Abstract
Product standards that greatly reduce the content of nicotine within cigarettes may result in improved public health. The present study used an animal model to investigate whether individuals who start smoking following implementation of regulation may be affected differently from current smokers who form the basis of most clinical studies. One group of adult male rats (n=14/group) acquired nicotine self-administration at a high nicotine dose (60 μg/kg/infusion) before experiencing a reduction to one of three low doses of nicotine (3.75, 7.5, or 15 μg/kg/infusion) or vehicle. Their self-administration behavior at the low doses was compared to a group of adult male rats given the opportunity to acquire nicotine self-administration at one of the same low doses or vehicle (n=7-14/group). Second, the self-administration behavior of the acquisition group of rats was compared to their own self-administration behavior following experience self-administering a high dose of nicotine. A cocktail of non-nicotine cigarette smoke constituents was included in the vehicle for all rats across all phases of the study. Rats with a history of self-administering a high dose of nicotine had a higher rate of self-administration across the low doses than rats with no history. Additionally, the number of earned infusions increased after rats experienced self-administration of a higher dose of nicotine. These data show that low-dose nicotine self-administration is higher following a dose reduction than during acquisition. If a nicotine reduction policy were implemented, this policy may be especially effective at reducing acquisition of smoking.
Keywords: nicotine reduction, acquisition, self-administration, non-nicotine cigarette constituents, rat
Tobacco control policies should aim to decrease the number of people who become smokers and better enable current smokers to quit smoking. Smoking initiation rates are still high, with one in four U.S. high school seniors smoking daily (US Department of Health and Human Services, 2012). Additionally, two thirds of current smokers express that they would like to quit smoking, but only 6.2% of smokers actually quit each year (CDC, 2011). In 2009, Congress passed the Family Smoking Prevention and Tobacco Control Act which permits the regulation of tobacco products and their constituents, including the reduction, but not elimination, of the nicotine content in cigarettes (US Congress, 2009). A policy that reduces the nicotine content of a cigarette below a threshold for addiction could substantially decrease initiation and increase smoking cessation (Benowitz and Henningfield, 1994), but such a policy requires an understanding of the dose range that supports initiation as well as maintenance of smoking behavior. Importantly, a history of smoking may shift the dose-response curve for the primary reinforcing effects of nicotine to the left (sensitization) or to the right (tolerance). In this case, a nicotine reduction policy may impact individuals who start smoking reduced nicotine content cigarettes (i.e., new smokers) differently from those who were smoking prior to policy implementation (i.e., current smokers). If a history of smoking normal nicotine content cigarettes increases sensitivity to low doses of nicotine, ongoing research on reduced nicotine cigarettes that uses current smokers as participants might underestimate the public health impact of regulation on individuals who try smoking for the first time after regulation. Understanding the impact of prior nicotine and tobacco use is critical in determining the likely effects of a reduction in nicotine content on smoking behavior.
Non-human research is valuable for studying how a nicotine reduction policy would differentially affect new and experienced cigarette smokers because ethical issues make experimentally manipulating the initiation of smoking impossible. Nicotine self-administration research, in which animals respond for intravenous (i.v.) infusions of nicotine, is likely to be particularly informative. Two dose-response curves, each plotting the rate of self-administration across low nicotine doses, can be compared: one for acquisition in which rats are first given the opportunity to respond for low doses of nicotine (analog for “new smokers”), and one following reduction after rats have a history of responding for a higher dose of nicotine (analog for “current smokers”). While the exact doses used are unlikely to translate to humans, the relation between the two dose-response curves may provide important information about the functional effects of a history of self-administering a higher dose of nicotine.
To date, the relationship between acquisition and reduction dose-response curves is unknown. While there are studies investigating self-administration across doses of acquisition (Chen, Matta, & Sharp, 2007; Cox, Goldstein, & Nelson, 1984; Donny et al., 1998; Sorge & Clarke, 2009) and reduction (Grebenstein, Burroughs, Zhang, & LeSage, 2013; Smith, Levin, Schassburger, Buffalari, Sved, & Donny, 2013), it is difficult to compare the results of these studies to previous studies investigating the acquisition dose-response curve because of methodological differences including strain of rat, nicotine-paired environmental stimuli, schedule of reinforcement, and daily duration of access (Donny et al., 2012). Research has shown that exposure to nicotine alters subsequent self-administration (Adriani et al., 2003; Shoaib, Schindler, & Goldberg, 1997), and in one small study, Cox et al. (1984), demonstrated a nicotine-dose reduction to 3 μg/kg/infusion produced an increase in behavior compared to the pre-reduction baseline of 30 μg/kg/infusion, even though 3 μg/kg/infusion failed to produce acquisition in a separate group of rats. If replicated, these data indicate that individuals with a history of smoking cigarettes with higher nicotine contents may smoke low-nicotine cigarettes at a higher rate than individuals who initiate smoking with low-nicotine cigarettes.
