Abstract
Intracranial germ cell tumours (GCTs) are a rare and diverse group of tumours. Histology determines the type of treatment, natural history and prognosis. Germinoma GCTs account for the majority and are very radiosensitive. Conversely, non-germinoma GCTs (NGGCTs) are heterogeneous, relatively radioresistant and have a high risk of relapse despite multimodality therapy. Treatment of recurrence in these patients remains a therapeutic challenge. In this report we review the current literature for treatment of recurrent NGGCTs and present a case of recurrent NGGCT who relapsed despite autologous stem cell transplant (ASCT). The patient was successfully cured using multimodality therapy, consisting of repeat ASCT, intensity modulated radiation therapy and stereotactic radiosurgery.
Background
Intracranial germ cell tumours (GCTs) are a rare and heterogeneous class of tumours, whose treatment and prognosis depend largely on histology. Germinoma GCTs are highly radiosensitive and are effectively managed with radical radiotherapy (RT) in the majority of patients. Non-germinoma germ cell tumours (NGGCTs) are histologically diverse, relatively radioresistant and carry with them a high risk of relapse despite multimodality treatment. Curative options in the management of recurrent NGGCTs are limited by prior treatment and pose a unique therapeutic challenge. This case report describes the curative treatment of a patient with a second NGGCT recurrence using multimodality therapy, consisting of intensity modulated radiation therapy (IMRT), stereotactic radiosurgery and high-dose chemotherapy with autologous stem cell transplant (ASCT).
Case presentation
A 27-year-old man presented with a 1 month history of persistent headaches, nausea and vomiting. The patient had no other associated neurological symptoms and had no history of significant medical problems.
Investigations
First presentation
A CT scan of the head showed a hyperdense, well-circumscribed 3.2×2.5 cm lesion situated in the region of the pineal gland with mass effect on the third ventricle and resultant obstructive hydrocephalus.
MRI scan confirmed a 3 cm mass in the pineal region that showed no evidence of craniospinal seeding (figure 1).
Figure 1.
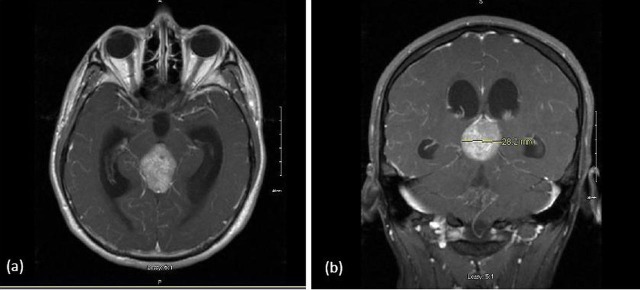
T1 postgadolinium MRI of the pineal region germinoma: (A) axial view and (B) coronal view.
Tumour markers, including alpha-fetoprotein (AFP) and B-human chorionic gonadotropin (b-HCG), were negative.
A stereotactic biopsy was carried out confirming the diagnosis of germinoma GCT.
Treatment
The patient received RT using a three-dimensional conformal technique to a dose of 5000 cGy in 25 fractions over 5 weeks. The treatment was well tolerated and resulted in the rapid resolution of clinical symptoms. Treatment response was confirmed by a CT scan at 1 month and an MRI at 2 months post-RT, demonstrating a 70% and 75% reduction in tumour size, respectively.
Outcome and follow-up
The clinical evaluation of the patient during follow-up consisted of neuroimaging and serological measurements of tumour markers every 3 months. The patient remained clinically well through the first year of follow-up.
First recurrence
Thirteen months after the start of RT the patient demonstrated elevated tumour markers (AFP=22.5, b-HCG=1451). An MRI of the brain revealed that while the initial pineal tumour had continued to regress, two new intracranial lesions had developed (figure 2). There was no evidence of spinal seeding or hematogenous metastases. The first tumour, in the subarachnoid space of the right lateral convexity, was successfully resected. Pathology revealed an NGGCT with leptomeningeal spread.
Figure 2.
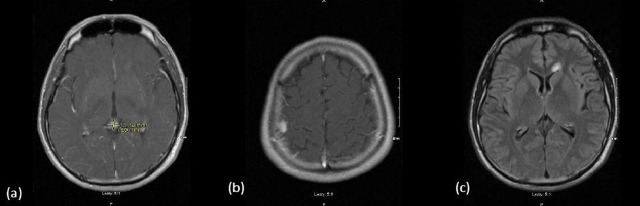
Imaging at first relapse. (A) Axial T1 postgadolinium image of regression of initial pineal germinoma, (B) Axial T1 postgadolinium image of relapse in right frontal region and (C) axial T2 FLAIR image of relapse in left frontal region.
