Abstract
Objective
Salt-induced elevation of the endogenous digitalis-like sodium pump ligand marinobufagenin (MBG) in the Dahl salt-sensitive rats (DS) resulted in elevated blood pressure (BP). Here, we tested, in humans, whether (1) MBG levels are related to ambulatory 24-hour BP (ABP), (2) controlled long term increase of salt-intake induces changes in MBG, and (3) any salt induced change in MBG is related to salt sensitivity.
Methods
Thirty-nine healthy subjects (53±11 years old; 20 males and 19 females) had a total daily NaCl intake of 50 mmol (low-salt) and 150 mmol (high-salt) for 4 weeks each, in random order. ABP and MBG in plasma and urine were measured at baseline (unstandardized salt intake) and after high- and low-salt intake.
Results
At baseline, plasma MBG (P-MBG) was related to 24-hour systolic (r= 0.43, P=0.007) and diastolic (r=0.32, P=0.047) BP, whereas 24-hour urinary excretion of MBG (UE-MBG) was related to 24-hour diastolic BP only (r=0.42, P=0.008). Gender specific analyses revealed that these relationships were significant in males only. Compared to low-salt, high-salt diet increased P-MBG (P=0.029), mainly driven by results in men. Male P-MBG responders vs. non-responders (above vs. below median of high-salt induced P-MBG increase) had markedly enhanced systolic (10.4±6.4 vs. 1.0±6.0 mmHg; P=0.003) and diastolic (6.7±5.0 vs. −0.6±3.6 mmHg; P=0.001) BP salt-sensitivity.
Conclusion
In males MBG increases with 24-hour ABP, and similar to DS, 4-weeks of high-salt induced MBG response is accompanied by marked salt sensitivity. However, these patterns seem to be gender specific and are not observed in females.
Keywords: Marinobufagenin, salt sensitivity, dietary sodium, gender specificity
INTRODUCTION
High salt-intake contributes to development of essential hypertension and salt intake reduction is one of the cornerstones in life style prevention and treatment of hypertension [1, 2]. The blood pressure elevating effect following increased salt intake, i.e. the degree of salt sensitivity, differs substantially among individuals [1] and has a Gaussian distribution [3], suggesting a multifactorial origin. It is known that increasing age, hypertension and low plasma renin concentration [1, 3–6] are independent factors associated with enhanced salt sensitivity of blood pressure.
Endogenous digitalis like cardiotonic steroids (CTS) have been implicated in sodium homeostasis and in blood pressure regulation through effects on the Na/K-ATPase in renal and cardiovascular tissue [7–10]. Human CTS include a bufadienolide marinobufagenin (MBG), a vasoconstrictor [11–13] which exhibits high affinity to α1-isoform of the Na/K-ATPase, the main Na/K-ATPase isoform in the renal tubules [14, 15]. In humans, elevated level of MBG has been observed in several pathological states of fluid retention such as heart failure, essential hypertension, chronic renal failure and primary aldosteronism [13, 16, 17].
Dahl salt sensitive rats (DS) have the impaired ion-pumping properties of renotubular Na/K-ATPase and thus exhibit several phenotypic similarities with human salt sensitive hypertension [18, 19]. Four weeks of high-salt intake in DS led to an elevation of MBG levels and blood pressure increase, whereas neither MBG nor blood pressure changed in the Dahl salt resistant rat (DR) [20]. Notably, the blood pressure elevation following high-salt intake in DS was attenuated by treatment with an anti-MBG antibody [20]. These results suggested that the in DS, long term high salt-intake gradually increases MBG in an attempt to enhance natriuresis by inhibiting the renal Na/K-ATPase, but that a blunted natriuretic response of the renal α1- Na/K-ATPase leads to excessive MBG production, inhibition of vascular smooth muscle Na/K-ATPase, vasoconstriction and blood pressure elevation. In our previous study in middle-aged human subjects with moderately elevated blood pressure, dietary sodium restriction reduced MBG excretion and systolic blood pressure [21]. While sub-acute NaCl loading of normotensive human subjects was reported to stimulate MBG [4], whether or not MBG is related to the salt-sensitivity of blood pressure in humans, remains unknown.
Here, we hypothesized that MBG and MBG responsiveness to salt may be involved in regulation of blood pressure and salt sensitivity in humans. To address this issue, we studied middle aged individuals who underwent two 4-week periods each of controlled high- (150 mmol/day) and low-salt (50 mmol/day) intake in random order, and who were monitored with 24-hour ambulatory blood pressure (ABP).
METHODS
The protocol of the study was approved by the ethics committee of Lund University, and all study participants gave written informed consent. The procedures were in accordance with institutional guidelines.
Subjects
The study protocol has been described in detail previously [1]. Briefly, 46 unmedicated study subjects without history of hypertension, diabetes or kidney disease were recruited via advertisements in local newspapers. Of these, 39 subjects (20 men and 19 women) completed the study. The mean age of these 39 subjects was 53 ± 11 years and body mass index (BMI) was 26.3 ± 3.1. Other baseline characteristics are shown in Table 1.
Table 1.
