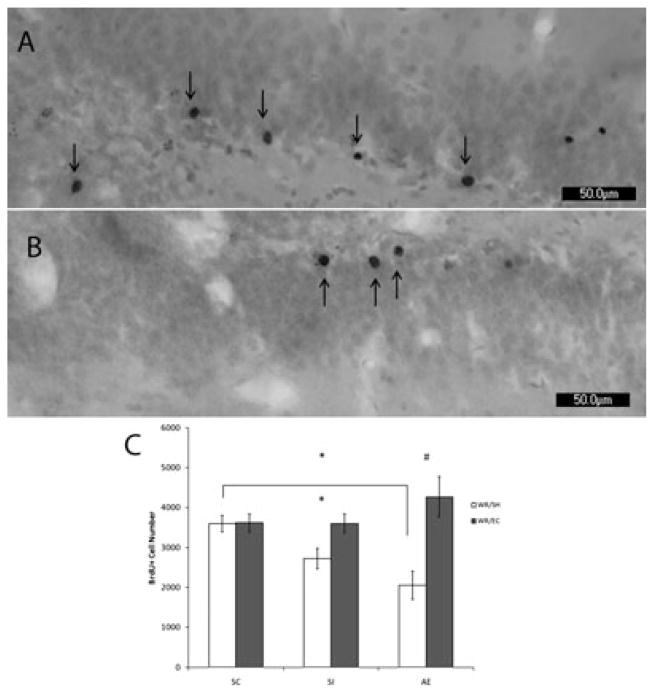
For Experiment 2, the number of surviving BrdU+ cells on PD72 (shown in Fig. 3) was analyzed. As in Experiment 1, no noticeable difference in stain quality or color was observed between groups. BrdU+ cells were primarily located within the granule cell layer in all animals, regardless of condition. Number of BrdU+ cells was analyzed with a two-way ANOVA with POSTNATAL TREATMENT (AE, SI, SC) and HOUSING CONDITION (EC vs SH) as the between-subject factors, followed by univariate ANOVA and post hoc comparisons (Tukey’s test) within each housing condition. There was a significant POSTNATAL TREATMENT x HOUSING CONDITION interaction (F1,29=3.870, p < 0.05) and a main effect of HOUSING CONDITION (F1,29=11.402, p < 0.01); the interaction appeared to be due to the greater effect of EC in the AE and SI groups than in the SC group. A follow-up one-way ANOVA of POSTNATAL TREATMENT in the SH animals revealed a significant main effect of treatment on the number of BrdU+ surviving cells (F1,19=3.727, p < 0.05). Post hoc analysis revealed that AE animals had significantly fewer BrdU+ surviving cells than SC animals (p < 0.05). In contrast, one-way ANOVA of POSTNATAL TREAMENT in the EC animals did not reveal a significant effect of treatment. Within each neonatal treatment group, the AE and the SI groups both showed significant increases in the number of BrdU+ cells in the EC condition relative to the SH condition (AE/EC vs AE/SH, Tukey HSD; p < 0.01; SI/EC vs SI/SH, Tukey HSD; p < 0.05); the environmental condition did not significantly affect the SC group. Survival of the cells generated on PD42 was affected by the neonatal alcohol exposure and by post-running environment. In the AE WR/SH group, the number of new cells labeled with BrdU on PD41 that survived until PD72 was only 71% of the total counted on PD42. However, when AE rats were placed in EC immediately after completion of 12-day WR period, the number of newly generated cells labeled with BrdU on PD41 and that survived until PD72 was 148% of the total counted on PD42. For both SC and SI animals placed in EC, the number of surviving cells relative the total counted on PD42 was increased to 129% and 128% respectively, whereas post-running housing in the social cage environment resulted in survival of 129% (SC) and 96% (SI) of the number of BrdU+ cells counted on PD42. In summary, these data demonstrate that the intervention consisting of WR followed by EC rescues the number of BrdU+ cells in DG of alcohol-exposed rats to SC levels (Fig. 3).
Figure 3.
