Abstract
BACKGROUND
Lung contusion (LC) is a common injury resulting from blunt thoracic trauma. LC is an important risk factor for the development acute lung injury, adult respiratory distress syndrome, and ventilator-associated pneumonia, all of which increase mortality from trauma. LC produces a nonspecific immune cellular response. Neutrophil recruitment is known to increase the severity of inflammation during LC. However, the exact role of macrophages in modulating the response to LC has not been well described.
METHODS
We used a cortical contusion impactor to induce unilateral LC in mice. Thoracic micro computed tomographic scans of these animals were obtained to document radiologic changes over time following LC. To understand the role of macrophages during LC, liposomal clodronate was used to deplete macrophage levels before traumatic insult. Acute inflammatory attributes after LC were assessed, by measuring pressure-volume mechanics; quantifying bronchial alveolar lavage levels of leukocytes, albumin, and cytokines; and finally examining lung specimen histopathology at 5, 24, 48, and 72 hours after injury.
RESULTS
After LC, alveolar macrophage numbers were significantly reduced and exhibited slowed recovery. Simultaneously, there was a significant increase in bronchial alveolar lavage neutrophil counts. The loss of macrophages could be attributed to both cellular apoptosis and necrosis. Pretreatment with clodronate increased the severity of lung inflammation as measured by worsened pulmonary compliance, increased lung permeability, amplification of neutrophil recruitment, and increases in early proinflammatory cytokine levels.
CONCLUSION
The presence of regulatory alveolar macrophages plays an important role in the pathogenesis of acute inflammation following LC.
Keywords: Macrophages, clodronate, lung contusion, murine, lung injury
Blunt thoracic trauma resulting in lung contusion (LC) is an important independent risk factor for the development of pneumonia, acute lung injury, and its more severe form known as adult respiratory distress syndrome. LC producing one of these respiratory complications results in mortality rates between 10% and 25% among adult trauma patients.1–3
Both macrophages and neutrophils are consistently recovered in bronchial alveolar lavage (BAL) fluid from patients with LC injury. While the importance of the neutrophils in the lung inflammatory response has been extensively studied, the role of alveolar macrophages (AMs) is a subject of debate.4,5 AMs are considered part of the nonspecific host immune system and have two main activation phenotypes as follows: M1 or classical phenotype mediates host defenses against bacteria, viruses, and protozoa. They are characterized by secreting a significant number of proinflammatory cytokines and have increased nitric oxide synthase activity resulting in the production of nitric oxide used for bacterial killing. Conversely, the M2 phenotype, also known as an alternative pathway, participates in helminth infections and probably has an important anti-inflammatory activity via interleukin 10 (IL-10) signaling. However, exuberant M2 activation along with their associated cytokine profile (IL-10, transforming growth factor β, and monocyte chemoattractant protein 1 [MCP-1]) has been implicated in persistent bacterial infection and pulmonary fibrosis.6–9
Previous experiments from our laboratory have shown that LC in mice significantly reduces the number of AMs present in BAL samples.10 Interestingly, we found that phagocytic capacity of AMs is improved and that polarization moves toward the M2 phenotype. Using CCR-2−/− mice, we found that some of these events are dependent on MCP-1 pathway.
Thus, in this present study, to understand the precise role of AMs during LC, we performed experiments using macrophage-depleting agent clodronate before traumatic injury. Importantly, we found an increased severity of lung inflammation with diminution of AMs after LC injury.
MATERIALS AND METHODS
Induction of Isolated LC
The institutional University Committee on Use and Care of Animals at the University of Michigan (Ann Arbor, Michigan) approved all protocols and procedures. Male, C57/BL6 mice (Charles River Laboratories, Wilmington, MA) 26 g to 30 g (8–12 weeks), were used and received intraperitoneal (IP) injection of ketamine/xylazine mixture for general anesthetic. For induction of LC injury, we followed method of Hoth et al.11 (2007) with minor modifications, and these methods are described in detail by the study of Machado-Aranda et al.12 (2013). Animals were allowed to spontaneously recover while being kept warm without auxiliary ventilator support. Groups of nine mice were studied at each different terminal time point (5, 24, 48, and 72 hours) after injury. In addition, nine uninjured mice were used as controls to obtained baseline values.
Computerized Tomography Imaging of LC
Three mice were injured as described earlier and immediately taken to the University of Michigan Core for Molecular Imaging and placed in the prone position with isoflurane anesthetic administered via a nose cone. High-resolution nongated midthoracic axial images were obtained using an Inveon Computerized Tomography (CT) scanner (Siemens, Tarrytown, NY). Animals were then allowed to recover and sent back to the vivarium. At additional time points (5, 24, and 48 hours as well as 7 days), these same animals were subjected to repeat CT scans under inhalational anesthesia to document sequential changes in the areas of known injury.
Measurement of Lung Mechanics
Lung mechanics were measured at each specified time point using a SCIREQ flexiVent (Montreal, Quebec, Canada). Postmortem ventilation was performed using a tidal volume of 10 mL/kg, respiratory rate of 150 breaths per minute, and positive end-expiratory pressure of 2 cm H2O, and specifics as regards data measurements have been described elsewhere.10,12,13 SCIREQ flexiVent measurements in animal models of lung injury have been validated in numerous studies and found comparable with results from other mechanical ventilator systems.14
BAL and Cell Counts
After lung mechanics measurements, BAL was performed by injecting 2 × 500-μL of ice cold phosphate-buffered saline (PBS). Both aliquots were pooled and centrifuged at 1,500 G for 3 minutes at 4°C. Supernatant was frozen at −80°C for later enzyme-linked immunosorbent assay (ELISA) analyses. The cell pellet was resuspended in PBS. A 50-μL was used for automatic cell counting with a Countess counter (Invitrogen, Carlsbad, CA). A second 100-μL fraction was spun into a Cytospin II slide (Shandon Scientific, Pittsburg, PA) for blot staining using Diff-Quik (Dade Behring Inc., Newark, DE). Differential counts were performed manually at high-power field. Remnant resuspended cells were centrifuged at 400 G for 8 minutes, and the supernatant was discarded. Cells were washed and resuspended in Dulbecco’s PBS with 1% fetal bovine serum and would be used later for antibody labeling and flow cytometric analysis.