The present experiment directly compared the dose-response curve for acquisition to the one for reduction, using both a between-subjects and a within-subjects approach. For the between-subjects comparison, a large group of rats acquired nicotine self-administration at a high nicotine dose (60 μg/kg/infusion) before experiencing a reduction to one of three low doses of nicotine (3.75, 7.5, or 15 μg/kg/infusion) or vehicle alone. The self-administration behavior of these rats at the low doses of nicotine may be thought of as an analog for current smokers who experience a large reduction in the nicotine content of their cigarettes. Their self-administration behavior at the low doses was compared to a group of rats who were given the opportunity to acquire nicotine self-administration for the first time at one of the same low doses or vehicle alone. The self-administration behavior of these rats may be thought of as an analog for new smokers who begin smoking after the nicotine content in cigarettes has been reduced. Second, we examined the effects of a history of high nicotine dose self-administration using a within-subjects comparison of self-administration behavior before and after experience self-administering a high dose of nicotine.
Techniques were employed to more appropriately model a policy scenario in which the nicotine content of cigarettes is greatly reduced. A light stimulus was paired with nicotine infusions to model the experience of smoker who experiences environmental cues paired with their smoking. Additionally, a cocktail of non-nicotine cigarette smoke constituents was included along with nicotine infusions to better model the experience of a smoker. Some of these constituents have been shown to have behavioral effects on their own or in combination with nicotine (Bardo, Green, Crooks, & Dwoskin, 1999; Belluzzi, Wang, & Leslie, 2005; Clemens, Caille, Stinus, & Cador, 2009; Guillem et al., 2005; Hoffman & Evans, 2013; Villegier, Lotfipour, McQuown, Belluzzi, & Leslie, 2007). Although these manipulations make it difficult to isolate the mechanism(s) responsible for a shift in self-administration, they have the advantage of being more directly relevant to the critical regulatory questions faced by the FDA that cannot be easily addressed in clinical samples (Donny et al., 2012).
Method
Subjects
Male Sprague-Dawley rats (Harlan-Farms, IN), weighing between 188 and 217 grams on the first day after arrival were used as subjects. Rats were approximately postnatal day 49 upon arrival and approximately postnatal day 84 at the start of self-administration. Rats were individually-housed in wire-mesh, hanging cages in a temperature-controlled room kept between 68 and 70 degrees Fahrenheit. Rats were kept on a reverse light-dark 12:12 hour schedule, and all testing took place during the dark phase of the cycle. Rats received free access to water in their home cages throughout the experiment, and were given unlimited access to Purina Rat Chow for the first week after arrival. After a week-long acclimation to our animal facility, rats were restricted to 20 g/day of chow for the remainder of the experiment, which has been shown to result in weight gain of approximately 10 g/week (Donny, Caggiula, Knopf, & Brown, 1995). All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Apparatus
Thirty-four commercial operant chambers (30.5cm × 24.1 cm × 21.0 cm; ENV-008CT; Med-Associates) were used in the present experiment. Each chamber was closed inside a sound-attenuating cubicle with a ventilation fan. The right wall of the chamber contained two nosepoke holes, each 2.5 cm in diameter. The bottom of each hole was 5 cm above the floor, and the inside edge of the two holes were spaced 14 cm apart. A white stimulus light, 3.5 cm in diameter, was located 6.25 cm above the top of each nosepoke hole. Each chamber also contained a houselight on the same wall, located 1 cm below the ceiling of the chamber, which was illuminated red during sessions. Outside of each chamber, a pump delivered i.v. infusions of nicotine or vehicle during the session through tubing that was connected to each rat’s catheter. The tubing was protected by a metal casing and was connected to a swivel that allowed virtually unrestricted movement in the chamber during sessions.