PD72 analysis of BrdU labeling using light microscope. Images of BrdU+ labeled cells recognized by reaction with diaminobenzidine and counterstained with Pyronin Y. Images taken at 20×. (A) is taken from an AE animal exposed to WR/EC. (B) is taken from an AE animal exposed to WR/SH. (C) demonstrates that the number of BrdU+ cells is significantly decreased in AE animals compared to SC when exposed to standard housing after running (p<0.05). Exposure to environmental complexity significantly increases the number of surviving new cells in both the SI (p < 0.05) and the AE (p < 0.01) groups compared to social housed littermates. All values represent mean +/− SEM. * p < 0.05; # p < 0.01.
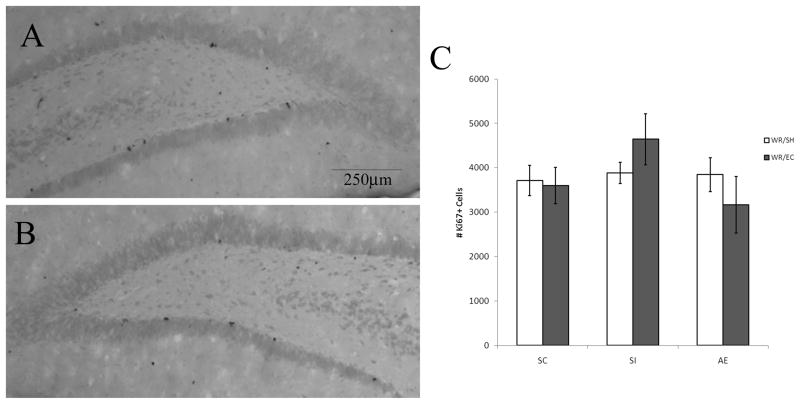
A two-way ANOVA was performed on PD72 Ki67 cell counts. No difference was found among groups in the number of Ki67+ cells on PD72 (Fig. 4) confirming that EC alone does not promote cell proliferation. Further, assessment of cell death was measured to ensure that WR had no impact on the net overall number of proliferating cells at both time points of interest. This analysis with the Hoechst nucleic acid stain revealed low levels of apoptosis were evident at both PD42 and PD72, but no difference in the number of apoptotic cells was found across conditions. On PD42, there was an average of 3.64± 0.44 apoptotic cells found per animal across conditions, while on PD72 there was an average of 2.15 ± 0.62 apoptotic cells detected per animal across conditions.
Figure 4.
Postnatal day (PD) 72 analysis of Ki67 labeling using light microscope. Images of Ki67+ cells recognized by reaction with diaminobenzidine and counterstained with Pyronin Y. Images taken at 5×. (A) is taken from an alcohol exposed (AE) animal exposed to running/environmental complexity. (B) is taken from an AE animal exposed to wheel running/standard housing. (C) indicates that neither prenatal alcohol exposure nor exposure to environmental complexity affect the number of proliferating Ki67+ cells on PD72. All values represent mean +/− SEM.