The clinical characteristics of study subjects on unstandardized salt intake at baseline, after 4 weeks of a high salt diet, and after 4 weeks of a low salt diet in all subjects (Table 1a), in males (Table 1b), and females (Table 1c).
| Table 1a | ||||
|---|---|---|---|---|
| All subjects (n=39) | Baseline | High-salt (150 mmol per day) | Low-salt (50 mmol per day) | P* (high- vs. low-salt) |
| 24-hour SBP (mmHg) | 139 ± 13.3 | 136 ± 12.7 | 131 ± 11.1 | <0.0001 |
| 24-hour DBP (mmHg) | 86.3 ± 7.4 | 85.0 ± 7.0 | 82.3 ± 6.6 | 0.004 |
| Body weight (kg) | 79.5 ± 11.2 | 77.4 ± 10.7 | 77.3 ± 10.6 | 0.43 |
| Urine-Na+ (mmol/24h) | 165 ± 67.4 | 140 ± 39.5 | 50.7 ± 17.3 | <0.0001 |
| Urine-K+ (mmol/24h) | 75.0 ± 22.9 | 50.8 ± 11.3 | 50.9 ± 14.1 | 0.94 |
| Urine-Crea (mmol/24h) | 13.1 ± 3.3 | 12.3 ± 2.7 | 12.2 ± 3.0 | 0.65 |
| Serum-Na+ (mmol/L) | 140 ± 1.8 | 141 ± 1.5 | 139 ± 1.7 | <0.0001 |
| Serum-K+ (mmol/L) | 3.7 ± 0.26 | 3.6 ± 0.25 | 3.7 ± 0.30 | 0.21 |
| Serum-Crea (nmol/L) | 78.4 ± 12.8 | 80.4 ± 12.8 | 84.1 ± 12.6 | 0.001 |
| Table 1b. | ||||
|---|---|---|---|---|
| Male subjects (n=20) | Baseline | High-salt | Low-salt | P* (high- vs. low-salt) |
| 24-hour SBP (mmHg) | 138 ± 12.4 | 135 ± 13.1 | 130 ± 11.5 | 0.004 |
| 24-hour DBP (mmHg) | 87.2 ± 6.3 | 85.4 ± 7.1 | 82.4 ± 6.4 | 0.026 |
| Body weight (kg) | 85.3 ± 9.4 | 83.1 ± 8.7 | 82.8 ± 9.0 | 0.35 |
| Urine-Na+ (mmol/24h) | 175 ± 56.7 | 143 ± 44.8 | 49.5 ± 19.2 | <0.0001 |
| Urine-K+ (mmol/24h) | 76.8 ± 23.7 | 50.2 ± 9.0 | 50.8 ± 9.4 | 0.89 |
| Urine-Crea (mmol/24h) | 15.0 ± 3.3 | 14.1 ± 2.2 | 13.9 ± 3.2 | 0.73 |
| Serum-Na+ (mmol/L) | 141 ± 2.1 | 141 ± 1.5 | 139 ± 1.9 | <0.0001 |
| Serum-K+ (mmol/L) | 3.7 ± 0.29 | 3.7 ± 0.25 | 3.7 ± 0.28 | 0.85 |
| Serum-Crea (nmol/L) | 85.6 ± 10.4 | 87.4 ± 9.9 | 92.0 ± 9.3 | 0.001 |
| Table 1c. | ||||
|---|---|---|---|---|
| Female subjects (n=19) | Baseline | High-salt | Low-salt | P* (high- vs. low-salt) |
| 24-hour SBP (mmHg) | 140 ± 14.5 | 138 ± 12.6 | 132 ± 10.9 | 0.002 |
| 24-hour DBP (mmHg) | 85.4 ± 8.6 | 84.5 ± 7.0 | 82.3 ± 7.1 | 0.075 |
| Body weight (kg) | 73.4 ± 9.7 | 71.1 ± 9.2 | 71.5 ± 9.1 | 0.89 |
| Urine-Na+ (mmol/24h) | 154 ± 77.3 | 136 ± 33.9 | 51.9 ± 15.5 | <0.0001 |
| Urine-K+ (mmol/24h) | 72.9 ± 22.6 | 51.4 ± 13.7 | 50.9 ± 18.4 | 0.87 |
| Urine-Crea (mmol/24h) | 11.1 ± 1.8 | 10.4 ± 1.8 | 10.4 ± 1.4 | 0.78 |
| Serum-Na+ (mmol/L) | 140 ± 1.3 | 140 ± 1.5 | 140 ± 1.3 | 0.10 |
| Serum-K+ (mmol/L) | 3.7 ± 0.24 | 3.5 ± 0.23 | 3.7 ± 0.33 | 0.14 |
| Serum-Crea (nmol/L) | 70.9 ± 10.7 | 72.8 ± 11.4 | 75.7 ± 10.1 | 0.16 |
Values are means ± standard deviation (SD). P* - Refer to individual change of variable on high- as compared with on low-salt. SBP, systolic blood pressure; DBP, diastolic blood pressure; Crea, Creatinine.