The second tumour, in the left frontal periventricular region, was treated with myeloablative high-dose chemotherapy and an ASCT. Peripheral blood stem cells were collected following one cycle of high-dose cyclophosphamide (4500 mg/m2) intravenous plus 10 days of daily filgrastim (300 μg subcutaneously). A total of 12.69×106 CD34 cells/kg were collected by apheresis and stored at the Canadian Blood Services for autologous stem cell rescue.
High-dose chemotherapy initially consisted of two cycles ifosfamide 2500 mg/m2 intravenous daily×4, carboplatin 500 mg/m2 intravenous daily×3 and etoposide 600 mg/m2 intravenous daily×3 separated by 1 month.
The patient was rescued by reinfusion of his peripheral blood stem cells containing 4.23×106 CD34 cells/kg given 72 h after the last dose of chemotherapy with each cycle. Filgrastim 300 μg daily subcutaneously was given starting 7 days after the stem cell rescue.
During high-dose chemotherapy he developed WHO Grade II–III mucositis/stomatitis, neutropaenic enterocolitis, neutropenia and thrombocytopenia as well as Grade II anaemia and fatigue. Successful stem cell rescue was confirmed by an absolute neutrophil count of >0.5 on day 11 after the stem cell rescue in the first transplant and day 10 after the stem cell rescue in the second transplant. Following the second transplant, the patient developed a fungal infection with Aspergillus flavus in his forearm, which was successfully treated with liposomal amphotericin-B.
Treatment response was evaluated by an MRI at 1 month and revealed significant tumour regression. The patient had a complete serological response to treatment, with normalisation of AFP and b-HCG tumour markers.
Second recurrence
Three months after the stem cell transplant the patient demonstrated elevated levels of AFP (1.9) and b-HCG (8). Tumour recurrence of the left frontal lesion was confirmed by brain MRI. The patient was managed aggressively with RT and an ASCT following one cycle of high-dose chemotherapy.
Radiotherapy details
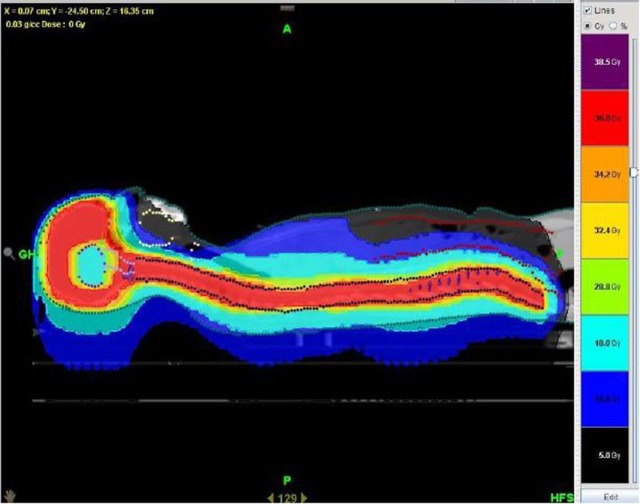
Craniospinal radiotherapy to a total dose of 3600 cGy was delivered in 20 fractions using IMRT on a helical Tomotherapy unit (figure 3). During treatment planning, the previously irradiated pineal region was defined as an organ at risk and the dose to this region was restricted to 1800 cGy. Daily, online treatment verification was carried out using megavolt CT imaging. During treatment, the frontal lobe tumour was also boosted using stereotactic radiosurgery on a linear accelerator to increase the chance for cure. The treatment boost was delivered using a four arc non-coplanar treatment plan with 15 Gy delivered to the 90% isodose line. Radiotherapy was well tolerated with grade 1 fatigue, nausea and alopecia.
Figure 3.
Craniospinal radiotherapy using Tomotherapy IMRT. The dose to the previously irradiated pineal region was restricted to 18 Gy.
High-dose chemotherapy and ASCT
High-dose chemotherapy consisted of ifosfamide 2500 mg/m2 intravenous daily×4, carboplatin 500 mg/m2 intravenous daily×3 and etoposide 600 mg/m2 intravenous daily×3 (ifosfamide 4125 mg intravenous daily×4, carboplatin 800 mg intravenous daily×3 and etoposide 960 mg intravenous daily×3). Stem cell rescue given 72 h after the last dose of chemotherapy and filgrastim 300 μg daily subcutaneously was started on day 7 after the stem cell rescue. Toxicities were assessed as with the first two transplants and successful stem cell rescue was confirmed by an absolute neutrophil count of >0.5 on day 10 after the stem cell rescue.
Nine months after the completion of salvage therapy, the patient developed MRI evidence of radiation necrosis at the site of radiosurgery, in the absence of clinical symptoms. The necrosis resolved radiologically following a 6-week tapering course of dexamethasone.
On long-term follow-up 30 months post second salvage treatment, the patient remains well with normal tumour markers and no evidence of disease recurrence or radiation necroses. The patient has a short-term memory deficit but no other neurocognitive deficits. He is currently pursuing a degree in accounting at college.