Drugs
Nicotine hydrogen tartrate salt (Sigma, St. Louis, MO), and a cocktail of other cigarette constituents including: acetaldehyde (Sigma, St. Louis, MO), harman (Sigma, St. Louis, MO), norharman (Sigma, St. Louis, MO), anabasine (Sigma, St. Louis, MO), anatabine (Toronto Research Chemicals, Inc), myosmine (Sigma, St. Louis, MO), cotinine (Sigma, St. Louis, MO), and nornicotine (Sigma, St. Louis, MO) were dissolved in 0.9% saline. The doses of nicotine used for self-administration were 3.75, 7.5, 15, and 60 μg/kg/infusion (expressed as base). The doses of nicotine were chosen to be a range that captures the threshold for maintaining behavior after reduction (Smith et al., 2013). However, at the beginning of the experiment, a dosing error was made and all rats were also exposed to doses of nicotine one tenth of the intended dose for the initial eight sessions.
The doses of the other constituents were either indexed to be proportional to the contents of cigarette smoke given a dose of nicotine that produces peak self-administration in rodents (30 μg/kg/infusions) (Herraiz, 2004) and/or because of their use in previous research (Clemens et al., 2009): 16 μg/kg/infusion (acetaldehyde), 0.1 μg/kg/infusion (harman), 0.3 μg/kg/infusion (norharman), 0.9 μg/kg/infusion (anabasine and nornicotine), 0.09 μg/kg/infusion (anatabine, myosmine and cotinine) (expressed as free-base).
The pH of all drug solutions was adjusted to 7.0 (±0.2) with dilute NaOH. All solutions, including the vehicle, were sterilized by being passed through a 0.22 μm filter. During sessions, the time to deliver an infusion ranged between 0.61 s and 1.04 s (dependent on each rat’s weight). Infusions averaged 0.1 ml/kg/infusion and had a standard deviation of 11% of the mean infusion volume.
Procedures
Surgery
After at least one week of acclimation, rats were anesthetized with isoflurane (2-3% in 100% O2) and implanted with jugular catheters as described previously (Donny et al., 1999). After surgery, rats recovered for a minimum of 5 days in their home cages. For the first three days after surgery, the catheters were flushed once daily with 0.1 mL of sterile saline containing heparin (30 U/mL), timentin (66.67 mg/ml) and streptokinase (9333 U/mL) to maintain catheter patency and prevent infection. After this initial post-surgery time period, the flushing solution contained only heparin and timentin. All rats included in the manuscript passed a patency test following the last day of self-administration, in which chloral hydrate (up to 60 mg per rat) was infused via the catheter into the bloodstream causing rapid loss of muscle tone in rats with patent catheters.
Self-Administration
Fulfilling the required number of nosepoke responses into the active (right) nosepoke hole resulted in one infusion of the assigned nicotine dose along with a cocktail of the other constituents discussed above. Each infusion resulted in the 15-s presentation of a stimulus light located above the nosepoke hole (cued paradigm) and a 1-min time-out (of which the remaining 45 s was unsignaled). Inactive (left) nosepoke responses were recorded, but had no scheduled consequences. Sessions lasted 1 hour and were conducted 7 days/week.
Between-Subjects Comparison
At the start of self-administration, rats were randomly assigned to receive a high acquisition dose of nicotine (60 μg/kg/infusion; n=57), or one of four lower acquisition doses: 15 (n=14), 7.5 (n=12), 3.75 (n=10), or 0.0 μg/kg/infusion (vehicle; n=12). Due to a technical error, all rats first experienced 8 sessions of exposure to one-tenth the assigned nicotine dose (fixed ratio (FR) 1, 1 session; FR2, 7 sessions). Upon discovery of the technical error, all rats were returned to FR1 at their intended dose. Rats experienced 1 session on an FR1 schedule of reinforcement and 7 sessions on an FR2 schedule of reinforcement before the ratio was escalated to an FR5. Rats assigned to one of the four lower doses of nicotine constitute the analog for “new smokers” and experienced 25 sessions on an FR5 at these doses. Rats assigned to the higher acquisition dose of nicotine (60 μg/kg/infusion) constitute the analog for current smokers and experienced 40 sessions on an FR5 schedule of reinforcement before the nicotine dose was reduced to one of the four lower doses. Rats were assigned to one of the four lower dose groups matched for average infusions earned over the last three sessions before reduction. These rats had their nicotine dose reduced to either 15 (n=15), 7.5 (n=14), 3.75 (n=14), or 0.0 (n=14) μg/kg/infusion for 16 sessions. For shorthand, groups are referred to using either ACQ or RED and their low nicotine dose (e.g., ACQ 7.5 for group that experienced 7.5 μg/kg/infusion during acquisition). The primary variable of interest was earned infusions at the low nicotine doses during acquisition (ACQ groups) and following reduction (RED groups).