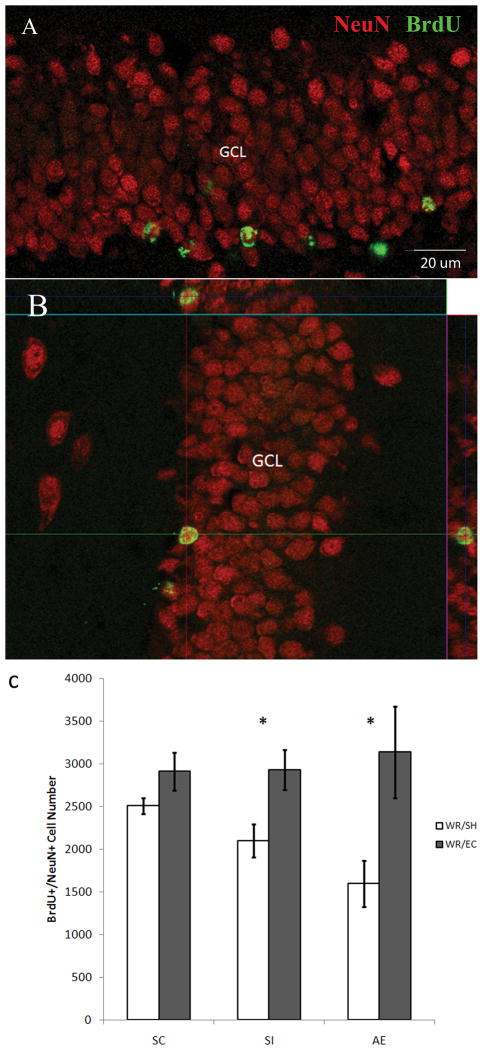
To determine whether the fate of surviving BrdU+ cells was neuronal, sections containing hippocampal DG were double-stained with anti-BrdU and anti-NeuN antibodies and were examined using confocal microscopy. Approximately 80% of the surviving cells in all conditions were colabeled with these two markers and thus were mature neurons. Further, a significant main effect of HOUSING CONDITION (F1,28=20.48, p < 0.001) was evident on the number of surviving BrdU+/NeuN+ cells in the DG of the hippocampus (Fig. 5). Post-hoc analysis demonstrated that the AE rats from the WR/SH condition had significantly fewer BrdU+/NeuN+ cells (1228±214) than the SC groups of the WR/SH condition (2506±64, p = 0.03). Survival of newly generated neurons was significantly enhanced by thirty days in EC in the SI (2928±237, p < 0.05) and AE (3136±536, p < 0.001) groups, compared with SH littermates from the same postnatal treatment (1946±147 and 1228±214, respectively). Housing in EC appeared to bring the number of surviving, adult-generated neurons in SC, SI and AE rats to similar levels, and differential housing did not significantly alter the extent of new neuron survival in SC rats (WR/EC: 2910±221 vs. WR/SH: 2506±65).
Figure 5.
Phenotype of Bromodeoxyuridine (BrdU)+ cells. (A, B) Confocal images demonstrate the BrdU+ labeling and distribution in the dentate gyrus. NeuN (red), mature neuronal marker; BrdU+ marker (green); GCL, granule cell layer. (C) The number of double labeled cells (BrdU+/NeuN+) is significantly increased in the AE (p < 0.05) and SI (p < 0.05) animals exposed to wheel running/environmental complexity compared to the number of BrdU+/NeuN+ cells present in the AE and SI animals exposed to the wheel running/standard housing. All values represent mean +/− SEM. * p < 0.05.
Discussion
This study is the first to demonstrate that exposure to EC for four weeks following voluntary WR exercise is sufficient to rescue the number of surviving newly generated neurons evident in AE animals exposed to WR alone. In addition, the results of this study confirm that WR enhances cell proliferation in AE and both control groups of animals. We previously showed that WR resulted in increased long-term cell survival only in SC animals, not in SI or AE rats (Helfer et al., 2009). Taken together, these results suggest that the postnatal alcohol treatment does not interfere with the generation of new cells in the adolescent DG but does result in impaired survival of newly generated cells, and that the maintenance in the EC environment after the WR experience particularly aided the AE rats in terms of survival of new BrdU+ cells and BrdU+/NeuN+ cells (Figs. 3, 5), that could be a result of their more successful integration in the hippocampal network (Aasebo et al.,2011). Overall, these findings provide evidence that EC can promote cell survival in the AE rats and in the SI rats, and yield long-term survival rates comparable to normally-reared SC groups.
Our previous study (Helfer et al., 2009) used multiple BrdU injections to demonstrate that twelve days of voluntary exercise significantly increased number of proliferating cells by the end of WR on PD42. The current study used a one-time 200 mg/kg BrdU injection at the end of the WR period. This dose of BrdU has been commonly reported in the literature as saturating for dividing cells and having minimal cellular toxicity (Eadie, Redila & Christie, 2005). It is worth noting that exercise can increase blood-brain barrier permeability and cerebral vascularity (Sharma, et al, 1991; Van der Borght et al., 2009), two factors which could affect bioavailability of BrdU and its incorporation into new cells. However, exercise’s effects on proliferation remain constant regardless of type of marker used (BrdU vs endogenous), ruling out altered BrdU incorporation following exercise as an alternate explanation of the results found here (Eadie, Redila & Christie, 2005; Van der Borght et al., 2009).