Subjects were first examined under baseline conditions on their habitual diets, i.e. on unstandardized salt-intake. Following the baseline visit, they were given all meals and drinks containing 50 mmol of NaCl (3 grams) and 50 mmol potassium per day during the entire study period of 8 weeks. All meals and drinks were provided from our metabolic ward. The diet was composed by a dietician and daily energy intake was adjusted according to body weight and gender (2000–2600 kcal/day). All foods were produced and packed at Findus R&D AB (Bjuv, Sweden). Subjects were advised to consume everything they received from us, and were prohibited to ingest anything else during the study apart from tap water. On top of the standardized diet, each subject was given 100 mmol (6 grams) of NaCl per day (3 × 500 mg capsules 4 times daily) for 4 weeks and corresponding number of placebo capsules for 4 weeks in random order with double-blind crossover design. Thus, all subjects underwent two different 4-week periods with 150 mmol NaCl (9 grams) intake per 24-hours (high-salt) and 50 mmol NaCl (3 grams) intake per 24-hours (low-salt), respectively. Production of NaCl capsules, placebo capsules as well as blinding, coding and packing procedures were performed by Apoteket AB (Swedish state pharmacy).
Subjects were examined with similar protocol at baseline and at the end of the low-salt and high-salt periods. Venous blood was drawn and anthropometric measures were obtained, and an ABPM 90207 device (Spacelabs Medical Inc, Redmond, WA, USA) was applied on the left arm for 24-hour ambulatory blood pressure (ABP) measurement. Two different cuff sizes were used depending on the arm circumference (24–32 cm and 32–42 cm, respectively) of the study subjects. Study subjects were advised to maintain the left arm relaxed along the body during each measurement. Twenty four hours urinary collections were also obtained during the 24-hours of ABP measurements. When subjects came to return the ABP device and the 24-hour urine collection one of the two different levels of salt intake (low salt or high salt) started or salt level changed. Degree of systolic and diastolic salt sensitivity was defined as the difference between 24-hour ABP after high-salt and 24-hour ABP after low-salt.
Biochemical Assays
Urine and serum concentrations of sodium, potassium and creatinine were measured by standard biochemical methods at the Department of Clinical Chemistry, Malmö University Hospital. Marinobufagenin concentration in plasma (P-MBG) and urine was measured using a solid-phase DELFIA fluoroimmunossay based on 4G4 anti-MBG mouse monoclonal antibody, as reported previously in detail [4]. Urinary excretion of marinobufagenin in urine during 24-hours was calculated (UE-MBG).
Statistics
All data was analyzed with SPSS statistical software (version 11.5, SPSS Inc. Chicago, Illinois, USA). Values are means ± standard deviation (SD) of not otherwise specified. Due to skewed distributions, P-MBG and UE-MBG levels were log transformed before any analyses. Significance of differences of paired variables (i.e. changes induced by different levels of salt intake) was tested by paired t-test or Wilcoxon’s paired rank test, where appropriate, whereas significance of differences between groups was tested with t-test. Pearson’s test of correlations (r) was used to calculate correlations. Linear regression models were used when crude correlations were significant with backward elimination of covariates at a retention P-value of <0.10. All tests were two-sided and throughout P< 0.05 was considered statistically significant.
RESULTS
The clinical characteristics of study subjects on unstandardized salt-intake at baseline, after 4 weeks of high- (150 mmol per day) and after 4 weeks of low-salt (50 mmol per day) diet are shown in Table 1. As expected, because of their low awareness of hypertension, some subjects had hypertensive levels of blood pressure despite no history of known hypertension. There was a highly significant increase of blood pressure after high- compared to after low-salt with an average systolic salt sensitivity of 5.8 ± 7.4 mmHg and diastolic salt sensitivity of 2.6 ± 5.3 mmHg. Both genders demonstrated similar changes in salt-sensitive changes in blood pressure: there was no difference in systolic blood pressure drop from high to low salt intake (i.e. the degree of salt sensitivity) between men and women (5.7±7.8 mmHg vs. 5.9±7.2 mmHg, P=0.92, correspondently; Table 1a and 1b). Urinary sodium excretion indicated good compliance to the standardized salt intake (Table 1).
At baseline, P-MBG was positively correlated to 24-hour systolic and diastolic blood pressure whereas UE-MBG was positively correlated to 24-hour diastolic blood pressure. Gender specific analyses, however, revealed that these correlations were only significant among males (Table 2). Correlations between MBG and 24-hour blood pressure were weaker and mainly present in males after 4 weeks of low- and high-salt (Table 2). There was a significant interaction between salt-induced change of P-MBG and gender on systolic salt sensitivity (P=0.034) and diastolic salt sensitivity (P=0.014), however, there was no significant interactions between baseline P-MBG and gender on baseline blood pressure, nor between UE-MBG and gender on baseline blood pressure.
Table 2.
