Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the loss of tolerance to nucleic acids and several of their binding proteins. Polymorphisms of key genes involved in the regulation of immunity and inflammation contribute to this loss of tolerance.1 Lupus patients can have high titers of IgG anti-nuclear autoantibodies years before they manifest clinical pathology2 that occurs when pathogenic autoantibodies damage organs by direct targeting of cells or by recruiting and activating inflammatory effector cells. In a recent issue of Nature Immunology, Doreau et al.3 show that inflammation can perpetuate autoimmunity through cytokine-driven activation of memory B cells in the absence of cognate T-cell help.
All individuals have ‘natural’ IgM antinuclear antibodies that enhance the noninflammatory clearance of circulating nucleic acid-containing material resulting in immune protection. A pathological response to nuclear antigens in SLE is characterized by class switching to IgG and, commonly, somatic mutations leading to increased affinity of the autoantibodies.4 Cognate T-cell help and costimulation through B7-CD28 are required to initiate secretion of pathogenic IgG autoantibodies in most murine SLE models.
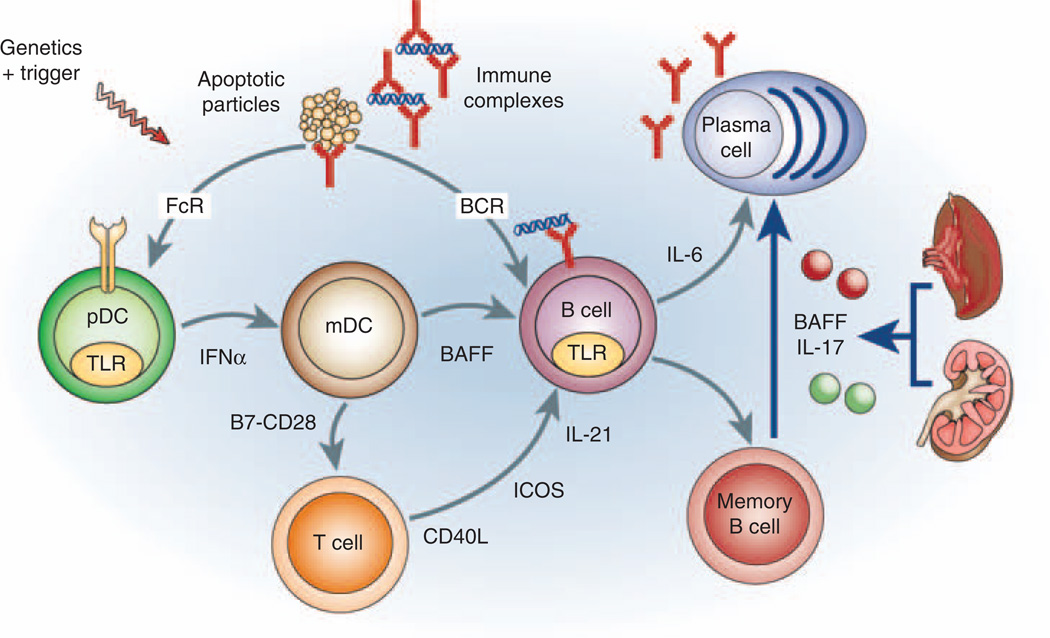
Immune complexes containing IgG antinuclear autoantibodies activate toll-like receptors (TLRs) within plasmacytoid dendritic cells (pDCs), monocytes and B cells.5 In pDCs, TLR engagement induces transcription of proinflammatory mediators, including type I interferons. These activate myeloid (m)DCs to costimulate T cells and to release both BAFF, necessary for B-cell homeostasis, and interleukin (IL)-6, which is required for induction of TH17 cells and optimal plasma cell differentiation.6,7 BAFF may also enhance DC maturation, TLR expression and IL-6 release.8 TLR engagement in B cells increases BCR-mediated signaling and upregulates BAFF receptors,9 resulting in preferential activation and survival of B cells that internalize nucleic acid-containing immune complexes. These amplification loops continue to drive the activation of autoreactive B cells and the production of autoantibodies that sustains inflammatory cascades. Both the innate and the adaptive immune response become dysregulated and interconnect through antigen-specific and nonspecific pathways (Figure 1). The recent failure of several immune-modulating treatments in SLE highlights the redundancy of the inflammatory cascades that operate during active disease.