Discussion
Intracranial GCT account for less than 5% of all paediatric tumours and tumours among young adults.1 Approximately two-thirds of these tumours are germinoma with the remaining one-third of tumours having non-germinomatous histology.2 While there is a lack of consensus on the standard management of intracranial GCT, radical irradiation to a dose of 50 Gy is generally regarded as an effective treatment for germinoma GCT.3 Many reports suggest 5-year survival rates of >90% in this patient population following radical radiation therapy.4 5
In the case presented herein, our patient was diagnosed with a primary germinoma GCT. Based on his early recurrence as a non-germinomatous GCT, we believe that he may have actually presented with a mixed tumour. Recent reports suggest that as many as 25% of paediatric intracranial GCT are mixed,6 the treatment of which can be challenging. Non-germinoma germ cell tumours are histologically diverse and carry with them a much worse prognosis than germinoma GCT.2 While some NGGCTs respond to RT, most NGGCTs are radioresistant. Craniospinal irradiation, with or without resection,2 7 has been shown to result in poor long-term survival, with 5-year rates of overall survival of less than 50%.2 5 8 Like their systemic counterparts, NGGCTs have a demonstrated chemosensitivity and respond well to carboplatin, cisplatin and etoposide.9 10
The high relapse rate and poor long-term survival of patients following chemotherapy alone,11 however, advocates for a more aggressive approach in the definitive management of primary NGGCT. Multimodality treatment with chemotherapy and craniospinal radiation has been shown to produce better long-term survival than monotherapy12–14 and is the most promising approach in the management of newly diagnosed NGGCT. Recurrent NGGCT pose a unique therapeutic challenge. Salvage therapies are often limited by prior treatment, but may include additional surgery, radiation therapy or high-dose chemotherapy with autologous stem cell rescue. Only the latter of these treatments is believed to carry curative potential in the management of recurrent NGGCT.15
Introduction of high-dose chemotherapy and ASCT in the management of intracranial GCT was based on the success of this treatment in the management of recurrent systematic GCT.16–18 By increasing passage through the blood–brain barrier, alkylating agents such as thiotepa,19 etoposide16 and cisplatin17 are believed to reduce chemotherapy resistance and improve treatment outcomes. Due to the myeloablative nature of high-dose chemotherapy, autologous stem cell rescue following treatment is required to reinstate haematopoiesis. In the management of intracranial GCT, high-dose chemotherapy and ASCT has typically been reserved for the treatment of recurrent disease.19 20 This approach has also met with some success in the management of primary NGGCT.21
While the treatment of recurrent intracranial GCT has been poorly documented, what limited data are available suggest that recurrent germinoma GCT has better treatment outcomes as compared with recurrent NGGCT following high-dose chemotherapy and ASCT.19
In a retrospective case series by Modak and colleagues, the results of 21 patients with recurrent intracranial GCT managed with high-dose chemotherapy and autologous stem cell rescue were reviewed. Of the nine patients with germinoma GCT, seven survived disease-free after a median of 48 months of follow-up.19 These results compare favourably to the survival of patients with NGGCT, two-thirds of whom (8/12) died of progressive disease within 17 months of treatment.19 While retrospective in nature, these results point to the differential effectiveness of this treatment regimen, based on histology, in the management of recurrent intracranial GCT. In the case of our patient, high-dose chemotherapy and ASCT for the first recurrence resulted in normalisation of tumour markers and a dramatic regression of the left frontal tumour. Treatment response, however, was not sustained. This raises the question as to whether a more aggressive treatment regimen to consolidate tumour remission and improve survival may be required for recurrent NGGCT. The poor survival of patients with recurrent NGGCT tumours following high-dose chemotherapy in the Modak study, described above, supports his view.
At the time of the second recurrence, our patient received multimodality therapy that consisted of IMRT and radiosurgery with high-dose chemotherapy and ASCT. The success of this treatment points to the potential utility of this aggressive approach in the treatment of recurrent NGGCT. Modern-day radiation therapy has evolved to the point where selective sparing and re-irradiation in patients previously treated with radical RT is now possible.
The prognosis of patients with NGGCT, particularly those with recurrent disease, is worrisomely low. Given the lack of standard treatment for NGGCT, case reports such as ours may provide important clues in guiding the future management of this high-risk population. Importantly, capitalising on the advances of modern technology in the re-treatment of patients may add to the armamentarium of curative treatment alternatives for recurrent NGGCT.
Learning points.
Prognosis of recurrent intracranial NGGCT is poor.
Myeloablative chemotherapy with autologous stem cell transplant is a curative option in these patients.