Flow Cytometry Experiments
Cell populations from BAL were assessed using fluorescence antibody staining and flow cytometry techniques. A total of 2 × 106 cells were used with viability staining performed with a Live/Dead staining kit (Invitrogen-Molecular Probes, Carlsbad, CA). The cells were washed and resuspended in Dulbecco’s PBS with 1% fetal bovine serum. Cells were divided into two equal sets containing 1 × 106 cells each. Fc receptors (CD16/CD32) were blocked using mouse Fc block antibodies (BioLegend, San Diego, CA) for 10 minutes at 4°C. Diluted fluorescent antibodies specific for the different surface markers or control antibodies were added to the cell suspension and incubated for 10 minutes at 4°C. The cells were washed, formalin fixed, washed again, and resuspended in flow buffer and assayed the same day. The following monoclonal antibodies were obtained from BD Biosciences (San Jose, CA): Gr-1-PE, CD11c-PE; from BioLegend: Ly6C-FITC, F4/80-Alexa Fluor 647, F4/80-Alexa Fluor 700, A-I/b-Alexa Fluor 647, CD11b-PE-Cy7, and CD11c-APC-Cy7. Data were collected using a LSR II BD Biosciences flow cytometer and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR). Forward light scatter low Gr-1+ CD11b+ F4/80− cells were identified as neutrophils and forward light scatter high F4/80+ CD11b+ cells were identified as macrophages.
Albumin and Cytokine Concentrations in BAL
Albumin concentrations in BAL were measured using ELISA with a polyclonal rabbit antimouse albumin antibody and detected by colorimetric assay using horseradish peroxidase labeled goat antirabbit IgG (BD Biosciences Pharmingen, San Diego, CA) and mouse albumin (Sigma, St. Louis, MO) as a standard. Soluble concentrations of BAL IL-1β, IL-6, IL-10, KC, MIP-2, and MCP-1 were measured using mouse ELISA kits from R&D Systems (Minneapolis, MN).
Histopathology
Two animals were sacrificed for at each time point. The trachea, lungs, and heart were removed en bloc via sternotomy. The right heart ventricle was flushed with 5 mL of ice cold PBS. Tissues were then fixed with 1% formalin drained into the lung by gravity, processed, stained with hematoxylin and eosin, and sectioned for microscopic examination.
Clodronate Experiments
Mice were treated with IP injection of 100 μL of liposomal complexed clodronate (Encapsula Nano Sciences, Nashville, TN) at a concentration of 0.2 mg/mL. This allowed approximately 85% reduction of AMs at 48 hours, a time point when LC injury was induced. An equivalent volume of liposomes (vehicle) in PBS was used as a control.4,15,16 IP injection as opposed to intratracheal injection allowed a more reliable delivery of clodronate without affecting baseline characteristics of lung volumes, mechanics, and/or creating preexisting inflammation that would obscure the results of LC-related inflammation (see Figures, Supplemental Digital Contents 1 and 2, http://links.lww.com/TA/A370 and http://links.lww.com/TA/A371).
Statistical Analyses
Data are expressed as mean ± SEM unless expressed otherwise. One-way analysis of variance (ANOVA) with Bonferroni’s post hoc test was used for all intergroup pairwise comparisons. Statistical significance was defined as p < 0.05. Analysis was performed and graphs were generated using GraphPad Prism 6.0 (La Jolla, CA).
RESULTS
CT Scan Evidence for Injury Evolution in a Murine Model of LC
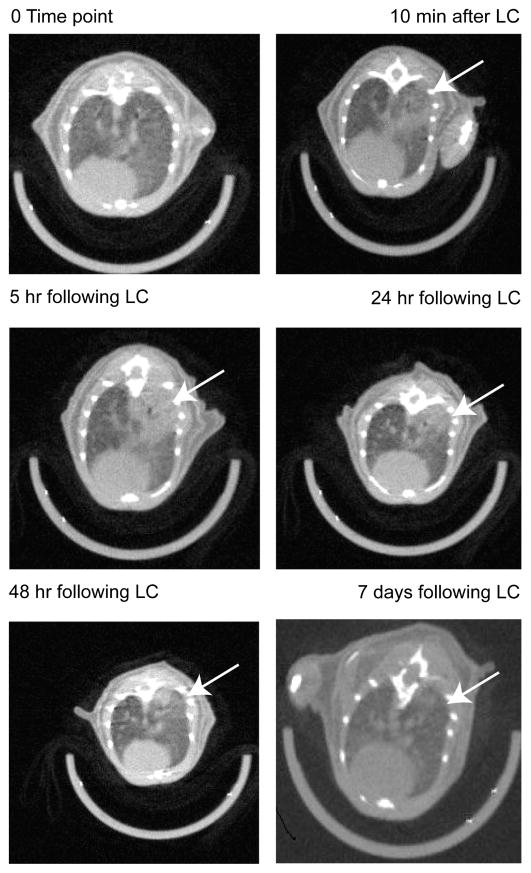
Micro CT scan images of the chest were obtained from three animals after sustaining unilateral LC. Evidence of pulmonary contusion was consistently seen as early as 10 minutes after injury. LC injury became more severe within 5 hours to 24 hours and showed resolution by 7 days (Fig. 1). The timing and radiologic pattern of LC in the mouse are similar to the clinical radiologic changes evident in human patients with LC following blunt chest injury.17–19
Figure 1.