Figure 1.
A new pathway for memory B-cell activation in systemic lupus erythematosus (SLE). Multiple amplification pathways (shown in gray) are activated in SLE. Doreau et al.3 describe a novel NF-κB-dependent pathway (shown in blue) by which circulating cytokines released from inflamed organs induce differentiation of memory B cells to plasma cells thus perpetuating the autoimmune response. IFN-α, interferon-α; IL, interleukin; mDC, myeloid dendritic cell; pDCs, plasmacytoid dendritic cells; TLRs, toll-like receptors.
The inflammatory milieu of chronic SLE profoundly alters T- and B-cell function. Decreased numbers of B cells10 and high levels of BAFF relax the stringency for B-cell-negative selection.11 An increased number of autoreactive B cells persist within the naïve repertoire even after treatment.12 Although the functional state of the escaping autoreactive B cells is unknown, even anergic cells pose a threat as they can still present antigen and because anergy is potentially reversible. Downregulation of the inhibitory FcγRIIB receptor on memory B cells,13 accumulation of an abnormal population of CD27−/IgD− memory cells14 and defects in the regulation of germinal center selection15 occur at later stages of B-cell ontogeny. T cells from SLE patients exhibit signaling defects that confer a lower threshold for activation and enhanced migratory capabilities. These changes may be persistent as they are associated with alterations in DNA methylation.16 These alterations in B- and T-cell selection and activation, and the sustained recruitment of the innate immune system, result in a trigger-happy immune system that is conducive to disease flares.
The recent paper by Doreau et al.3 identifies a novel mechanism for B-cell activation in SLE patients. The authors show that the threshold for naïve and memory B-cell activation, proliferation and Ig secretion is lowered by the combination of BAFF and IL-17 and that this combination can substitute for either TLR9 or CD40 engagement in BCR-stimulated B cells. IL-17 and BAFF impede apoptosis and induce B-cell differentiation to a plasma cell phenotype through a novel NF-κB-dependent mechanism, different from the pathways activated by engagement of CD40 or BAFF-R. Cognate T-cell help is not necessary in this pathway. They further show that IL-17 and BAFF are B-cell-activating components of SLE serum.
Although the role of excess BAFF in SLE has been clearly shown in murine models,17 the contribution of IL-17 is less well studied. In human SLE, it is released by both CD4+ and CD3+ double-negative T cells and by effector T cells in inflamed organs in which it enhances release of other proinflammatory mediators, and recruitment of other effector cells.18 Studies of the effect of IL-17 on B cells are in their infancy. It has previously been proposed that IL-17 has a role in germinal center development through a direct effect on B cells.19,20 The study by Doreau et al.3 shows that under inflammatory circumstances, IL-17 can also foster the differentiation of memory cells to plasma cells. This article, along with recent studies showing synergy between IL-21 and BAFF in human memory B-cell differentiation,21 shows a unique role for BAFF in T cell-independent, and, in the case of IL-21, antigen-independent production of high-affinity antibodies. As BAFF, IL-17 and IL-21 are elaborated in lymphoid and inflamed organs in SLE, these findings establish a link between systemic inflammation and enhanced production of pathogenic autoantibodies (Figure 1).