24-hour ambulatory blood pressure in relation to marinobufagenin under non-standardized salt intake (baseline)
| Baseline (unstandardized salt-intake) | ||||
|---|---|---|---|---|
| r Pearson* | Pcrude * | Padjusted *† | β (mmHg per 1 SD increase) *† | |
| 24-h SBP (All subjects, n=39) | ||||
| P-MBG | 0.43 | 0.007 | 0.007 | 5.7 |
| UE-MBG | 0.21 | 0.19 | ||
| 24-h DBP (All subjects, n=39) | ||||
| P-MBG | 0.32 | 0.047 | 0.047 | 2.4 |
| UE-MBG | 0.42 | 0.008 | 0.005 | 3.2 |
| 24-h SBP (Males, n=20) | ||||
| P-MBG | 0.45 | 0.047 | 0.047 | 4.7 |
| UE-MBG | 0.37 | 0.11 | ||
| 24-h DBP (Males, n=20) | ||||
| P-MBG | 0.45 | 0.048 | 0.048 | 2.4 |
| UE-MBG | 0.49 | 0.029 | 0.029 | 2.9 |
| 24-h SBP (Females, n=19) | ||||
| P-MBG | 0.43 | 0.064 | ||
| UE-MBG | 0.09 | 0.70 | ||
| 24-h DBP (Females, n=19) | ||||
| P-MBG | 0.27 | 0.26 | ||
| UE-MBG | 0.36 | 0.13 | ||
| High salt intake (150 mmol/day) | ||||
|---|---|---|---|---|
| r Pearson* | Pcrude * | Padjusted *† | β (mmHg per 1 SD increase) *† | |
| 24-h SBP (All subjects, n=39) | ||||
| P-MBG | 0.22 | 0.18 | ||
| UE-MBG | 0.14 | 0.39 | ||
| 24-h DBP (All subjects, n=39) | ||||
| P-MBG | 0.31 | 0.056 | ||
| UE-MBG | 0.38 | 0.019 | 0.026 | 2.4 |
| 24-h SBP (Males, n=20) | ||||
| P-MBG | 0.34 | 0.14 | ||
| UE-MBG | 0.45 | 0.047 | 0.046 | 5.9 |
| 24-h DBP (Males, n=20) | ||||
| P-MBG | 0.52 | 0.019 | 0.019 | 4.2 |
| UE-MBG | 0.61 | 0.004 | 0.004 | 4.6 |
| 24-h SBP (Females, n=19) | ||||
| P-MBG | 0.17 | 0.49 | ||
| UE-MBG | −0.11 | 0.66 | ||
| 24-h DBP (Females, n=19) | ||||
| P-MBG | 0.11 | 0.65 | ||
| UE-MBG | 0.13 | 0.60 | ||
| Low salt intake (50 mmol/day) | ||||
|---|---|---|---|---|
| r Pearson* | Pcrude * | Padjusted *† | β (mmHg per 1 SD increase) *† | |
| 24-h SBP (All subjects, n=39) | ||||
| P-MBG | 0.23 | 0.16 | ||
| UE-MBG | 0.18 | 0.26 | ||
| 24-h DBP (All subjects, n=39) | ||||
| P-MBG | 0.22 | 0.19 | ||
| UE-MBG | 0.35 | 0.031 | 0.031 | 2.3 |
| 24-h SBP (Males, n=20) | ||||
| P-MBG | 0.29 | 0.22 | ||
| UE-MBG | 0.32 | 0.17 | ||
| 24-h DBP (Males, n=20) | ||||
| P-MBG | 0.42 | 0.067 | ||
| UE-MBG | 0.52 | 0.019 | 0.019 | 3.1 |
| 24-h SBP (Females, n=19) | ||||
| P-MBG | 0.15 | 0.54 | ||
| UE-MBG | 0.06 | 0.79 | ||
| 24-h DBP (Females, n=19) | ||||
| P-MBG | −0.03 | 0.91 | ||
| UE-MBG | 0.17 | 0.48 | ||
P-MBG, plasma concentration of marinobufagenin; UE-MBG, 24-hour urinary excretion of marinobufagenin
P-MBG and UE-MBG transformed with the natural logarithm
P-MBG and UE-MBG adjusted for age, sex and body mass index using linear regression with backward elimination retaining independent variables in the model at P<0.10
Overall, compared to low-salt, high-salt intake induced a small but significant elevation in P-MBG, whereas UE-MBG remained the same, and again, this pattern was driven by males (Table 3). P-MBG was significantly lower during unstandardized salt intake when compared to conditions of high- as well as low-salt intake in both gender (P<0.001 for all), whereas UE-MBG tended to be higher during unstandardized salt intake and was significantly higher in males when compared to low-salt intake (Table 3).
Table 3.
Changes in plasma MBG (P-MBG) and urinary MBG (U-MBG) at baseline, high-salt, and low-salt intake in all subjects, and in males and females separately.
| Baseline | High-salt | Low-salt | P* (high- vs. low-salt) | |
|---|---|---|---|---|
| All subjects (n=39) | ||||
| P-MBG (pmol/L) | 111 (67.5–155) | 262 (175–367) | 233 (159–303) | 0.029 |
| UE-MBG (pmol/24 hours) | 1224 (796–1483) | 1080 (824–1321) | 1078 (709–1224) | 0.21 |
| Males (n=20) | ||||
| P-MBG (pmol/L) | 84.5 (63.0–158) | 279 (215–366) | 250 (124–305) | 0.067 |
| UE-MBG (pmol/24 hours) | 1301 (825–1722) | 1187 (935–1468) | 1133 (793–1362) | 0.15 |
| Females (n=19) | ||||
| P-MBG (pmol/L) | 124 (83.0–150) | 225 (146–367) | 214 (166–274) | 0.21 |
| UE-MBG (pmol/24 hours) | 1063 (780–1380) | 973 (678–1135) | 980 (634–1180) | 0.78 |
Values are medians (interquartile range). P* - Refer to individual change of variable on high- as compared with on low-salt. P-MBG, plasma concentration of marinobufagenin; UE-MBG, 24-hour urinary excretion of marinobufagenin
Next we studied the correlations of P-MBG and UE-MBG changes (calculated as a difference of values between high-salt intake and low-salt intake at the end of each condition, i.e. ΔP-MBG and ΔUE-MBG) and 24-hour blood pressure changes (i.e. degree of salt sensitivity). There was a significant correlation between ΔP-MBG and degree of both systolic and diastolic blood pressure salt sensitivity in males but not in females. There was no relationship between ΔUE-MBG and the degree of salt sensitivity in any gender (Table 4). After adjustment for ambulatory systolic and diastolic ABP respectively, delta P-MBG remained significantly associated with both systolic (P=0.044) and diastolic (P=0.028) salt sensitivity.