Evolution of unilateral LC injury in mice. Images were captured in nongated micro CT scanner with midthoracic axial cuts. Infiltrates (white arrow) can be seen as early as 10 minutes and are most severe between 24 hours and 48 hours. If no additional insult was added, lung infiltrates will show almost complete resolution by 7 days.
LC Reduces Macrophage and Increases Neutrophil Cell Population(s) in BAL After Injury
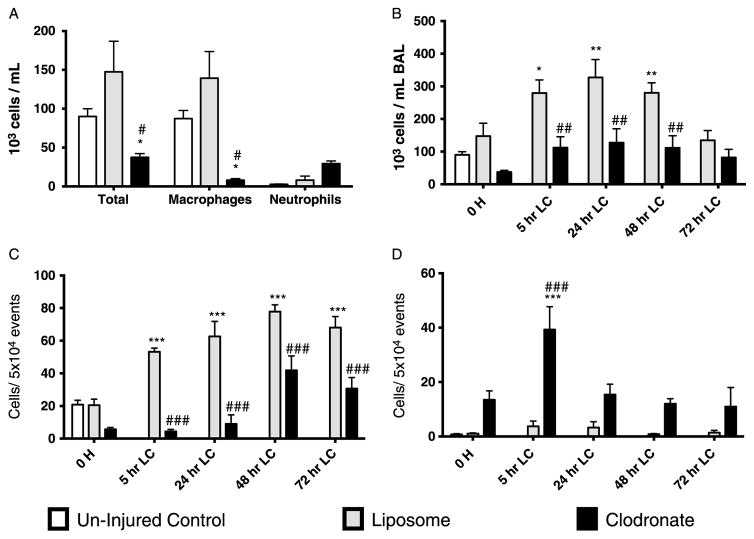
In our model of LC, we found a twofold reduction in AMs present in BAL samples obtained throughout the duration of our experiment. By 72 hours after LC, the level of AMs had still not rebounded compared with uninjured controls (Fig. 2A). Simultaneously, there was a larger-fold increase in the number of neutrophils collected in BAL following LC–peaking at 24 hours after injury and declining afterward, still remaining elevated by 72 hours in comparison with the control. Dendritic cells were also measured and had an up-trending signal after LC, but these changes were not statistically significant.
Figure 2.
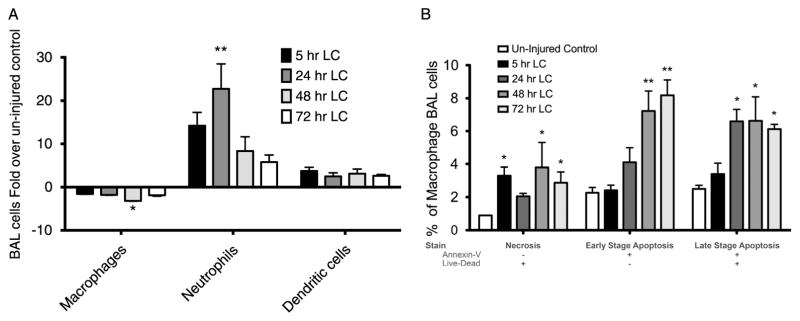
Macrophages are lost during LC injury as a result of increased necrosis and apoptosis. A, BAL cells were counted and later manually differentiated at different time points following LC. Macrophages showed a persistent reduction in numbers when compared with uninjured controls at all time points, whereas neutrophils showed up to 20-fold increases as compared with baseline. Dendritic cells had a modest increased that was not statically significant. B, BAL cells were analyzed using flow cytometry. Necrosis as characterized by Live-Dead+/Annexin V− double stain was present in all time points assessed in F480+/Gr− surface markers present in activated macrophages. Early-stage (Live-Dead−/Annexin V+) and late-stage (Live-Dead+/Annexin V+) apoptoses were also present but mostly so at 48 hours and 72 hours. One-way ANOVA versus uninjured control (n = 9, *p < 0.05. **p < 0.01. ***p < 0.001).
LC-Mediated Reduction in AM Levels Is Associated With Necrosis and Apoptosis
Using flow cytometry, we found a threefold increase in macrophage necrosis (Live-Dead+/Annexin V−) in LC animals compared with control. These observations were present as early as 5 hours after injury and continued at 72 hours (Fig. 2B). In addition, AMs also showed apoptotic activation following LC. Both early-stage (Live-Dead−/Annexin V+) and late-stage (Live-Dead+/Annexin V+) apoptosis were present in BAL AMs, most importantly at 48-hour and 72-hour time points.
