Abstract
We describe a case of commotio cordis in which the patient had an extensive cardiac evaluation, including ECGs, a coronary angiogram, a left ventriculogram, repeated echocardiography and cardiovascular MRI (CMRI). A healthy 17-year-old boy sustained an open-handed blow to the anterior part of the chest from a friend with whom he was playing. On admission ECG was performed that showed ST-T alterations and a TNI increase, with echocardiographic evidence of a localised pericardial effusion associated with a persistent myocardial blush at selective angiography. In addition, CMRI confirmed a local delayed enhancement in the same zone. An echocardiogram examination performed 30 days after discharge showed a complete disappearance of pericardial effusion and an improvement on ECG alterations. This is the first case report of a patient with commotio cordis, who did not show any arrhythmias and did not receive any resuscitation procedure, and was extensively studied by imaging methods.
Background
One of the most increasingly reported causes of sudden cardiac death in young adults is that of commotio cordis. This phenomenon may be a ‘pure’ instance of mechanically induced arrhythmia. With the increased awareness of this condition over the last years, the number of reported cases of commotio has dramatically risen. The Commotio Cordis Registry has over 130 patients and approximately 10 new cases are reported each year.1 Still, it is probable that the actual number of cases is greater than reported because of the lack of awareness of this devastating condition and underreporting to the Commotio Cordis Registry. Collapse in most cases is immediate, although some report up to a minute of presyncope prior to collapse. At autopsy, no acute or chronic cardiac pathology, or damage to the ribs, sternum or heart have been found that would account for the sudden death. An absence of structural cardiac injury distinguishes commotio cordis from cardiac contusion, in which high-impact blows result in traumatic damage to myocardial tissue and the overlying thorax.
Commotio cordis occurs primarily in children, adolescents and young adults, most often during participation in certain recreational or competitive sports, with rare occurrences during normal, routine daily activities.1–8 We describe a case of commotio cordis in which the patient had an extensive cardiac evaluation, including ECGs, a coronary angiogram, a left ventriculogram, repeated echocardiography and cardiovascular MRI (CMRI). These clinical details suggest that the mechanism of commotio cordis may be more complex than solely ventricular fibrillation.
Case presentation
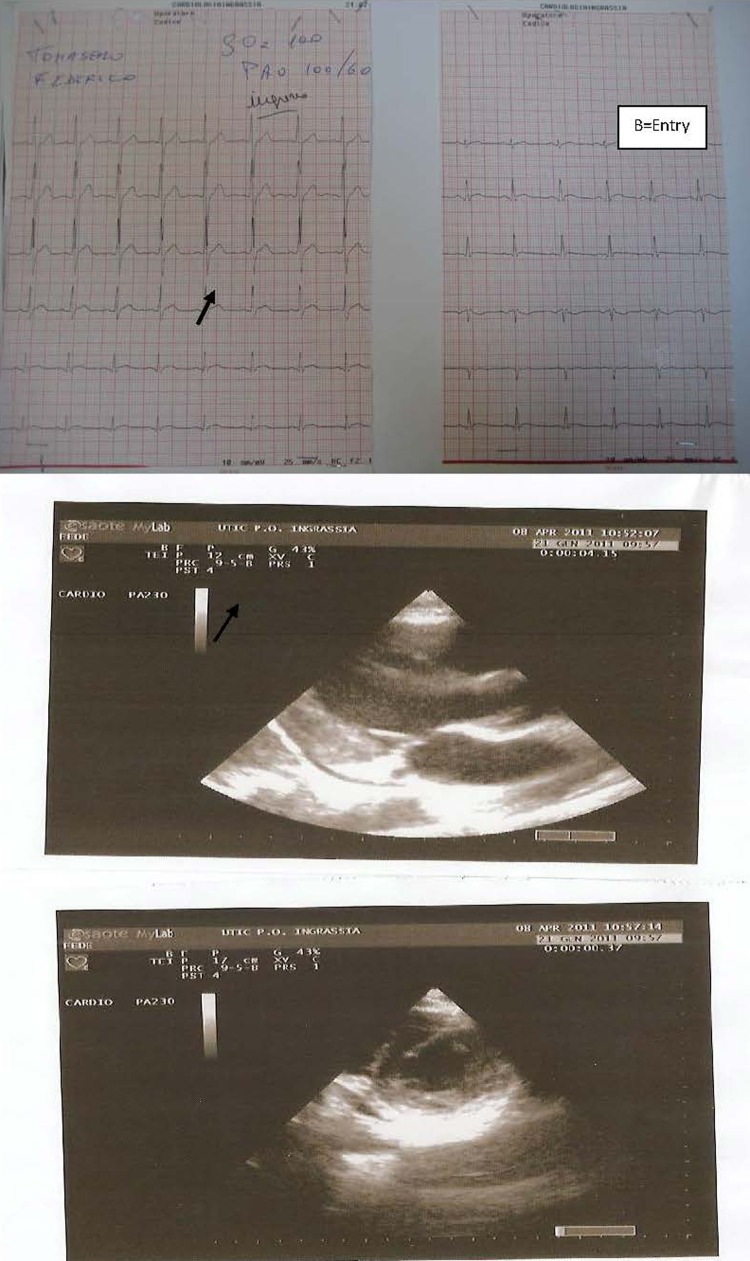
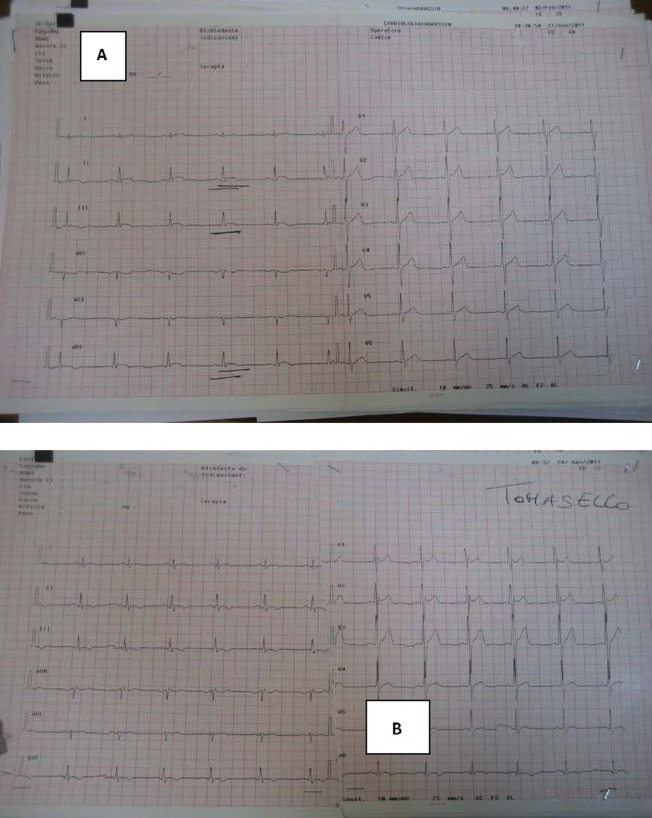
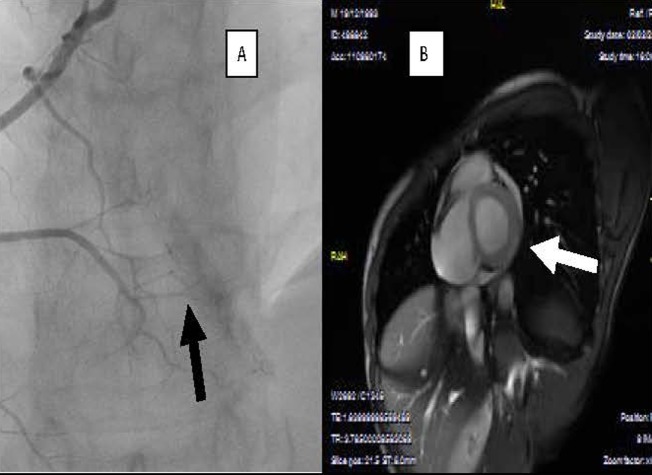