Table 4.
Relationship between salt sensitivity of 24-hour ambulatory blood pressure (difference between blood pressure after high-salt diet and blood pressure after low-salt diet) and high-salt induced increase in plasma marinobufagenin.
| r Pearson* | Pcrude * | Padjusted *† | β (mmHg per 1 SD increase) *† | |
|---|---|---|---|---|
| 24-h SBP salt sensitivity (All subjects, n=39) | ||||
| Δ P-MBG | 0.22 | 0.17 | ||
| ΔUE-MBG | 0.23 | 0.17 | ||
| 24-h DBP salt sensitivity (All subjects, n=39) | ||||
| Δ P-MBG | 0.22 | 0.19 | ||
| ΔUE-MBG | 0.24 | 0.14 | ||
| 24-h SBP salt sensitivity SBP (Males, n=20) | ||||
| Δ P-MBG | 0.54 | 0.014 | 0.014 | 4.0 |
| ΔUE-MBG | 0.16 | 0.51 | ||
| 24-h DBP salt sensitivity (Males, n=20) | ||||
| Δ P-MBG | 0.55 | 0.013 | 0.013 | 3.0 |
| ΔUE-MBG | 0.25 | 0.30 | ||
| 24-h SBP salt sensitivity (Females, n=19) | ||||
| Δ P-MBG | −0.15 | 0.53 | ||
| ΔUE-MBG | 0.30 | 0.21 | ||
| 24-h DBP salt sensitivity (Females, n=19) | ||||
| Δ P-MBG | −0.25 | 0.31 | ||
| ΔUE-MBG | 0.22 | 0.36 | ||
ΔP-MBG, difference in plasma concentration of marinobufagenin after high-salt versus that after low-salt; Δ UE-MBG, difference in 24-hour urinary excretion of marinobufagenin after high-salt versus that after low-salt diet;
ΔP-MBG and ΔUE-MBG transformed with the natural logarithm
ΔP-MBG and ΔUE-MBG adjusted for age, sex and body mass index at baseline using linear regression with backward elimination retaining independent variables in the model at P<0.10
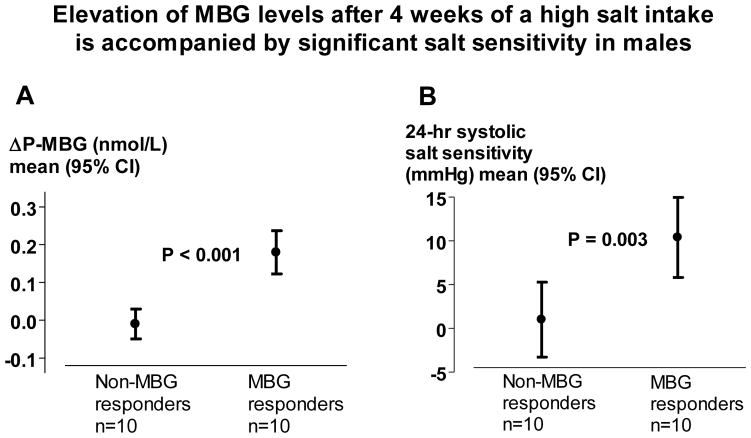
To discriminate blood pressure salt sensitivity in males who changed and who did not change P-MBG in response on a high-salt diet, we divided the male subjects by the median (inter-quartile range) of ΔP-MBG into two subgroups: 10 “P-MBG responders” with median 129 (116 to 161) pmol/L change of P-MBG on a high-salt vs. low-salt intake, and 10 “P-MBG non-responders” with virtually no change of P-MBG on a high-salt compared to low-salt intake (−27.6; −71.1 to 30.1 pmol/L). Moreover, whereas P-MBG-responders displayed pronounced systolic (10.4 ± 6.4 mmHg, P=0.001) and diastolic (6.7 ± 5.0 mmHg, P=0.002) salt sensitivity, P-MBG non-responders had neither significant systolic (1.0 ± 6.0 mmHg, P=0.61) nor diastolic (−0.6 ± 3.6 mmHg, P=0.61) salt sensitivity. The difference between two groups was highly significant (Figure 1). The significant difference remained after adjustment for baseline ambulatory systolic blood pressure (P=0.008).
Figure 1.
Elevation of plasma MBG (P-MBG) after 4 weeks of a high salt intake is accompanied by significant salt sensitivity in males. Differences in P-MBG between high and low salt intake in male non-MBG responders and MBG responders; P < 0.001; t-test (a). Salt-sensitivity presented as a difference of systolic blood pressure between high and low salt intake parameters measured at the end of each 4-weeks period in male non-MBG responders and MBG-responders; P = 0.003; t-test (b). Values are means ± standard deviation (SD).