Overall, analysis of BrdU+ cell number in the DG on PD42 supports our previous data (Helfer et al, 2009). The same duration of WR (as in Helfer et al., 2009) significantly enhanced DG cell proliferation of all three postnatal treatment conditions. AE/SH animals did not significantly differ in the number of proliferating BrdU+ cells in DG on PD42 when compared to SI and SC animals, supporting the hypothesis that developmental alcohol exposure does not have a detrimental effect on the generation of new cells. In addition, analysis of Ki67+ cells on PD72 (30 days after the end of the WR) also revealed no significant difference in the number of proliferating cells in SC, SI and AE rats regardless of housing condition (Fig. 4), indicating that the effect of EC on adolescent cell proliferation is subtle compared to that of exercise.
The combined effect of sequential WR/EC on the AE rats is due primarily to the promotion of neurogenesis, since previous studies (van Praag et al., 1999; Brown et al., 2003; Helfer et al., 2009) along with the current study demonstrate that the vast majority (approximately 80%) of new cells in DG express neuronal markers 30 days after their birth. In the current study, neither postnatal treatment group nor adolescent housing condition affected the relative number of newly generated cells that differentiated into neurons. Thus, the increased number of surviving BrdU+ cells seen following exposure to WR/EC does not solely reflect an increase in numbers of mature neurons. More work is needed to specifically examine the effects of AE and WR/EC on the various stages of cellular proliferation, migration and differentiation in the DG.
Alcohol exposure is known to negatively affect the production and survival of progenitor cells through a variety of factors such as the inhibition of growth factors, which regulate each stage of neurogenesis (Davis et al., 1999; Developmental exposure to alcohol results in significant changes in expression of neurotrophins that depend on the timing of insult and area of the brain. In the rat, both NGF and BDNF levels were elevated, while expression of their receptors was significantly decreased, in hippocampus and cortex after postnatal alcohol exposure on PD4-10 (Heaton et al., 2000; Moore et al., 2004). While transient, these changes in neurotrophic factors and their receptors during the critical period of the development could result in the abnormal cell differentiation and maturation. Other extrinsic factors affect AN. In particular, exercise, which improves spatial memory and enhances long term potentiation (van Praag et al., 1999;), has consistently been shown to increase the number of proliferating cells in the DG compared to non-exercising control animals (van Praag et al., 1999; Lou et al., 2008). In addition, exercise enhances long-term cell survival (Helfer et al., 2009). Physical activity is associated with the upregulation of β-endorphin expression, as well as the expression of insulin-like growth factor 1 (IGF 1) (Carro et al., 2000; Llorens-Martin et al., 2010), vascular endothelial growth factor (VEGF) (Fabel et al., 2003; Lou et al., 2008) and brain derived neurotrophic factor (BDNF) (Neeper et al., 1999; Boehme et al., 2011). Increases in these factors may play a role in the higher number of proliferating cells as well as the increased rate of cell survival seen in WR animals. For example, BDNF expression is directly related to the amount of exercise in which an animal engages (Adlard et al., 2004). Moreover, BDNF concentrations within the DG are significantly increased after exposure to exercise (Griffin et al., 2009). Interestingly, BDNF protein level remains elevated after cessation of exercise returning to baseline level after a week or two (Berchtold et al., 2005). Hippocampal cell proliferation is reported to return to normal rates within 24 hours following termination of WR in mice (Van de Borght et al., 2009), suggesting that the sustained levels of BDNF may play a critical role in promoting neuronal maturation and survival. VEGF has indirect effects on AN through its role in angiogenesis and formation of neurovascular niches necessary for proliferation within the SGZ (Palmer, Willhoite & Gage, 2000), as well as direct effects on cell proliferation and maturation. Application of VEGF enhances AN both in cultured cells and in vivo (Jin et al, 2002). Additionally, the VEGF receptor Flk-1 is colocalized on immature neurons within the DG, suggesting a continued role for VEGF in cell maturation. Both BDNF and VEGF have been found to promote the proliferation of neuronal precursors (Neeper et al., 1999; Lou et al., 2008). Together, these data suggest that exercise enhances the levels of proliferating cells in the hippocampus and may do so through its influence over a variety of neurotrophic factors. It needs to be mentioned that, while the effect of exercise on cell proliferation appears well documented, the effect of exercise on survival is less clear but it is likely that the growth factors mentioned above continue to have an impact on cell maturation.