Macrophage Depletion With Clodronate Before LC Worsens Lung Function and Increases Albumin Permeability Caused by LC Injury
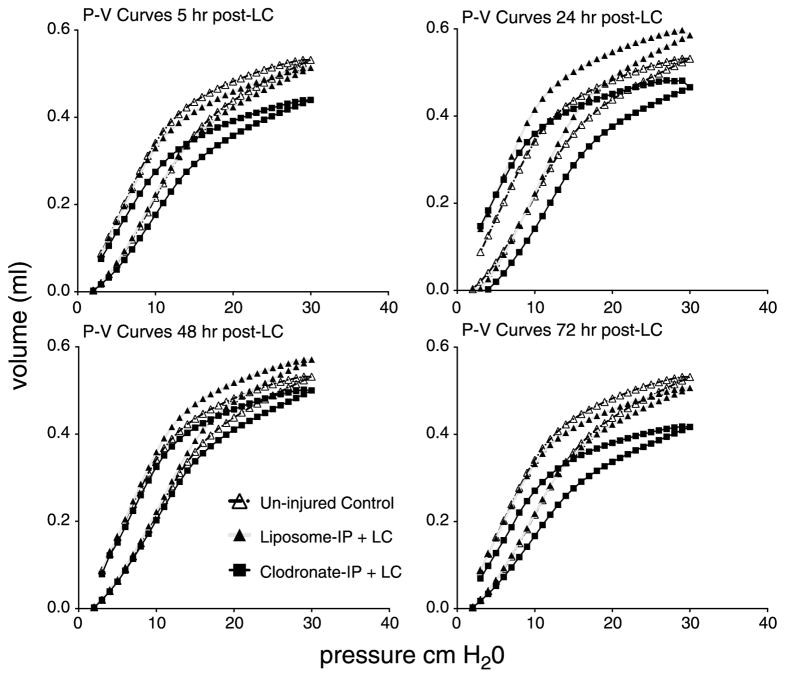
Following pretreatment with clodronate or its liposome (vehicle) control, mice were subjected to LC. Mechanical injury was assessed at specified time points after insult by measuring pressure-volume (P/V) curves as shown in Figure 3. After LC, clodronate-IP group showed decreased pulmonary compliance as measured by smaller and more downshifted P/V curves. Unexpectedly, liposome-IP group (closed triangles) demonstrated better compliance after LC at 24 hours and 48 hours compared with uninjured controls (open triangles). This phenomenon could reflect the surfactant-altering properties of liposomes. The TLC-30, a value also dependent on pulmonary compliance, was also significantly diminished in macrophage-depleted animals (see Figure, Supplemental Digital Content 3, http://links.lww.com/TA/A372).
Figure 3.
Clodronate pretreatment decreased volumes for pressure changes in mechanically ventilated mice following LC. Uninjured controls are shown in open triangles, Liposome-pretreated animals are shown in solid triangles and clodronate-pretreated animals are in solid boxes. “Quasi-static” closed-chest P/V curves were measured at 5, 24, 48, and 72 hours after injury. Each curve was generated by more than 4,000 data points for each animal, and SEM for nine animals is not shown to allow clear visualization of the curves.
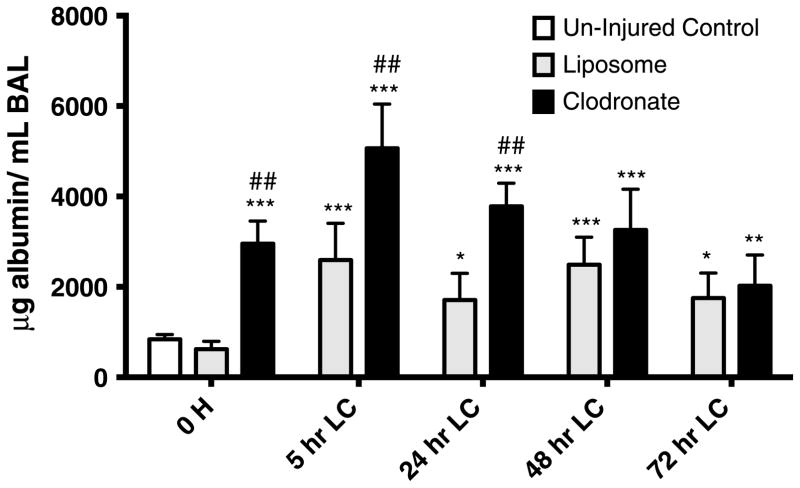
BAL albumin, a surrogate of permeability injury, was also measured in animals as treated as earlier (Fig. 4). The permeability injury was increased at all time points in the clodronate-IP group. Liposome-IP group, although with better lung mechanics, still had a significant permeability injury present if compared with baseline.
Figure 4.
Clodronate pretreatment worsens permeability injury in mice with LC. Mice receiving clodronate pretreatment (100 μL of 0.2 mg/mL) IP injection 48 hours before LC showed increase alveolar/endothelial permeability to albumin recovered in BAL. These effects were mostly noted at 5-hour and 24-hour peak closely following other inflammatory attributes (P/V curves, BAL cellularity). One-way ANOVA versus uninjured control (n = 9, *p < 0.05. **p < 0.01. ***p < 0.001). One-way ANOVA versus liposome (n = 9, ##p < 0.01).
Macrophage depletion with clodronate reduces the total number of inflammatory cells and increases the amount of neutrophils in BAL fluid after LC
A single dose of IP clodronate 48 hours before LC produced an 85% reduction in BAL macrophages (Fig. 5A) and did not rebound until after 48 hours LC, known time of which clodronate has been cleared by the host.20–22 Animals treated with clodronate before LC had a decrease in total BAL cells both at baseline and all of time points assessed after LC injury (Fig. 5B) compared with their vehicle control. With macrophage depletion, neutrophils became the predominant cell in BAL after LC confirmed by flow cytometry (Fig. 5C and D). In clodronate-IP group, neutrophils were significantly increased at 5 hours after LC, indicating a robust proinflammatory response. Interestingly, liposome-IP had increased levels of BAL macrophages before insult, effect that was continued and even augmented after LC.
Figure 5.
Effect on BAL cellular composition with macrophage-depleting agent clodronate in the evolution of LC injury. A, An additional group of animals were sacrificed 48 hours after pretreatment injection; values show the composition of BAL before LC. B, Evolution of the total number of cells present in BAL after LC. Clodronate treatment decreased the total number of cells present in BAL at all compared time points. C, With the use of F4/80+/CD11b+ antibody labeling, macrophage numbers were measured by flow cytometry. Decreases in total number of cells were attributed overall to diminished number of total macrophages after clodronate treatment. D, Neutrophils measured by flow cytometry using Gr-1+/CD11b+/F4/80− labeling showed that within the clodronate group, there was a significant, more than 100-fold increase of neutrophilic at 5 hours. Neutrophilia was persistent at all time points. One-way ANOVA versus uninjured control (n = 9, *p < 0.05. **p < 0.01. ***p < 0.001). One-way ANOVA versus liposome (n = 9, ##p < 0.01. ###p < 0.001).