In our previous study, in middle-aged normotensive females, UE-MBG was negatively related to age and to the NaCl-induced pressor response, suggesting that a relative deficiency in MBG response to NaCl loading may be related to salt-sensitivity [4]. In the present study we examined the relationship between these parameters, and while on a high-salt intake UE-MBG in subjects of both sexes was negatively related to age, the magnitude of UE-MBG response to a high-salt intake (high-salt / low-salt ratio) exhibited a positive correlation with age in males, but not in females (Figure 2).
Figure 2.
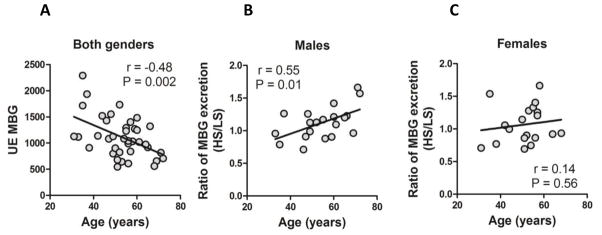
Correlation of aging and urinary MBG during a high salt diet. Correlation between 24-hr urinary MBG excretion (UE-MBG) and age in both genders (a). Correlation between magnitude of MBG response (UE-MBG-HS/UE-MBG-LS) and age in males (b) and females (c). HS – high-salt intake; LS – low-salt intake.
DISCUSSION
We examined the relationship between MBG, 24-hour blood pressure and salt sensitivity in middle-aged individuals during long term controlled conditions of a high-sodium (150 mmol/day) and a low-sodium (50 mmol/day) intake. We found evidence that (i) P-MBG and UE-MBG increased in high- vs. low-salt intake and were correlated to salt-sensitivity of 24-hour systolic and diastolic blood pressure in males, and that (ii) males who responded with increase in P-MBG on high- vs low-salt intake are highly salt sensitive, whereas males who did not respond with a P-MBG increase are salt resistant. Intriguingly, among females no such relationships could be found.
The results in males showed several similarities to those observed in previous studies of male Dahl rats [20]. (i) We observed the most consistent correlations between blood pressure and MBG at measurement points when salt intake was high (i.e. on high-salt diet and at baseline) and in DS, blood pressure is MBG-dependent only during high salt intake [20]. (ii) Not all males responded with marked MBG elevation when moving from the 4 weeks of controlled low-salt intake to the 4 weeks of controlled high-salt intake. This is likely due to the complex genetic background of humans which is expected to resemble a mix of the genetic background of the DS, which responded with marked MBG elevation following 4 weeks of high salt, and the DR, in which MBG did not change after high-salt intake [20]. (iii) The degree of P-MBG elevation was positively correlated to the degree of blood pressure elevation (i.e. salt sensitivity) at the end of a high-salt diet compared to a low-salt diet. Importantly, males with a DS-like P-MBG response also resembled DS by having pronounced salt sensitivity of blood pressure, whereas those with a DR-like P-MBG response resembled DR by having blood pressure resistant to high salt intake [20].
In salt-sensitive hypertension, an endogenous MBG increased with an adaptive aim to stimulate natriuresis by inhibiting renal Na/K-ATPase. This primary adaptive mechanism of stimulation MBG biosynthesis at the scenario of an inadequate sodium excretion will lead to an excessive MBG production, which induces maladaptive pro-hypertensive effect by inhibiting of vascular Na/K-ATPase and by potentiating vasoconstriction [22]. Interestingly, in our previous study, dietary sodium restriction (low salt vs. normal salt) reduced MBG excretion, and urinary MBG was positively correlated to SBP in middle-aged human subjects with moderately elevated blood pressure [21]. Although in the previous [21] and in the present studies we used different levels of salt intake, the results of both studies are in agreement with each other, because the magnitude and direction of changes in MBG levels and MBG association with blood pressure between different dietary/salt conditions are the same in both cases.
The blood pressure variance explained in males by MBG at baseline during unstandardized salt-intake did not seem to be cofounded by age and body mass index. The reason why there was no salt induced change of MBG and no relationship between MBG and blood pressure and salt sensitivity in women is not clear. Importantly, this finding agrees with our previous observation that in middle-aged normotensive females NaCl-induced MBG response was negatively related to magnitude of blood pressure during high NaCl intake [4]. It is possible that progesterone, which exerts natriuretic effects [23], and competes with CTS for the binding sites on sodium pump [24], could influence MBG response to high NaCl intake in women. Importantly, the interaction between salt-induced changes of plasma MBG and gender on systolic and diastolic salt-sensitivity was observed on a high salt diet, and not at the baseline. Thus the difference between genders and MBG vs. blood pressure at non-standardized diet should be interpreted with caution.
One unexpected observation was that during the study subjects’ habitual nonstandardized diets at baseline, where 24-hour sodium excretion, and thus presumably salt intake, was the highest, P-MBG was significantly lower than on both high- and low-salt intake in both sexes whereas UE-MBG tended to be higher at baseline, at least in males. The lower baseline P-MBG observed on a non-standardized diet may be a result of the higher potassium content of the food during the dietary non-controlled part of the study with lesser need of potassium sparing MBG blockade of the renal Na/K-ATPase. Given the tendency of higher UE-MBG at baseline, increased renal excretion of MBG may at least partially explain the lower P-MBG at the same time point. Previously it was demonstrated that potassium supplement activated Na/K-ATPase [25], lowered blood pressure [26], and increased sodium excretion [27, 28], and this antihypertensive effect of potassium is attributed to its impact on natriuresis [27, 28]. These findings indicated on the causative link between potassium and MBG, an endogenous natriuretic hormone and Na/K-ATPase inhibitor. In any case, in the present experiment, the diet was identical except for sodium content during the controlled part of the study, when we have found salt-dependent changes of MBG (Table 3) and significant correlations of MBG and salt-sensitivity in males (Table 2).