Environmental complexity has been shown to increase AN. Whereas WR promotes proliferation of progenitors in adult neurogenic niches, EC appears to positively impact the survival of progenitor cells (Brown et al., 2003; Olson et al., 2006;). Brown and colleagues (2003) demonstrated that the increased levels of surviving cells as a result of exposure to EC was specific to the DG, as no difference was found in the olfactory bulb. Similar to exercise, exposure to EC results in an increase in neurotrophins such as BDNF, glia-derived neurotrophic factor (GDNF), neuronal growth factor (NGF) and neurotrophin-3 (NT3) (Olson et al., 2006; Angelucci et al., 2009). In addition, inhibition of VEGF blocks the environmental induction of neurogenesis (During & Cao, 2006). Thus, the same neurotrophic factors that enhance proliferation also add to survival of new neurons after the exposure to complex environment. The factors that are adding to neuronal survival in AE animals from EC are yet to be determined.
The current study supports our previous work using multiple BrdU injections showing that access to WR increases the level of hippocampal cell proliferation in rats postnatally exposed to alcohol (Helfer et al., 2009). In that study, it was found that the influence of voluntary exercise alone is not sufficient to enhance the survival of newly generated neurons in rats exposed to alcohol postnatally; whereas the addition of a complex environment immediately following voluntary exercise successfully promotes the survival of adult generated neurons. Prenatal exposure to alcohol has been found to impair the neurogenic response to EC. While control mice reared in an enriched environment after weaning doubled the number of adult generated neurons, alcohol-exposed animals had impaired survival of new neurons in DG (Choi et al., 2005). Thus, neither WR nor exposure to a complex environment appear solely sufficient to overcome the negative effects of developmental exposure to alcohol on AN. Instead, it appears that exercise primarily influences proliferation levels and EC affects differentiation of newly generated cells and their survival (van Praag, 1999; Brown et al., 2003; Olson et al., 2006). Along with many other effects, exercise is suggested to prime a “molecular memory” (for growth factors’ expression, e.g. BDNF) of such experience. In doing so, the prior experience works to increase the readiness of the hippocampus for a future BDNF response to exercise (Berchtold et al., 2005). This results in a memory of an event that produces a greater response when reexposed to the stimulus (i.e., exercise).
Finally, the results of our study demonstrate that voluntary exercise followed by exposure to a complex environment successfully promoted the survival of adult-generated hippocampal neurons that is otherwise impaired following postnatal alcohol exposure in rats. This suggests that a combined treatment of exercise and EC may significantly improve deficits in hippocampal-dependent learning and memory known to result from heavy prenatal alcohol exposure. However, whether the increased neurogenesis resulting from this combined treatment has beneficial consequences has yet to be established. If it does, we believe that a complex intervention strategy could be utilized in the future to improve behavioral and anatomical outcomes in children with FASD.
Acknowledgments
The authors thank Jennifer Helfer for her contribution to the generation of animals and Stephen St. Cyr for his contribution to the data collection.
Grant sponsor: NIH; Grant number: AA09838.