Macrophage Depletion With Clodronate Increases BAL Levels of Early Proinflammatory Cytokines Before and After LC
BAL in clodronate-IP before LC showed increased levels of early proinflammatory cytokines (IL-1β, IL-6, tumor necrosis factor α MIP-2, KC) (see Table, Supplemental Digital Content 4, http://links.lww.com/TA/A373). After 24 hours after injury, most of these measured cytokine levels rapidly decreased. In contrast, anti-inflammatory cytokine MCP-1 had a persistent elevation during assessed time points. Liposome-IP–treated groups did not produce any major changes in cytokine levels, suggesting an alternative mechanism of action.
Clodronate Pretreatment Resulted in Increased Pulmonary Inflammatory Injury Following LC
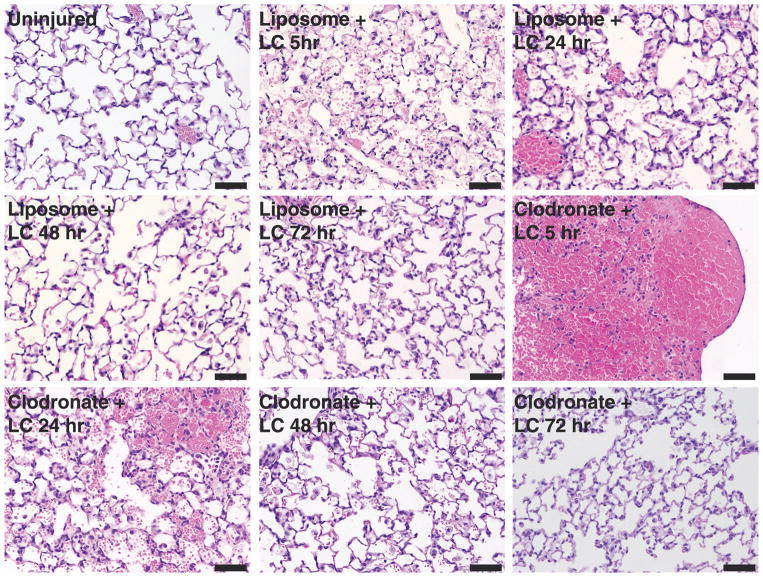
Figure 6 shows the evolution in histologic changes after LC. In the clodronate group, there was extensive intra-alveolar hemorrhage early after LC injury (5 hours and 24 hours). By 48 hours, the alveolar septa were thickened owing to congestion and accumulation of inflammatory cells (predominantly neutrophils) with cellular debris. These markers of inflammatory severity were still present at 72 hours. In contrast, the liposome group showed mild inflammatory characteristics in the earlier time points (5 hours and 24 hours) with significant improvement toward recovery afterward (48 hours and 72 hours).
Figure 6.
Evolution of histologic changes in the presence of macrophage-depleting agent clodronate following LC injury. The right lungs at the area of grossly identified contusion were fixed and processed for hematoxylin and eosin staining. Clodronate groups showed increase inflammatory attributes (hemorrhage, cellular infiltrates, alveolar edema) in comparison with respective controls at early time points (5, 24, and 48 hours). At 72 hours, both groups (liposome and clodronate) seem to have similar recovery from LC injury. See text for details. Bar, 50 μm.
DISCUSSION
New designs in passive vehicular safety such as steering wheel airbags have decreased the overall incidence of bilateral thoracic blunt trauma.23,24 However, lateral impacts still remain a significant problem, and the present study serves as an animal model to characterize the features present in a unilateral LC. When the ideal target area of impact is achieved, the cortical contusion impactor created a consistent injury to the right pulmonary lobes. The CT scan observations found in this study share results similar to those seen in clinical practice with LC. Although the model as presented is nonlethal, we have previously reported that the combination of LC with acid and gastric particulate aspiration can produce a more severe inflammatory and lethal response as opposed to either insult(s) alone25–27 (for additional details, please see Text, Supplemental Digital Content 5, http://links.lww.com/TA/A374).
After blunt thoracic trauma, the combination of forces elegantly described by Clemedson et al.28,29 results in the integrity loss of the epithelial barrier ensuing into hemorrhagic flooding of alveolar airspaces. In our study, we note these findings at 5 hours in our micro CT scans. It is at this time point when maximal levels of albumin are also detected from the BAL, indicating increased permeability injury. The hemorrhage is rapidly substituted at 24 hours by a neutrophilic in-filtration of the affected alveoli in which chemokines that are either neutrophil dependent or participatory in their regulation are also increased (IL-1β, IL-6, KC). The loss of ventilation in flooded and inflamed alveoli observed between 5 hours and 48 hours may contribute to the reductions in lung compliance seen during this study period in our model. The shifted inflation curves suggest that the recruitment of alveoli with optimal positive end-expiratory pressure might play an important therapeutic role in LC and accelerate recovery.30
In similar experiments performed by our laboratory, using vinblastine depletion of neutrophils, we have shown that inflammation after LC is neutrophil dependent.30 The neutrophilic inflammation sustained by its related pro-inflammatory cytokines IL-1beta and IL-6 is probably beneficial in pneumonia whereas in LC, these cytokines likely indicate severity of injury. It is entirely possible that AMs are required for the subsequent return of these cytokines to baseline values.25,30–33
In the current study, the population of resident AMs was lost early in the course of LC either through necrosis or apoptosis. By using a pharmacologic depletion strategy (clodronate), diminished number of resident AMs before the insult (LC) showed that the expected neutrophilic response was greatly enhanced and, as a consequence, had an increased severity of all measured inflammatory attributes (mechanical ventilation alterations, permeability injury, and proinflammatory cytokine profile). Previous results from our laboratory have documented that during LC, AMs undergo differentiation toward the alternative M2 phenotype, mediated by CCL-2 cytokines.10,27,31 This phenotype has an increased phagocytic capacity especially in the removal of necrotic cells and debris, which are known neutrophilic chemotactic signals to the lung. It is possible that the lack of clearance of these signals caused by the loss of “phagocytic-capable” macrophages could be contributing to the increase severity of neutrophilic-driven inflammatory response.