Within-Subjects Comparison
The within-subjects design involved three phases and only included the rats in the ACQ groups. Following 25 sessions at one of the four low doses of nicotine (now referred to as Phase 1), rats in the acquisition groups were given experience self-administering a high dose of nicotine (60 μg/kg, 15 sessions-Phase 2) and then placed back on the low dose of nicotine experienced in Phase 1 (0-15 μg/kg/infusion, 16 sessions-Phase 3). Although human smokers would be unlikely to experience a low-high-low nicotine dose change, these dose manipulations examine the effect of a history of a higher nicotine self-administration on low nicotine dose self-administration using a within-subjects design. The primary variable of interest was the number of infusions earned during acquisition (in Phase 1) and following reduction (in Phase 3).
Data Analysis
At the end of the first 25 FR5 sessions, a group was considered stable if the difference between average earned infusions over the last three days and the preceding three days was less than 10% of the mean of all six days, or the overall average from those days was less than 2.5 infusions. Throughout all analyses, rats were considered to have met criteria for self-administration if they received an average of at least 5 infusions over the previous three sessions and had twice as many average active as inactive responses over the same time (Smith et al., 2013). The proportion of rats that met criteria at each dose of nicotine was compared using the Fisher Exact Test (α=0.005). The speed at which the criteria were met was compared using the first session in which the 3-session moving average for infusions earned was at least five and active responses were at least twice inactive responses (log-transformed values due to positive skew).
For both the between-subjects and within-subjects comparisons, all rats that failed to meet the criteria for self-administration prior to reduction were removed because they failed to experience the manipulation of interest (experience self-administering a higher dose of nicotine). Eight rats were excluded: 2 ACQ 7.5 rats, 3 ACQ 3.75 rats, 2 ACQ 0.0 rats, and 1 RED 15 rat. All subsequent analyses focused on the average earned infusions over the last three sessions of acquisition or reduction. Data were analyzed using ANOVA. All post-hoc tests employed a Bonferroni correction to keep family-wise Type 1 error rate less than 0.05.
Results
Acquisition Analyses
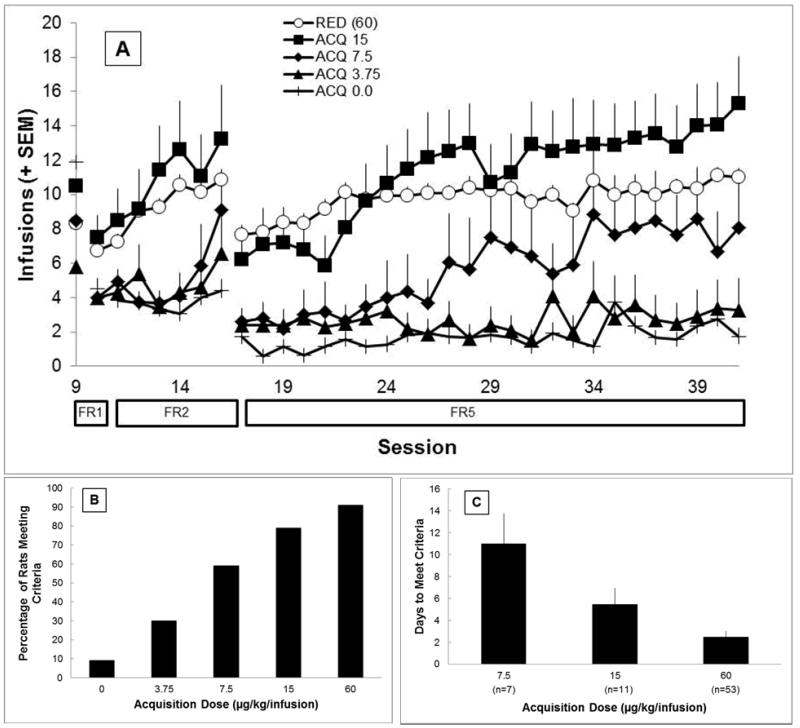
Figure 1 describes initial acquisition behavior for rats in all groups. At the end of the 25 FR5 acquisition sessions, rats in the 60 μg/kg/infusion group earned significantly more infusions than rats in the ACQ 3.75, and ACQ 0.0 groups (Figure 1A; all ps<0.0125), but not from any other dose groups. At the end of the 25 acquisition sessions, all nicotine groups met the stability criterion indicating that behavior was unlikely to change given more experience in the acquisition phase. Rats in higher-dose groups were more likely to meet the criteria for self-administration than rats in lower-dose groups. A higher percentage of rats in the 60 μg/kg/infusion group (RED rats) met the criteria than rats in the 3.75 μg/kg/infusion and vehicle groups. A higher percentage of rats in the 15 μg/kg/infusion group met the criteria than rats in the vehicle group (all ps < 0.005). There were no other significant differences. Among the three groups in which more than half of the rats met the criteria for self-administration (60, 15, and 7.5 μg/kg/infusion), the time to acquire was dose dependent with rats self-administering larger doses acquiring more quickly. Independent samples t-tests confirm that rats in the 60 μg/kg/infusion group met criteria significantly faster than rats in either the 15 μg/kg/infusion or the 7.5 μg/kg/infusion dose groups (p<0.017). The speed of acquisition did not significantly differ between the 15 and 7.5 μg/kg/infusion groups, p=0.182. The rate of self-administration among those rats meeting the criteria for self-administration did not differ significantly among groups where more than half of the rats met criteria for self-administration (p>0.0167)
Figure 1.