References
- Aasebo IEJ, Blankvoort S, Tashiro A. Critical maturational period of new neurons in adult dentate gyrus for their involvement in memory formation. European Journal of Neuroscience. 2011;33:1094–1100. doi: 10.1111/j.1460-9568.2011.07608.x. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neuroscience Letters. 2004;363(1):43–48. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Angelucci F, De Bartolo P, Gelfo F, Foti F, Cutuli D, Bossù P, Caltagirone C, Petrosini L. Increased concentrations of nerve growth factor and brain-derived neurotrophic factor in the rat cerebellum after exposure to environmental enrichment. Cerebellum. 2009;8(4):499–506. doi: 10.1007/s12311-009-0129-1. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167:588–597. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10:94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Boehme F, Gil-Mohapel J, Cox A, Patten A, Giles E, Brocardo PS, Christie BR. Voluntary exercise induces adult hippocampal neurogenesis and BDNF expression in a rodent model of fetal alcohol spectrum disorders. Eur J Neurosci. 2011;33(10):1799–811. doi: 10.1111/j.1460-9568.2011.07676.x. [DOI] [PubMed] [Google Scholar]
- Branchi I, D’Andrea I, Sietzema J, Fiore M, Di Fausto V, Aloe L, Alleva E. Early social enrichment augments adult hippocampal BDNF levels and survival of BrdU-positive cells while increasing anxiety- and “depression”-like behavior. J Neurosci Res. 2006;83(6):965–73. doi: 10.1002/jnr.20789. [DOI] [PubMed] [Google Scholar]
- Brandt MD, Maass A, Kempermann G, Storch A. Physical exercise increases Notch activity, proliferation and cell cycle exit of type-3 progenitor cells in adult hippocampal neurogenesis. Eur J Neurosci. 2010;32(8):1256–64. doi: 10.1111/j.1460-9568.2010.07410.x. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. European Journal of Neuroscience. 2003;17(10):2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nature Genetics. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Carro E, Nuñez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20(8):2926–33. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Allan AM, Cunningham LA. Moderate fetal alcohol exposure impairs the neurogenic response to an enriched environment in adult mice. Alcoholism, Clinical and Experimental Research. 2005;29(11):2053–2062. doi: 10.1097/01.alc.0000187037.02670.59. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol. 2004;33(1):63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137(2):437–45. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Darmopil S, Petanjek Z, Mohammed AH, Bogdanovi N. Environmental enrichment alters dentate granule cell morphology in oldest-old rat. Journal of Cellular and Molecular Medicine. 2009;13(8B):1845–1856. doi: 10.1111/j.1582-4934.2008.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MI, Szarowski D, Turner JN, Morrisett RA, Shain W. In vivo activation and in situ BDNF-stimulated nuclear translocation of mitogen-activated/extracellular signal-regulated protein kinase is inhibited by ethanol in the developing rat hippocampus. Neurosci Lett. 1999;272(2):95–8. doi: 10.1016/s0304-3940(99)00572-8. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature Reviews Neuroscience. 2010;11(5):339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Human Development. 1979;311:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- During MJ, Cao L. VEGF, a mediator of the effect of experience on hippocampal neurogenesis. Current Alzheimer Research. 2006;3(1):29–33. doi: 10.2174/156720506775697133. [DOI] [PubMed] [Google Scholar]
- Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Progess in Neurobiology. 2005;75(5):321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, et al. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. The Journal of Comparative Neurology. 2005;486(1):39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75(5):321–41. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. European Journal of Neuroscience. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Fiore M, Laviola G, Aloe L, di Fausto V, Mancinelli R, Ceccanti M. Early exposure to ethanol but not red wine at the same alcohol concentration induces behavioral and brain neurotrophin alterations in young and adult mice. Neurotoxicology. 2009;30(1):59–71. doi: 10.1016/j.neuro.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Gelfo F, De Bartolo P, Giovine A, Petrosini L, Leggio MG. Layer and regional effects of environmental enrichment on the pyramidal neuron morphology of the rat. Neurobiology of Learning and Memory. 2009;91(4):353–365. doi: 10.1016/j.nlm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicology and Teratology. 1997;19(6):435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Griffin EW, Bechara RG, Birch AM, Kelly AM. Exercise enhances hippocampal-dependent learning in the rat: Evidence for a BDNF-related mechanism. Hippocampus. 2009;19:973–980. doi: 10.1002/hipo.20631. [DOI] [PubMed] [Google Scholar]