Doreau et al.3 add to the growing body of evidence that the immune dysregulation in SLE is the consequence of activation of multiple converging and intersecting inflammatory pathways of both the innate and the adaptive immune system. Both antigen-specific responses and tissue-specific inflammatory pathways drive an escalating immune response in which the ability to control cellular activation by normal regulatory mechanisms is lost. Where does this leave us in terms of therapy for SLE? With so many inflammatory pathways activated, the clinician is currently forced to choose between excessive immune suppression and continuing inflammation and progressive organ damage. Studies of mouse models have taught us that early intervention aimed at preventing expansion of autoreactive B cells is much more effective than intense therapy aimed at tissue effector cells. Once pathogenic IgG autoantibodies arise and tissue inflammation is initiated, therapies directed at single cytokines or activation pathways often fail and more aggressive drug combinations that may be unacceptably immunosuppressive in humans are required to induce remission. Earlier intervention and a more aggressive approach to remission maintenance may reduce morbidity from incremental tissue damage and prolonged immunosuppression.
References
- 1.Crow MK. Collaboration, genetic associations, lupus erythematosus. N Engl J Med. 2008;358:956–961. doi: 10.1056/NEJMe0800096. [DOI] [PubMed] [Google Scholar]
- 2.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 3.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 4.Wellmann U, Letz M, Herrmann M, Angermüller S, Kalden JR, Winkler TH. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci USA. 2005;102:9258–9263. doi: 10.1073/pnas.0500132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrat FJ, Coffman RL. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol Rev. 2008;223:271–283. doi: 10.1111/j.1600-065X.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 6.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pascual V, Farkas L, Banchereau J. Systemic lupus erythematosus: all roads lead to type I interferons. Curr Opin Immunol. 2006;18:676–682. doi: 10.1016/j.coi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Lai Kwan Lam Q, King Hung Ko O, Zheng BJ, Lu L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc Natl Acad Sci USA. 2008;105:14993–14998. doi: 10.1073/pnas.0806044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treml LS, Carlesso G, Hoek KL, Stadanlick JE, Kambayashi T, Bram RJ, et al. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J Immunol. 2007;178:7531–7539. doi: 10.4049/jimmunol.178.12.7531. [DOI] [PubMed] [Google Scholar]
- 10.Odendahl M, Jacobi A, Hansen A, Feist E, Hiepe F, Burmester GR, et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol. 2000;165:5970–5979. doi: 10.4049/jimmunol.165.10.5970. [DOI] [PubMed] [Google Scholar]
- 11.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Wardemann H, Nussenzweig MC. B-cell self-tolerance in humans. Adv Immunol. 2007;95:83–110. doi: 10.1016/S0065-2776(07)95003-8. [DOI] [PubMed] [Google Scholar]
- 13.Mackay M, Stanevsky A, Wang T, Aranow C, Li M, Koenig S, et al. Selective dysregulation of the FcgammaIIB receptor on memory B cells in SLE. J Exp Med. 2006;203:2157–2164. doi: 10.1084/jem.20051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, et al. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol. 2007;178:6624–6633. doi: 10.4049/jimmunol.178.10.6624. [DOI] [PubMed] [Google Scholar]
- 15.Pugh-Bernard AE, Silverman GJ, Cappione AJ, Villano ME, Ryan DH, Insel RA, et al. Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J Clin Invest. 2001;108:1061–1070. doi: 10.1172/JCI12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crispin JC, Kyttaris VC, Juang YT, Tsokos GC. How signaling and gene transcription aberrations dictate the systemic lupus erythematosus T cell phenotype. Trends Immunol. 2008;29:110–115. doi: 10.1016/j.it.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Ramanujam M, Davidson A. BAFF blockade for systemic lupus erythematosus—will the promise be fulfilled? Immunol Rev. 2008;223:156–174. doi: 10.1111/j.1600-065X.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- 18.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarlinton D. IL-17 drives germinal center B cells? Nat Immunol. 2008;9:124–126. doi: 10.1038/ni0208-124. [DOI] [PubMed] [Google Scholar]
- 20.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 21.Ettinger R, Sims GP, Robbins R, Withers D, Fischer RT, Grammer AC, et al. IL-21 and BAFF/BLyS synergize in stimulating plasma cell differentiation from a unique population of human splenic memory B cells. J Immunol. 2007;178:2872–2882. doi: 10.4049/jimmunol.178.5.2872. [DOI] [PubMed] [Google Scholar]