A healthy 17-year-old boy sustained an open-handed blow to the anterior part of the chest from a friend with whom he was playing. The patient, 4 h after the chest blow, complained of chest pains with resolution by paracetamol consumption. Owing to the recurrence of chest pain he underwent medical control and thorax Rx which did not reveal any thoracic alteration. The following day the patient continued to complain of thorax discomfort and was referred to our emergency department. On admission ECG showed ST-T alterations (figure 1). In addition, troponin I (TNI) plasma determination showed a significant increase (18 pg/ml) and the patient was hospitalised in the coronary care unit (CCU). On admission to the CCU an echocardiogram was performed and it showed a localised pericardial effusion (inferior-posterior) and complete absence of segmentary kinetic alterations (figure 1). Serial ECGs, and echocardiography, during hospitalisation, showed ST-T changes and localised pericardial effusion as diagnosed at entry to the emergency department, while laboratory analysis revealed a TNI increase (30 pg/ml). No arrhythmic event was observed. In addition, all the laboratory parameters were in normal range, also including inflammatory indices (high sensitivity C reactive protein, white blood cell count, erythrocyte sedimentation rate, etc). Anti-inflammatory treatment (indomethacin 50 mg twice daily) was immediately started, but 12 h after admission the patient continued to complain of chest pains. For this reason a thoracic spiral CT was performed, which did not show any pulmonary vascular or lung damage. About 30 min after the spiral CT the patient showed an increase in chest pain; however, the echocardiogram remained unmodified whereas the ECG showed an increase in ST-T alterations (figure 2). Under the assumption that coronary damage had occurred, a coronary and left ventricle angiography was performed. The coronary tree was normal, while the selective angiography of the right coronary showed an important delayed and persistent myocardial blush in the inferior-posterior zone, on the same side as the pericardial effusion revealed by the echocardiogram (figure 3). After the coronary angiography and analgesic administration the chest pain ceased completely and the ECG showed T-wave inversion in inferior leads (figure 2). The patient continued anti-inflammatory treatment without any problem in the subsequent days, and 4 days after admission normalisation in TNI plasma levels was observed associated with a reduction of the localised pericardial effusion diagnosed on entry. Before discharge (7 days after admission), in order to obtain a thorough understanding of the patient's condition a cardiovascular CMRI (figure 3) was performed. The CMRI showed a persistent local delayed enhancement in the inferior-posterior myocardial wall, and this finding was in line with the evidence of myocardial blush and the pericardial effusion observed by echocardiography in this zone. The ECG and the echocardiogram control performed 30 days after discharge showed the complete disappearance of pericardial effusion and an improvement on ECG alterations.
Figure 1.
(A) Echocardiogram on admission. (B) ECG on admission.
Figure 2.
(A) ECG during chest pain and before coronary angiography. (B) ECG postcoronary angiography.
Figure 3.
(A) Myocardial blush regarding right coronary territory and (B) predischarge, cardiovascular MRI.
Learning points.