Aggressive management of local relapses in this patient population with ASCT can result in cure and should be pursued using advances in technology to minimise toxicity.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Bloom HJ. Primary intracranial germ cell tumors. Clin Oncol 1983;2: 233–57. [Google Scholar]
- 2.Jennings MT, Geiman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg 1985;63:155–67. [DOI] [PubMed] [Google Scholar]
- 3.Echevarria ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: a review. Oncologist 2008;13:690–9. [DOI] [PubMed] [Google Scholar]
- 4.Bamberg M, Kortmann RD, Calaminus G, et al. Radiation therapy for intracranial germinoma: results of the German cooperative prospective trials MAKEI 83/86/89. J Clin Oncol 1999;17:2585–92. [DOI] [PubMed] [Google Scholar]
- 5.Matsutani M, Sano K, Takakura K, et al. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg 1997;86:446–55. [DOI] [PubMed] [Google Scholar]
- 6.Calaminus G, Garre M. Germ-cell tumors of the central nervous system. In: Walker PA, Perilongo G, Punt JAG, et al, eds. Brain and spinal tumors of childhood. London: Arnold, 2004:345–56. [Google Scholar]
- 7.Dearnaley DP, A'Hern RP, Whittaker S, et al. Pineal and CNS germ cell tumors. Royal Marsden hospital experience 1962–1987. Int J Radiat Oncol Biol Phys 1990;18:773–81. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman HJ, Otsubo H, Hendrick EB, et al. Intracranial germ-cell tumors in children. J Neurosurg 1991;74:545–51. [DOI] [PubMed] [Google Scholar]
- 9.Allen JC, Walker R, Luks E, et al. Carboplatin and recurrent childhood brain tumors. J Clin Oncol 1987;5:459–63. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida J, Sugita K, Kobayashi T, et al. Prognosis of intracranial germ cell tumors: effectiveness of chemotherapy with cisplatin and etoposide (CDDP adn VP-16). Acta Neurochir (Wien) 1993;120:111–17. [DOI] [PubMed] [Google Scholar]
- 11.Kellie SJ, Boyce H, Dunkel IJ, et al. Primary chemotherapy for intracranial nongerminomatous germ cell tumors: results of the second international CNS germ cell study group protocol. J Clin Oncol 2004;22:846–53. [DOI] [PubMed] [Google Scholar]
- 12.Robertson PL, DaRosso RC, Allen JC. Improved prognosis of intracranial non-germinoma germ cell tumors with multimodality therapy. J Neurooncol 1997;32:71–80. [DOI] [PubMed] [Google Scholar]
- 13.Matsutani M. Combined chemotherapy and radiation therapy for CNS germ cell tumors: the Japanese experience. J Neurooncol 2001;54:311–16. [DOI] [PubMed] [Google Scholar]
- 14.Calaminus G, Bamberg M, Baranzelli MC, et al. Intracranial germ cell tumors: a comprehensive update of the European data. Neuropediatrics 1994;25:26–32. [DOI] [PubMed] [Google Scholar]
- 15.Gardner SL. Application of stem cell transplant for brain tumors. Pediatr Transplant 2004;8Suppl. 5:28–32. [DOI] [PubMed] [Google Scholar]
- 16.Motzer RJ, Mazumdar M, Bosl GL, et al. High-dose carboplatin, etoposide, and cyclophosphamide for patients with refractory germ cell tumors: treatment results and prognostic factors for survival and toxicity. J Clin Oncol 1996;14:1098–105. [DOI] [PubMed] [Google Scholar]
- 17.Nichols CR, Andersen J, Lazarus HM, et al. High-dose carboplatin and etoposide with autologous bone marrow transplantation in refractory germ cell cancer: an Eastern Cooperative Oncology Group protocol. J Clin Oncol 1992;10:556–63. [DOI] [PubMed] [Google Scholar]
- 18.Beyer J, Kramar A, Mandanas R, et al. High-dose chemotherapy as salvage treatment in germ cell tumors: a multivariate analysis of prognostic variables. J Clin Oncol 1996;14:2638–45. [DOI] [PubMed] [Google Scholar]
- 19.Modak S, Gardner S, Dunkel IJ, et al. Thiotepa-based high-dose chemotherapy with autologous stem-cell rescue in patients with recurrent or progressive CNS germ cell tumors. J Clin Oncol 2004;22:1934–43. [DOI] [PubMed] [Google Scholar]
- 20.Bouffet E, Baranzelli MC, Patte C, et al. on behalf of the Society Francaise d'Oncologie Pediatrique (SFOP). High dose etoposide and thiotepa for refractory and recurrent malignant intracranial germ cell tumors (CNS-GCT). Neurooncol 2000;2Suppl. 2:s72. [Google Scholar]
- 21.Tada T, Takizama T, Nakazato F, et al. Treatment of intracranial nongerminomatous germ-cell tumor by high-dose chemotherapy and autologous stem-cell rescue. J Neurooncol 1999;44:71–6. [DOI] [PubMed] [Google Scholar]