- Gundersen H, Jensen E. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Jensen EBV, KiÊU K, Nielsen J. The efficiency of systematic sampling in stereology — reconsidered. Journal of Microscopy. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with fetal alcohol syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behavioural Brain Research. 2003;143(1):85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Hannigan J, O’Leary-Moore S, et al. Postnatal environmental or experiential amelioration of neurobehavioral effects of perinatal alcohol exposure in rats. Neuroscience & Biobehavioral Reviews. 2007;31(2):202–211. doi: 10.1016/j.neubiorev.2006.06.019. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacology, Biochemistry and Behavior. 2007;86(2):327–333. doi: 10.1016/j.pbb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Mitchell JJ, Paiva M, Walker DW. Ethanol-induced alterations in the expression of neurotrophic factors in the developing rat central nervous system. Developmental Brain Research. 2000;121:97–107. doi: 10.1016/s0165-3806(00)00032-8. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Madorsky I, Shaw G. Ethanol effects on neonatal rat cortex: comparative analyses of neurotrophic factors, apoptosis-related proteins, and oxidative processes during vulnerable and resistant periods. Brain Res Dev Brain Res. 2003;145(2):249–62. doi: 10.1016/j.devbrainres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Helfer JL, Goodlett CR, Greenough WT, Klintsova AY. The effects of exercise on adolescent hippocampal neurogenesis in a rat model of binge alcohol exposure during the brain growth spurt. Brain Research. 2009;1294:1–11. doi: 10.1016/j.brainres.2009.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J Neurosci Res. 2004;76(2):216–22. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Single alcohol exposure in early life damages hippocampal stem/progenitor cells and reduces adult neurogenesis. Neurobiology of Disease. 2007;26(3):597–605. doi: 10.1016/j.nbd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. PNAS. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TB, Goodlett CR. Selective and enduring deficits in spatial learning after limited neonatal binge alcohol exposure in male rats. Alcoholism: Clinical & Experimental Research. 2002;26(1):83–93. [PubMed] [Google Scholar]
- Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT. Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcoholism, Clinical and Experimental Research. 2007;31(12):2073–2082. doi: 10.1111/j.1530-0277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gross CG, Kopil C, Battaglia L, McBreen M, Stranahan AM, Gould E. Experience induces structural and biochemical changes in the adult primate brain. Proceeding of the National Academy of Science USA. 2005;102(48):17478–17482. doi: 10.1073/pnas.0508817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Waddell J, Gould E, Shors TJ. Temporal discontiguity is neither necessary nor sufficient for learning-induced effects on adult neurogenesis. Journal of Neuroscience. 2006;26(52):13437–13442. doi: 10.1523/JNEUROSCI.2781-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annual Review of Pharmacology and Toxicology. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Liu HL, Zhao G, Cai K, Zhao HH, Shi LD. Treadmill exercise prevents decline in spatial learning and memory in APP/PS1 transgenic mice through improvement of hippocampal long-term potentiation. Behav Brain Res. 2011;218(2):308–14. doi: 10.1016/j.bbr.2010.12.030. [DOI] [PubMed] [Google Scholar]
- Llorens-Martín M, Torres-Alemán I, Trejo JL. Exercise modulates insulin-like growth factor 1-dependent and -independent effects on adult hippocampal neurogenesis and behaviour. Mol Cell Neurosci. 2010;44(2):109–17. doi: 10.1016/j.mcn.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Lou SJ, Liu JY, Chang H, Chen PJ. Hippocampal neurogenesis and gene expression depend on exercise intensity in juvenile rats. Brain Res. 2008;1210:48–55. doi: 10.1016/j.brainres.2008.02.080. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15(3):176–92. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Mirochnic S, Wolf S, Staufenbiel M, Kempermann G. Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of Alzheimer disease. Hippocampus. 2009;19(10):1008–18. doi: 10.1002/hipo.20560. [DOI] [PubMed] [Google Scholar]