Interestingly, we found that liposome-treated animals had better parameters of P/V compliance. We speculate that these phospholipids with a chemical structure similar to pulmonary surfactant and its precursors may confer stabilization properties to the surfactant with an effect on the surface tension and improvement in lung mechanics.12,26,33 Certainly, this could constitute an interesting avenue of research, as we are the first to report this beneficial effect with the IP delivery of these compounds in a lung injury model.
There are limitations to the current study. The use of liposome-encapsulated clodronate, which can cause a reduction in the number of alveolar and systemic macrophages, may induce a compensatory innate systemic immunologic response, thus confusing our results.22 Other strategies that reduce the number of AMs have also met important limitations. The use of antibodies against colony stimulating factor 1 receptor (CSF1R) can deplete resident macrophage populations in the peritoneum, gastrointestinal tract, liver, kidney, and skin but does not seem to affect those located in the lung or female reproductive organs.34 Similarly, antibodies against macrophage specific surface receptor CD11b/CD18 (MAC-1) have been used to selectively deplete macrophages but simultaneously produce a significant elevation (approximately 2.5) of white blood cell count.35 In the current study, we were able to show that the use of IP injection was sufficient to deplete resident AMs (approximately 85% reduction) and was simpler and as effective as aerosolized delivery or intratracheal delivery without the possible inconsistencies of the former21–36 and the added surgical insult of the later.37 The effects of liposome treatment have been discussed at length throughout the article.
In summary, we report a model for closed chest unilateral LC where the CT scan changes mimic those of humans with LC. The injury is most severe at early stages (5–24 hours) with signs of resolution seen by 72 hours. The relatively rapid resolution of LC injury both in mice and our previous experiments in rats suggests that other important factors such as extrapulmonary trauma and concomitant or sequential insults such as gastric aspiration may explain why a selective group of patients will progress into more severe forms of respiratory failure after blunt thoracic injury. An increased number of AMs are lost in the early stages of acute inflammation following LC as a result of necrosis and apoptosis. Depletion of AMs by clodronate significantly increased the magnitude of permeability injury and inflammation following LC. These differences in resident macrophages and numbers may account for the unregulated and robust increase in unchecked neutrophilic inflammation representative of the progressive nature of lung injury seen in some patients with LC.
Supplementary Material
TABLE 1.
Effect on Cytokine Profile With Clodronate Pre-treatment in Injured Mice After LC
| Group | 0 h | 5 h | 24 h | 48 h | 72 h | |
|---|---|---|---|---|---|---|
| IL-1β | Saline | 3.15 ± 0.56 | ||||
| Liposome + LC | 4.85 ± 1.18 | 8.93 ± 1.47 | 8.30 ± 2.71 | 6.14 ± 1.23 | 3.94 ± 2.96 | |
| Clodronate | 55.23 ± 15.51***### | 51.65 ± 12.9**## | 20.73 ± 16.7 | 5.08 ± 1.78 | 3.35 ± 1.89 | |
| IL-6 | Saline | 4.15 ± 1.29 | ||||
| Liposome + LC | 8.59 ± 2.93 | 28.76 ± 9.81 | 8.29 ± 2.31 | 14.69 ± 4.53 | 15.77 ± 7.76 | |
| Clodronate | 80.45 ± 21.79***### | 109.0 ± 21.18***### | 14.7 ± 0.66 | 10.41 ± 2.85 | 11.18 ± 3.05 | |
| MCP-1 | Saline | 3.08 ± 1.72 | ||||
| Liposome + LC | 6.45 ± 1.32 | 47.7 ± 16.09 | 38.76 ± 11.46 | 6.74 ± 2.05 | 14.76 ± 12.76 | |
| Clodronate | 57.84 ± 3.81*## | 64.87 ± 15.68* | 80.59 ± 8.53*** | 61.18 ± 15.25**### | 39.19 ± 6.21 | |
| MIP-2 | Saline | 5.12 ± 2.0 | ||||
| Liposome + LC | 8.65 ± 2.27 | 42.64 ± 27.38 | 9.24 ± 7.87 | 48.25 ± 33.85 | 4.0 ± 4.0 | |
| Clodronate | 126.2 ± 36.14*## | 192.31 ± 32.98***### | 36.65 ± 18.51 | 15.26 ± 6.55 | 22.0 ± 17.9 | |
| KC | Saline | 4.0 ± 2.0 | ||||
| Liposome + LC | 4.32 ± 3.44 | 35.72 ± 17.34 | 13.23 ± 6.12 | 19.6 ± 7.57 | 32.0 ± 27.08 | |
| Clodronate | 104.4 ± 26.2***### | 84.1 ± 13.71**# | 20.25 ± 7.75 | 9.29 ± 2.52 | 8.38 ± 2.66 | |
| Tumor necrosis factor α | Saline | 5.92 ± 4.42 | ||||
| Liposome + LC | 8.56 ± 4.19 | 6.31 ± 1.33 | 30.78 ± 18.62 | 19.43 ± 5.35 | 30.84 ± 26.19 | |
| Clodronate | 57.19 ± 21.33# | 48.96 ± 23.41 | 15.28 ± 6.55 | 18.47 ± 4.74 | 11.61 ± 0.61 |
Several pro-inflammatory cytokines were elevated when compared to baseline prior to lung contusion in clodronate pre-treated animals. These levels drop as macrophages numbers increased in later time points. MCP-1 remained persistently high at all time points. One-way ANOVA vs. Un-injured control (n=9; * p < 0.05; ** p < 0.01, *** p < 0.001). One-way ANOVA vs. liposome (n=9, # p < 0.05; ## p < 0.01, ### p < 0.001).