Recent experimental laboratory studies conducted under controlled conditions with pigs, dogs and rabbits,9–13 have provided insights into the underlying mechanisms of commotio cordis that are consistent with its clinical profile and have dispelled the notion that sudden death after a blow to the chest is a mysterious phenomenon.1 2
The cellular (and subcellular) mechanisms responsible for commotio cordis appear to be multifactorial and complex, and they remain incompletely defined. Information obtained from experimental and animal models13–17 has led to hypotheses concerning specific mechanistic pathways. It is believed that the mechanical force generated by precordial blows during repolarisation causes left ventricular intracavitary pressure.10 14 15 18
It has been hypothesised that this elevation in pressure causes the cell membranes to stretch, activating ion channels and increasing transmembrane current flow by means of mechanical–electric coupling.9 10 13 16 Autopsies on commotio cordis victims are notable for the lack of coronary artery damage, coronary artery thrombosis or myocardial infarction.2 12 Another possibility is that myocardial contusion causes the ECG and clinical abnormalities. However, despite frequent ECG abnormalities and contusions in animal models of chest wall trauma, ventricular fibrillation is only rarely seen.19 In human commotio cordis victims, occasional myocardial haemorrhage is seen, but it is never extensive and is usually ascribed to resuscitation efforts.2 This case report gives a rare glimpse into the pathophysiological characteristics of commotio cordis because of the relatively complete and serial studies of the patient's cardiac anatomy and physiological features. Clinically the description of the patient is typical of previous descriptions of commotio cordis victims. He is young and healthy, but did not have sudden cardiac death following the chest wall trauma. In this patient who did not present any arrhythmic event and received no resuscitation procedure, the ECG, echocardiographic, angiographic and cardiovascular MRI (CRMI) findings all suggest that the sudden death in commotio cordis for ventricular fibrillatory arrest could also have other causes. Prolonged ST-T abnormalities are unusual in a primary ventricular fibrillation arrest and ST-T abnormalities would be more common with myocardial ischaemia or myocardial contusion. Similarly, although ventricular fibrillation frequently causes transient global hypokinesis, a regional, reversible wall motion abnormality would be more commonly associated with transient myocardial ischaemia or myocardial contusion. In this case report, even if localised pericardial effusion, late and persistent myocardial blush and persistent and delayed enhancement were present, no wall motion abnormality was observed. This case may also provide some explanation for the high mortality of commotio cordis victims. The ECG, echocardiographic, coronary angiography, CRMI and TNI findings suggest that in patients with commotio cordis a small local myocardial injury can be verified by intrawall bleeding that may trigger fatal arrhythmias.