- Moore BD, Madorsky I, Paiva M, Barrow Heaton M. Ethanol exposure alters neurotrophin receptor expression in the rat central nervous system: Effects of prenatal exposure. Journal of Neurobiology. 2004;60:101–113. doi: 10.1002/neu.20009. [DOI] [PubMed] [Google Scholar]
- Morleo M, Woolfall K, Dedman D, Mukherjee R, Bellis MA, Cook PA. Under-reporting of foetal alcohol spectrum disorders: an analysis of hospital episode statistics. BMC Pediatr. 2011 Feb 8;11:14. doi: 10.1186/1471-2431-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. Journal of Neurochemistry. 2002;83(5):1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Norman AL, Crocker N, Mattson SN, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Developmental Disabilities Research Reviews. 2009;15(3):209–217. doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16(3):250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. The Journal of Comparative Neurology. 2000;425(4):479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Redila VA, Olson AK, Swann SE, Mohades G, Webber AJ, Weinberg J, Christie BR. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus. 2006;16(3):305–311. doi: 10.1002/hipo.20164. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Social grouping cannot account for cerebral effects of enriched environments. Brain Research. 1978;153(3):563–576. doi: 10.1016/0006-8993(78)90340-2. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56(5):317–26. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Cervos-Navarro J, Dey PK. Increased blood–brain barrier permeability following acute short-term swimming exercise in conscious normotensive young rats. Neuroscience Research. 1991;10:211–221. doi: 10.1016/0168-0102(91)90058-7. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Slomianka L, West MJ. Estimators of the precision of stereological estimates: An example based on the CA1 pyramidal cell layer of rats. Neuroscience. 2005;136:757–767. doi: 10.1016/j.neuroscience.2005.06.086. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus. 1997;(7):232–238. doi: 10.1002/(SICI)1098-1063(1997)7:2<232::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. Journal of Neuroscience. 1999;19(14):5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwani RJ, Buckmaster PS, Varma S, Cosgaya JM, Wu Y, Suri C, Shooter EM. BDNF overexpression increases dendrite complexity in hippocampal dentate gyrus. Neuroscience. 2002;114(3):795–805. doi: 10.1016/s0306-4522(02)00301-9. [DOI] [PubMed] [Google Scholar]
- Trouche SP, Bontempi B, Roullet P, Rampon C. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proceedings of the National Academy of Sciences. 2009;106:5919–5924. doi: 10.1073/pnas.0811054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Borght K, Kobor-Nyakas DE, Klauke K, Eggen BJL, Nyakas C, Van der Zee EA, Meerlo P. Physical exercise leads to rapid adaptations in hippocampal vasculature: Temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009;19(10):928–36. doi: 10.1002/hipo.20545. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceeding of the National Academy of Science USA. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nature Reviews Neuroscience. 2000;1(3):191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: something to chew on. Trends in Neurosciences. 2009;32(5):283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19(4):283–95. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Walsh RN. Effects of environmental complexity and deprivation on brain anatomy and histology: a review. International Journal of Neuroscience. 1981;12(1):33–51. doi: 10.3109/00207458108990671. [DOI] [PubMed] [Google Scholar]
- Yancey SL, Overton JM. Cardiovascular responses to voluntary and treadmill exercise in rats. J Appl Physiol. 1993;75:1334–1340. doi: 10.1152/jappl.1993.75.3.1334. [DOI] [PubMed] [Google Scholar]
- Zucca S, Valenzuela CF. Low concentrations of alcohol inhibit BDNF-dependent GABAergic plasticity via L-type Ca2+ channel inhibition in developing CA3 hippocampal pyramidal neurons. J Neurosci. 2010;30(19):6776–81. doi: 10.1523/JNEUROSCI.5405-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivotosky B, Orrenius S. Assessment of apoptosis and necrosis by DNA fragmentation and morphological criteria. Curr Protoc Cell Biol. 2001;Chapter 18(Unit 18.3) doi: 10.1002/0471143030.cb1803s12. [DOI] [PubMed] [Google Scholar]