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
AUTHORSHIP
D.M.-A. provided the P/V curves, performed the statistical analysis, and drafted the manuscript. S.M.V. performed the immunoassays. B.Y. performed the initial LC experiments and the histologic analysis. V.D. performed the flow cytometry analysis and the subsequent statistical interpretation. M.R.H. and K.R. conceived the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
DISCLOSURE
This study was supported by the NIH-NIGMS HL102013-04 (K.R.) and AAST Research and Education Scholarship Foundation (D.M.-A.).
References
- 1.Miller PR, Croce MA, Kilgo PD, Scott J, Fabian TC. Acute respiratory distress syndrome in blunt trauma: identification of independent risk factors. Am Surg. 2002;68:845–850. discussion 850–851. [PubMed] [Google Scholar]
- 2.Cohn SM. Pulmonary contusion: review of the clinical entity. J Trauma. 1997;42:973–979. doi: 10.1097/00005373-199705000-00033. [DOI] [PubMed] [Google Scholar]
- 3.Kollmorgen DR, Murray KA, Sullivan JJ, Mone MC, Barton RG. Predictors of mortality in pulmonary contusion. Am J Surg. 1994;168:659–663. doi: 10.1016/s0002-9610(05)80140-0. discussion 663–664. [DOI] [PubMed] [Google Scholar]
- 4.Bem RA, Farnand AW, Wong V, Koski A, Rosenfeld ME, van Rooijen N, Frevert CW, Martin TR, Matute-Bello G. Depletion of resident alveolar macrophages does not prevent Fas-mediated lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L314–L325. doi: 10.1152/ajplung.00210.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989;170(2):499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young LR, Borchers MT, Allen HL, Gibbons RS, McCormack FX. Lung-restricted macrophage activation in the pearl mouse model of Hermansky-Pudlak syndrome. J Immunol. 2006;176:4361–4368. doi: 10.4049/jimmunol.176.7.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belperio JA, Keane MP, Burdick MD, Lynch JP, 3rd, Xue YY, Berlin A, Ross DJ, Kunkel SL, Charo IF, Strieter RM. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest. 2001;108:547–556. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogaboam CM, Gallinat CS, Bone-Larson C, Chensue SW, Lukacs NW, Strieter RM, Kunkel SL. Collagen deposition in a non-fibrotic lung granuloma model after nitric oxide inhibition. Am J Pathol. 1998;153:1861–1872. doi: 10.1016/S0002-9440(10)65700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broug-Holub E, Toews GB, van Iwaarden JF, Strieter RM, Kunkel SL, Paine R, 3rd, Standiford TJ. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997;65:1139–1146. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suresh MV, Yu B, Machado-Aranda D, Bender MD, Ochoa-Frongia L, Helinski JD, Davidson BA, Knight PR, Hogaboam CM, Moore BB, Raghavendran K. Role of macrophage chemoattractant protein-1 in acute inflammation after lung contusion. Am J Respir Cell Mol Biol. 2012;46(6):797–806. doi: 10.1165/rcmb.2011-0358OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoth JJ, Hudson WP, Brownlee NA, Yoza BK, Hiltbold EM, Meredith JW, McCall CE. Toll-like receptor 2 participates in the response to lung injury in a murine model of pulmonary contusion. Shock. 2007;28:447–452. doi: 10.1097/shk.0b013e318048801a. [DOI] [PubMed] [Google Scholar]
- 12.Machado-Aranda D, Wang Z, Yu B, Suresh MV, Notter RH, Raghavendran K. Increased phospholipase A2 and lysophosphatidylcholine levels are associated with surfactant dysfunction in lung contusion injury in mice. Surgery. 2013;153:25–35. doi: 10.1016/j.surg.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machado-Aranda DA, Suresh MV, Yu B, Raghavendran K. Electroporation-mediated in vivo gene delivery of the Na+/K+-ATPase pump reduced lung injury in a mouse model of lung contusion. J Trauma Acute Care Surg. 2012;72:32–39. doi: 10.1097/TA.0b013e31823f0606. discussion 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanoirbeek JA, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, Hoet PH, Verbeken E, Decramer M, Nemery B, Janssens W. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol. 2010;42:96–104. doi: 10.1165/rcmb.2008-0487OC. [DOI] [PubMed] [Google Scholar]
- 15.Berg JT, Lee ST, Thepen T, Lee CY, Tsan MF. Depletion of alveolar macrophages by liposome-encapsulated dichloromethylene diphosphonate. J Appl Physiol. 1993;74:2812–2819. doi: 10.1152/jappl.1993.74.6.2812. [DOI] [PubMed] [Google Scholar]
- 16.Farley KS, Wang LF, Razavi HM, Law C, Rohan M, McCormack DG, Mehta S. Effects of macrophage inducible nitric oxide synthase in murine septic lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1164–L1172. doi: 10.1152/ajplung.00248.2005. [DOI] [PubMed] [Google Scholar]
- 17.Daly M, Miller PR, Carr JJ, Gayzik FS, Hoth JJ, Meredith JW, Stitzel JD. Traumatic pulmonary pathology measured with computed tomography and a semiautomated analytic method. Clin Imaging. 2008;32:346–354. doi: 10.1016/j.clinimag.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Miller PR, Croce MA, Bee TK, Qaisi WG, Smith CP, Collins GL, Fabian TC. ARDS after pulmonary contusion: accurate measurement of contusion volume identifies high-risk patients. J Trauma. 2001;51:223–228. doi: 10.1097/00005373-200108000-00003. discussion 229–230. [DOI] [PubMed] [Google Scholar]