Behavior during acquisition
A) Earned infusions across acquisition for all groups (only first 25 FR5 sessions shown for rats in RED groups and excluding the initial eight dosing-error sessions). Error bars represent standard error. B) Proportion of rats in each acquisition groups that met criteria for self-administration (see text). C) The number of days to meet criteria for each acquisition group in which more than half of the rats met criteria. Only rats that were classified as meeting criteria at the end of acquisition are included.
Between-Subjects Comparison
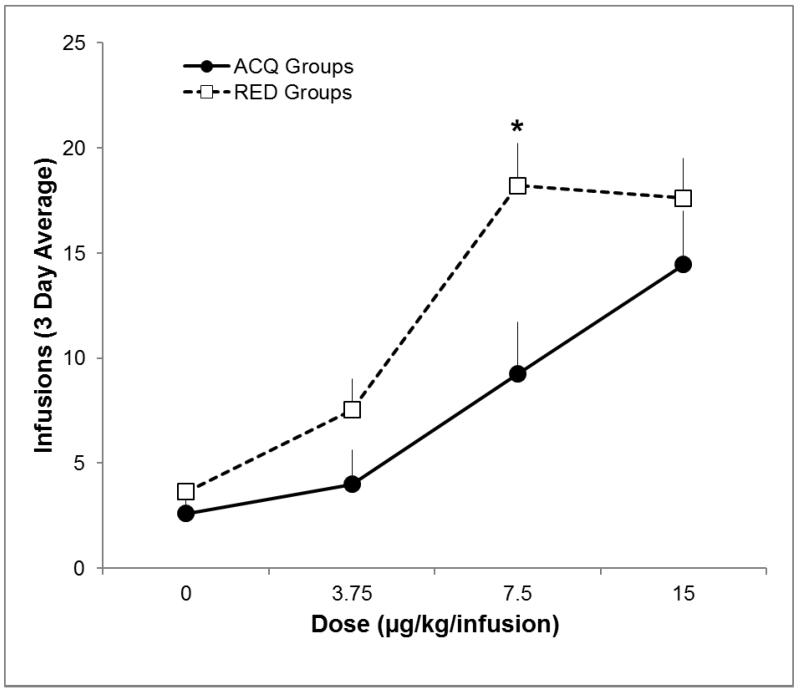
Figure 2 shows the average earned infusions at each of the four low doses during acquisition (ACQ groups) and following reduction (RED groups). The ANOVA revealed a significant main effect of dose and significant main effect of history (ACQ vs RED; ps<0.05), but not a significant interaction (p=0.177). Follow-up tests revealed that the RED 7.5 group earned significantly more infusions following reduction than the ACQ 7.5 group earned during acquisition (p<0.0125). The difference in infusions at 7.5 μg/kg/infusion was larger than the small difference observed for vehicle (p=0.017).
Figure 2.
Earned infusions at each nicotine dose during acquisition (ACQ groups), and reduction (RED groups)
Earned infusions over the last three sessions of acquisition (ACQ groups, filled circles) and reduction (RED groups, open squares). Bars represent standard errors. Significantly more infusions earned for RED group, and significant nicotine × history interaction is represented by *.