In conclusion, the clinical details of this case suggest that commotio cordis is more complex than a primary ventricular fibrillatory arrest and that myocardial contusion may also play a role. This is the first case report of a patient with commotio cordis who did not show any arrhythmias and where no resuscitation was required. Clearly, more information is needed to delineate the cause and pathophysiological findings of commotio cordis.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Maron BJ, Gohman TE, Kyle SB, et al. Clinical profile and spectrum of commotio cordis. JAMA 2002;287:1142–6. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Poliac L, Kaplan JA, et al. Blunt impact to the chest leading to sudden death from cardiac arrest during sports activities. N Engl J Med 1995;333:337–42. [DOI] [PubMed] [Google Scholar]
- 3.Nesbitt AD, Cooper PJ, Kohl P. Rediscovering commotio cordis. Lancet 2001;357:1195–7. [DOI] [PubMed] [Google Scholar]
- 4.Zangwill SD, Strasburger JF. Commotio cordis. Pediatr Clin North Am 2004;51:1347–54. [DOI] [PubMed] [Google Scholar]
- 5.Boden BP, Tacchetti R, Mueller FO. Catastrophic injuries in high school and college baseball players. Am J Sports Med 2004;32:1189–96. [DOI] [PubMed] [Google Scholar]
- 6.Maron BJ. Sudden death in young athletes. N Engl J Med 2003;349:1064–75. [DOI] [PubMed] [Google Scholar]
- 7.Maron BJ, Doerer JJ, Haas TS, et al. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation 2009;119:1085–92. [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ, Doerer JJ, Haas TS, et al. Commotio cordis and the epidemiology of sudden death in competitive lacrosse. Pediatrics 2009;124:966–71. [DOI] [PubMed] [Google Scholar]
- 9.Kohl P, Nesbitt AD, Cooper PJ, et al. Sudden cardiac death by commotio cordis:role of mechano-electric feedback. Cardiovasc Res 2001;50:280–9. [DOI] [PubMed] [Google Scholar]
- 10.Bode F, Franz MR, Wilke I, et al. Ventricular fibrillation induced by stretch pulse: implications for sudden death due to commotio cordis. J Cardiovasc Electrophysiol 2006;17:1011–17. [DOI] [PubMed] [Google Scholar]
- 11.Cooper GJ, Pearce BP, Stainer MC, et al. The biomechanical response of the thorax to nonpenetrating impact with particular reference to cardiac injuries. J Trauma 1982;22:994–1008. [DOI] [PubMed] [Google Scholar]
- 12.Liedtke AJ, Allen RP, Nellis SH. Effects of blunt cardiac trauma on coronary vasomotion, perfusion, myocardial mechanics, and metabolism. J Trauma 1980;20:777–85. [DOI] [PubMed] [Google Scholar]
- 13.Madias C, Maron BJ, Supron S, et al. Cell membrane stretch and chest blow-induced ventricular fibrillation (commotio cordis). J Cardiovasc Electrophysiol 2008;19:1304–9. [DOI] [PubMed] [Google Scholar]
- 14.Link MS, Wang PJ, VanderBrink BA, et al. Selective activation of the K +ATP channel is a mechanism by which sudden death is produced by low energy chest wall impact (commotio cordis). Circulation 1999;100:413–18. [DOI] [PubMed] [Google Scholar]
- 15.Link MS, Maron BJ, Wang PJ, et al. Upper and lower limits of vulnerability to sudden arrhythmic death with chest wall impact (commotio cordis). J Am Coll Cardiol 2003;41:99–104. [DOI] [PubMed] [Google Scholar]
- 16.Garan AR, Maron BJ, Wang PJ, et al. Role of streptomycin sensitive stretch activated channel in chest wall impact induced sudden death (commotio cordis). J Cardiovasc Electrophysiol 2005;16:433–8. [DOI] [PubMed] [Google Scholar]
- 17.Stout CW, Maron BJ, Vanderbrink BA, et al. Importance of the autonomic nervous system in an experimental model of commotio cordis. Med Sci Monit 2007;13:BR11–15. [PubMed] [Google Scholar]
- 18.Link MS, Maron BJ, VanderBrink BA, et al. Impact directly over the cardiac silhouette is necessary to produce ventricular fibrillation in an experimental model of commotio cordis. J Am Coll Cardiol 2001;37:649–54. [DOI] [PubMed] [Google Scholar]
- 19.Bright EF, Beck CS. Nonpenetrating wounds of the heart: a clinical and experimental study. Am Heart J 1935;10:293–321. [Google Scholar]