- 19.Becher RD, Colonna AL, Enniss TM, Weaver AA, Crane DK, Martin RS, Mowery NT, Miller PR, Stitzel JD, Hoth JJ. An innovative approach to predict the development of adult respiratory distress syndrome in patients with blunt trauma. J Trauma Acute Care Surg. 2012;73(5):1229–1235. doi: 10.1097/TA.0b013e31825b2124. [DOI] [PubMed] [Google Scholar]
- 20.Elder A, Johnston C, Gelein R, Finkelstein J, Wang Z, Notter R, Oberdörster G. Lung inflammation induced by endotoxin is enhanced in rats depleted of alveolar macrophages with aerosolized clodronate. Exp Lung Res. 2005;31:527–546. doi: 10.1080/019021490944223. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto S, Pittet JF, Hong K, Folkesson H, Bagby G, Kobzik L, Frevert C, Watanabe K, Tsurufuji S, Wiener-Kronish J. Depletion of alveolar macrophages decreases neutrophil chemotaxis to Pseudomonas airspace infections. Am J Physiol. 1996;270:L819–L828. doi: 10.1152/ajplung.1996.270.5.L819. [DOI] [PubMed] [Google Scholar]
- 22.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174(1–2):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 23.O’Connor JV, Kufera JA, Kerns TJ, Stein DM, Ho S, Dischinger PC, Scalea TM. Crash and occupant predictors of pulmonary contusion. J Trauma. 2009;66:1091–1095. doi: 10.1097/TA.0b013e318164d097. [DOI] [PubMed] [Google Scholar]
- 24.Gayzik FS, Martin RS, Gabler HC, Hoth JJ, Duma SM, Meredith JW, Stitzel JD. Characterization of crash-induced thoracic loading resulting in pulmonary contusion. J Trauma. 2009;66:840–849. doi: 10.1097/TA.0b013e318186251e. [DOI] [PubMed] [Google Scholar]
- 25.Raghavendran K, Davidson BA, Huebschmann JC, Helinski JD, Hutson AD, Dayton MT, Notter RH, Knight PR. Superimposed gastric aspiration increases the severity of inflammation and permeability injury in a rat model of lung contusion. J Surg Res. 2009;155:273–282. doi: 10.1016/j.jss.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raghavendran K, Davidson BA, Knight PR, Wang Z, Helinski J, Chess PR, Notter RH. Surfactant dysfunction in lung contusion with and without superimposed gastric aspiration in a rat model. Shock. 2008;30:508–517. doi: 10.1097/SHK.0b013e3181673fc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolgachev VA, Yu B, Reinke JM, Raghavendran K, Hemmila MR. Host susceptibility to gram-negative pneumonia after lung contusion. J Trauma Acute Care Surg. 2012;72:614–622. doi: 10.1097/TA.0b013e318243d9b1. discussion 622–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clemedson CJ, Hultman H, Gronberg B. Respiration and pulmonary gas exchange in blast injury. J Appl Physiol. 1953;6:213–220. doi: 10.1152/jappl.1953.6.4.213. [DOI] [PubMed] [Google Scholar]
- 29.Clemedson CJ, Pettersson H. Genesis of respiratory and circulatory changes in blast injury. Am J Physiol. 1953;174:316–320. doi: 10.1152/ajplegacy.1953.174.2.316. [DOI] [PubMed] [Google Scholar]
- 30.Raghavendran K, Davidson BA, Woytash JA, Helinski JD, Marschke CJ, Manderscheid PA, Notter RH, Knight PR. The evolution of isolated bilateral lung contusion from blunt chest trauma in rats: cellular and cytokine responses. Shock. 2005;24:132–138. doi: 10.1097/01.shk.0000169725.80068.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raghavendran K, Davidson BA, Mullan BA, Hutson AD, Russo TA, Manderscheid PA, Woytash JA, Holm BA, Notter RH, Knight PR. Acid and particulate-induced aspiration lung injury in mice: importance of MCP-1. Am J Physiol Lung Cell Mol Physiol. 2005;289:L134–L143. doi: 10.1152/ajplung.00390.2004. [DOI] [PubMed] [Google Scholar]
- 32.Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Crit Care Med. 2011;39:818–826. doi: 10.1097/CCM.0b013e31820a856b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raghavendran K, Notter RH, Davidson BA, Helinski JD, Kunkel SL, Knight PR. Lung contusion: inflammatory mechanisms and interaction with other injuries. Shock. 2009;32:122–130. doi: 10.1097/SHK.0b013e31819c385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, Kuns R, Pettit AR, Clouston A, Wainwright B. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955–3963. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- 35.Pradilla G, Wang PP, Legnani FG, Ogata L, Dietsch GN, Tamargo RJ. Prevention of vasospasm by anti-CD11/CD18 monoclonal antibody therapy following subarachnoid hemorrhage in rabbits. J Neurosurg. 2004;101(1):88–92. doi: 10.3171/jns.2004.101.1.0088. [DOI] [PubMed] [Google Scholar]
- 36.Yeo LY, Friend JR, McIntosh MP, Meeusen EN, Morton DA. Ultrasonic nebulization platforms for pulmonary drug delivery. Expert Opin Drug Deliv. 2010;7(6):663–679. doi: 10.1517/17425247.2010.485608. [DOI] [PubMed] [Google Scholar]
- 37.Driscoll KE, Costa DL, Hatch G, Henderson R, Oberdorster G, Salem H, Schlesinger RB. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: uses and limitations. Toxicol Sci. 2000;55(1):24–35. doi: 10.1093/toxsci/55.1.24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.