Within-Subjects Comparison
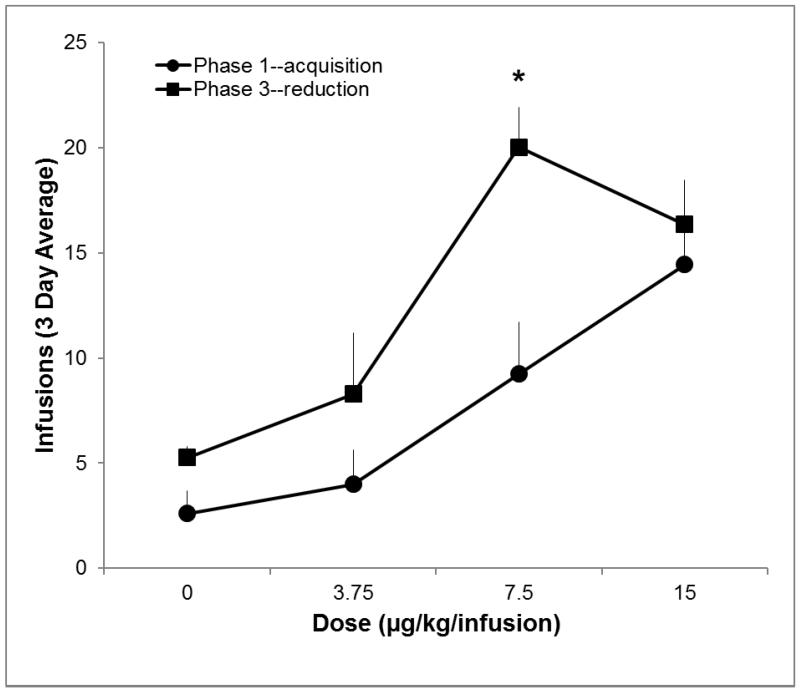
Figure 3 shows the average earned infusions for the ACQ groups at each of the four low doses during Phase 1 (same ACQ data shown in Figure 2) and during Phase 3. Rats earned significantly more infusions following self-administration of 60 μg/kg/infusion nicotine (Phase 3) than during Phase 1 (p<0.01). There was also a significant main effect of dose (p<0.01), and a dose by phase interaction (p<0.05). Infusions earned during Phase 3 were significantly greater than during Phase 1 for ACQ 7.5 rats (p<0.0125); this difference was also greater than the change in responding for vehicle (p<0.017). If rats that failed to meet criterion in Phase 1 are excluded, earned infusions are greater in Phase 3 than in Phase 1 for the ACQ 7.5 group, p < 0.05 (analysis only conducted for the ACQ 15 and ACQ 7.5 groups where more than half of the rats met criteria in Phase 1).
Figure 3.
Earned infusions at each nicotine dose during acquisition (Phase 1) and reduction (Phase 3).
Earned infusions over the last three sessions of acquisition (Phase 1, filled circles) and reduction (Phase 3, filled squares).Significant increase in infusions earned for from Phase 1 to Phase 3 (p<0.0125), and significant nicotine × phase interaction (p<0.017) denoted by *. Bars represent standard errors
Comparing low-dose nicotine self-administration after different lengths of high-dose experience
One additional comparison that could be made is whether infusions of low-dose nicotine earned following reduction depend on the length of experience at the high dose of nicotine. There were no significant differences in the infusions of low-dose nicotine earned following 15 (i.e., ACQ groups during Phase 3) compared to 40 (i.e., RED groups) sessions of experience at 60 μg/kg/infusion.
Discussion
The present study provides important information regarding the impact of a history of high dose nicotine self-administration on subsequent self-administration of lower doses. Rats self-administered low doses of nicotine at a higher rate following reduction from self-administration of a high dose of nicotine than during acquisition. This history had the greatest impact at 7.5 μg/kg/infusion nicotine. These data are reassuring from a policy perspective. If the nicotine content in cigarettes was reduced, there would likely be a substantial clinical literature for regulators to rely on to predict how a nicotine reduction policy might affect current smokers. However, little or no clinical data on relatively naïve individuals will be available to predict the likely impact on individuals who may start smoking for the first time at the reduced level. These data suggest that following a nicotine reduction policy, individuals who try smoking for the first time are likely to have lower smoking rates and a lower smoking prevalence than individuals with a history of smoking cigarettes with regular nicotine content.