The present study has some limitations. First of all, the number of study subjects, especially when stratified by gender, is limited. This is the main reason why the present finding cannot be generalized, although the present data are in agreement with our previous observations. Previously we have demonstrated, that salt-dependent increase of MBG levels in population of middle-age and older women inversely related to NaCl sensitivity and SBP [4]. At the same time, in the clinical study with a crossover design by Jablonski at al. [21], in which the gender mixed sample was mostly presented by men, NaCl-dependent MBG levels positively correlated to SBP. In the present study, the absence of correlations of MBG to blood pressure in women and positive correlations of these parameters in men are echoed in the above previous observations. It will be important to replicate our findings in larger populations. Furthermore, although we tried to mimic and translate the previous experiment of DS and DR [20] by controlling the salt diet rigorously for 4-week periods and applying 24-hour blood pressure measurements, it was impossible to avoid environmental noise, which affected the blood pressure data, and although 24-hour sodium excretion indicated good compliance, one cannot assume complete compliance. Also, the salt doses given to the rats during high salt intake were much higher than what would be possible to administer to human subjects. Finally, we do not have sequential MBG and 24-hour blood pressure measurements, e.g. weekly, during the 4 week periods which would have been informative in assessing temporal relationships between variations of salt intake, MBG and blood pressure.
In conclusion, in males MBG increases in parallel with blood pressure and the MBG response to high-salt is heterogeneous. This, together with the fact that salt sensitivity is dependent on a DS-like MBG response, suggests that MBG is an important factor in mediating salt-induced elevations of blood pressure in males. The relatively large effect sizes on 24-hour blood pressure variance by MBG in males merits further investigation, and larger studies including prospective ones are warranted to replicate this finding and prospectively examine whether MBG independently predicts hypertension and hypertension-related complications. However, the fact that we did not find any evidence of relations between MBG, blood pressure and salt sensitivity in females suggesting different gender-dependent underlying mechanisms for salt-sensitivity, which would merit additional studies.
Acknowledgments
Supported in part by Intramural Research Program, National Institute on Aging, NIH. Authors gratefully acknowledge excellent technical support by Anton Bzhelyanskiy.
Footnotes
Conflicts of interest: There are no conflicts of iterest.
Supplementary digital content files: none
References
- 1.Melander O, Wowern F, Frandsen E, Burri P, Willsteen G, Aurell M, et al. Moderate salt restriction effectively lowers blood pressure and degree of salt sensitivity is related to baseline concentration of renin and N-terminal atrial natriuretic peptide in plasma. J Hypertens. 2007;25(3):619–27. doi: 10.1097/HJH.0b013e328013cd50. [DOI] [PubMed] [Google Scholar]
- 2.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8(6 Pt 2):127–134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DE, Fedorova OV, Morrell CH, Longo DL, Kashkin VA, Metzler JD, et al. Endogenous sodium pump inhibitors and age-associated increases in salt sensitivity of blood pressure in normotensives. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1248–R1254. doi: 10.1152/ajpregu.00782.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Midgley JP, Matthew AG, Greenwood CM, Logan AG. Effect of reduced dietary sodium on blood pressure: a meta-analysis of randomized controlled trials. Jama. 1996;275(20):1590–1597. doi: 10.1001/jama.1996.03530440070039. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger MH, Fineberg NS. Sodium and volume sensitivity of blood pressure: age and pressure change over time. Hypertension. 1991;18:67–71. doi: 10.1161/01.hyp.18.1.67. [DOI] [PubMed] [Google Scholar]
- 7.Blaustein MP. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. Am J Physiol. 1977;232(5):C165–C173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- 8.Dahl LK, Knudsen KD, Iwai J. Humoral transmission of hypertension: evidence from parabiosis. Circ Res. 1969;24(5) Suppl:21–33. [PubMed] [Google Scholar]
- 9.Haddy FJ, Overbeck HW. The role of humoral agents in volume expanded hypertension. Life Sci. 1976;19(7):935–947. doi: 10.1016/0024-3205(76)90284-8. [DOI] [PubMed] [Google Scholar]