More than half of the rats given the opportunity to acquire nicotine self-administration did so when the nicotine dose was 7.5 μg/kg/infusion or greater. The proportion of rats that acquired self-administration and the time to acquire were also dose-dependent. As the dose used for acquisition increased, the probability of any rat meeting the criteria for self-administration increased, and the time it took to meet these criteria decreased, consistent with what was reported by Peartree et al. (2012). These data indicate that regulation aimed at decreasing the content of nicotine within cigarettes is likely to result in a smaller proportion of individuals acquiring stable smoking behavior and a longer acquisition period for those that do acquire.
Some of the increase in self-administration following high-dose self-administration is almost certainly related to the association of the operant chamber and light cue with nicotine. Even after nicotine is removed, the operant chamber and active nosepoke continue to function as discriminative stimuli, signaling the availability of nicotine (Skinner, 1953). Also, the presentation of the light cue (which was paired with 60 μg/kg/infusion prior to reduction) likely maintains responding as a conditioned reinforcer. For smokers, distal (e.g.,a bar) and proximal smoking (e.g., taste of a cigarette) cues will continue to maintain behavior for some time after nicotine reduction. In the present study, self-administration rate was reduced quickly following nicotine reduction, but behavior did not return to the acquisition baseline, even over 16 1-hour sessions. Another study showed that responding was maintained for over 60 sessions even after nicotine was removed and only decreased further when the cues were removed (Cohen, Perrault, Griebel, & Soubrie, 2005). These data suggest that the process of extinction could be very prolonged. Furthermore, rat self-administration models may overestimate the speed of extinction because learning and extinction occur in one context, the operant chamber. For human smokers, nicotine has been paired with many cues in many contexts, and extinction would likely need to take place in many or all of those contexts, increasing the time until behavior is extinguished (Wing & Shoaib, 2008). The present study illustrates the need for research investigating methods that may facilitate extinction (Donny et al., 2012).
The effect of a history of high dose nicotine self-administration was larger at 7.5 μg/kg/infusion, a dose that was on the ascending portion of the dose-response curve during acquisition. The larger increase in self-administration at this dose may be indicative of a leftward shift in the dose-response curve. A leftward shift in the dose-response curve would indicate that experience self-administering a high nicotine dose caused rats to become more sensitive to the reinforcing effects of nicotine than they were prior to that experience. Another possible explanation would be that the history of high-dose self-administration resulted in decreased sensitivity to the rate-limiting effects of nicotine typically considered responsible for the descending limb of the dose-response curve. Another mechanism for the increase in self-administration rate at 7.5 μg/kg/infusion may be that for this group, nicotine enhanced the reinforcing value of the light cue following reduction, whereas it did not during acquisition (Donny et al., 2003; Palmatier et al., 2006). During acquisition, the cue light would have had little, if any, reinforcing value for nicotine to enhance in the 7.5 μg/kg/infusion group, but likely because a conditioned reinforcer in Phase 2, causing nicotine to enhance the reinforcing value of the light cue following reduction. Regardless of the mechanism, these data suggest that self-administration of large doses of nicotine might have the greatest impact on behavior that is near the threshold for self-administration.
Limitations and Future Directions
As discussed above, a dosing error occurred at the very start of acquisition. It is possible that the rats’ pharmacological and behavioral experience during these initial eight sessions influenced the results of the study by sensitizing the rats to nicotine or slowing down learning about the cue contingencies (i.e., latent inhibition). However, the within-subjects design employed in the study provides confidence that history of high-dose self-administration experience, and not the dosing error, is responsible for the shift in self-administration during reduction.
In this study, the vehicle contained a cocktail of other cigarette constituents. Thus far, there is nothing in the literature to suggest that nicotine may shift the reinforcing value of the constituents used here. However, research on these constituents is sparse, and experiments have not been designed to test this possibility. The five minor alkaloids employed here (anabasine, anatabine, myosmine, cotinine, and nornicotine) have a similar chemical structure to nicotine (Huang & Hsieh, 2007), and it is possible that a history of nicotine exposure could increase sensitivity to these constituents. While it is impossible to know what influence the non-nicotine vehicle may have had on the results of the present study, the use of these constituents may be viewed as a strength of the present study because it more appropriately models a policy scenario in which nicotine is reduced, but the doses of other constituents are held constant. Furthermore, in the present study, the effects of high dose nicotine self-administration are confounded with the effects of extended self-administration experience. While it will always be possible that the results of the present study may be due to the increased number of sessions self-administering, and not high dose self-administration, it is unlikely given that rats were given a large number of acquisition sessions (25 FR5 sessions), and self-administration met a criterion for stability at the end of those sessions. Furthermore, extended self-administration experience before reduction appropriately models a policy scenario because current smokers would have more smoking experience than naïve individuals. If the extended self-administration experience in Phase 2 did indeed contribute to the observed effect, escalation of smoking in naïve smokers might be expected if these individuals experiment with low nicotine content cigarettes over an extended period of time for reasons other than the primary reinforcing effects. Additionally, the present study employed only adult male rats. Future research should use adolescent and female rats to investigate low-dose nicotine self-administration, as there is likely to be little human research on acquisition in these populations.