- 10.Ludens JH, Clark MA, DuCharme DW, Harris DW, Lutzke BS, Mandel F, et al. Purification of an endogenous digitalislike factor from human plasma for structural analysis. Hypertension. 1991;17(6 Pt 2):923–929. doi: 10.1161/01.hyp.17.6.923. [DOI] [PubMed] [Google Scholar]
- 11.Bagrov AY, Fedorova OV, Dmitrieva RI, Howald WN, Hunter AP, Kuznetsova EA, et al. Characterization of a urinary bufodienolide Na+,K+-ATPase inhibitor in patients after acute myocardial infarction. Hypertension. 1998;31(5):1097–1103. doi: 10.1161/01.hyp.31.5.1097. [DOI] [PubMed] [Google Scholar]
- 12.Fedorova OV, Dorofeeva NA, Lopatin DA, Lakatta EG, Bagrov AY. Phorbol diacetate potentiates Na(+)-K(+) ATPase inhibition by a putative endogenous ligand, marinobufagenin. Hypertension. 2002;39(2):298–302. doi: 10.1161/hy0202.104344. [DOI] [PubMed] [Google Scholar]
- 13.Lopatin DA, Ailamazian EK, Dmitrieva RI, Shpen VM, Fedorova OV, Doris PA, et al. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J Hypertens. 1999;17(8):1179–1187. doi: 10.1097/00004872-199917080-00018. [DOI] [PubMed] [Google Scholar]
- 14.Fedorova OV, Bagrov AY. Inhibition of Na/K ATPase from rat aorta by two Na/K pump inhibitors, ouabain and marinobufagenin: evidence of interaction with different alpha-subunit isoforms. Am J Hypertens. 1997;10(8):929–935. doi: 10.1016/s0895-7061(97)00096-4. [DOI] [PubMed] [Google Scholar]
- 15.Fedorova OV, Lakatta EG, Bagrov AY. Endogenous Na,K pump ligands are differentially regulated during acute NaCl loading of Dahl rats. Circulation. 2000;102(24):3009–3014. doi: 10.1161/01.cir.102.24.3009. [DOI] [PubMed] [Google Scholar]
- 16.Gonick HC, Ding Y, Vaziri ND, Bagrov AY, Fedorova OV. Simultaneous measurement of marinobufagenin, ouabain, and hypertension-associated protein in various disease states. Clin Exp Hypertens. 1998;20(5–6):617–627. doi: 10.3109/10641969809053240. [DOI] [PubMed] [Google Scholar]
- 17.Tomaschitz A, Piecha G, Ritz E, Meinitzer A, Haas J, Pieske B, et al. Marinobufagenin in essential hypertension and primary aldosteronism: a cardiotonic steroid with clinical and diagnostic implications. Clin Exp Hypertens. 2014 Apr 30; doi: 10.3109/10641963.2014.913604. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Herrera VL, Xie HX, Lopez LV, Schork NJ, Ruiz-Opazo N. The alpha1 Na,K-ATPase gene is a susceptibility hypertension gene in the Dahl salt-sensitive HSD rat. J Clin Invest. 1998;102(6):1102–1111. doi: 10.1172/JCI3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orosz DE, Hopfer U. Pathophysiological consequences of changes in the coupling ratio of Na,K-ATPase for renal sodium reabsorption and its implications for hypertension. Hypertension. 1996;27(2):219–227. doi: 10.1161/01.hyp.27.2.219. [DOI] [PubMed] [Google Scholar]
- 20.Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. Endogenous ligand of alpha(1) sodium pump, marinobufagenin, is a novel mediator of sodium chloride--dependent hypertension. Circulation. 2002;105(9):1122–1127. doi: 10.1161/hc0902.104710. [DOI] [PubMed] [Google Scholar]
- 21.Jablonski KL, Fedorova OV, Racine ML, Geolfos CJ, Gates PE, Chonchol M, et al. Dietary sodium restriction and association with urinary marinobufagenin, blood pressure, and aortic stiffness. J Am Soc Nephrol. 2013;8(11):1952–1959. doi: 10.2215/CJN.00900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharm Rev. 2009;61:9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mujais SK, Nora A, Chen Y. Regulation of the renal Na:K pump: role of progesterone. J Am Soc Nephrol. 1993;(3):1488–1495. doi: 10.1681/ASN.V381488. [DOI] [PubMed] [Google Scholar]
- 24.Morrill GA, Kostellow AB, Askari A. Progesterone binding to the alpha-1-subunit of the Na/K-ATPase on the cell surface: Insights from computational modeling. Steroids. 2008;73(1):27–40. doi: 10.1016/j.steroids.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helou CM, Araujo M, Seguro AC. Effect of low and high potassium diets on H+-K+-ATPase and Na+-K+-ATPase activities in rat inner medullary collecting duct cells. Renal Physiol Biochem. 1994;17:21–26. doi: 10.1159/000173784. [DOI] [PubMed] [Google Scholar]
- 26.MacGregor GA, Smith SJ, Markandu ND, Banks RA, Sagnella GA. Moderate potassium supplementation in essential hypertension. Lancet. 1982;2(8298):567–570. doi: 10.1016/s0140-6736(82)90657-2. [DOI] [PubMed] [Google Scholar]
- 27.Mano M, Sugawara A, Nara Y, Nakao K, Horie R, Endo J, et al. Potassium accelerates urinary sodium excretion during salt loading without stimulating atrial natriuretic polypeptide secretion. Clin Exper Pharmacol Physiol. 1992;19:795–801. doi: 10.1111/j.1440-1681.1992.tb00417.x. [DOI] [PubMed] [Google Scholar]
- 28.Smith SR, Klotman PE, Svetkey LP. Potassium chloride lowers blood pressure and causes natriuresis in older patients with hypertension. J Am Soc Nephrol. 1992;2(8):1302–1309. doi: 10.1681/ASN.V281302. [DOI] [PubMed] [Google Scholar]