If clinical data from current smokers support nicotine reduction as an effective strategy for reducing public harm, preclinical data like these will be important in determining the potential impact on individuals who have not yet started smoking. Future animal research should continue to prioritize research questions related to acquisition of low-dose nicotine self-administration, which cannot be evaluated in clinical studies.
Acknowledgments
Research reported in this publication was supported by the National Institute on Drug Abuse and FDA Center for Tobacco Products (CTP) (U54 DA031659 awarded to E.C.D.) The funding source had no other role other than financial support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
All authors contributed significantly to this manuscript, and all authors have read and approved the final version.
The authors would like to thank Maysa Gharib, Josh Alberts, Emily Pitzer, Angela Lutheran, Melinda Moran, and Richard Jacobson for their assistance in data collection.
Footnotes
A portion of these data were also presented as part of a symposium at the Society for Research on Nicotine and Tobacco’s annual meeting on March 16th, 2013
Disclosures
The authors have no competing interests to declare.
Contributor Information
Tracy T. Smith, Department of Psychology, University of Pittsburgh
Rachel L. Schassburger, Department of Neuroscience, University of Pittsburgh.
Deanne M. Buffalari, Department of Neuroscience, University of Pittsburgh
Alan F. Sved, Departments of Psychology and Neuroscience, University of Pittsburgh
Eric C. Donny, Department of Psychology, University of Pittsburgh
References
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23(11):4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl) 1999;146(3):290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30(4):705–712. doi: 10.1038/sj.npp.1300586. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. New England Journal of Medicine. 1994;331(2):123–125. doi: 10.1056/NEJM199407143310212. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- CDC Quitting smoking among adults -- United States, 2001-2010. Morbidity and Mortality Weekly Report. 2011;60:1513–1519. [PubMed] [Google Scholar]
- Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32(3):700–709. doi: 10.1038/sj.npp.1301135. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Caille S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. International Journal of Neuropsychopharmacology. 2009;12(10):1355–1366. doi: 10.1017/S1461145709000273. doi: 10.1017/S1461145709000273. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmacology. 2005;30(1):145–155. doi: 10.1038/sj.npp.1300541. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Cox BM, Goldstein A, Nelson WT. Nicotine self-administration in rats. British Journal of Pharmacology. 1984;83(1):49–55. doi: 10.1111/j.1476-5381.1984.tb10118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122(4):390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, McCallum SE. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147(2):135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl) 1998;136(1):83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169(1):68–76. doi: 10.1007/s00213-003-1473-3. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Donny EC, Taylor TG, Lesage MG, Levin M, Buffalari DM, Joel D, Sved AF. Impact of Tobacco Regulation on Animal Research: New Perspectives and Opportunities. Nicotine and Tobacco Research. 2012 doi: 10.1093/ntr/nts162. doi: 10.1093/ntr/nts162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenstein P, Burroughs D, Zhang Y, LeSage MG. Sex differences in nicotine self-administration in rats during progressive unit dose reduction: Implications for nicotine regulation policy. Pharmacology, Biochemistry, and Behavior. 2013 doi: 10.1016/j.pbb.2013.10.020. http://dx.doi.org/10.1016/j.pbb.2013.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. Journal of Neuroscience. 2005;25(38):8593–8600. doi: 10.1523/JNEUROSCI.2139-05.2005. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AC, Evans SE. Abuse potential of non-nicotine tobacco smoke components: acetaldehyde, nornicotine, cotinine, and anabasine. Nicotine and Tobacco Research. 2013;15(3):622–632. doi: 10.1093/ntr/nts192. doi: 10.1093/ntr/nts192. [DOI] [PubMed] [Google Scholar]
- Huang HY, Hsieh SH. Analyses of tobacco alkaloids by cation-selective exhaustive injection sweeping microemulsion electrokinetic chromatography. Journal of Chromatography A. 2007;1164(1-2):313–319. doi: 10.1016/j.chroma.2007.06.065. doi: 10.1016/j.chroma.2007.06.065. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006;184(3-4):391–400. doi: 10.1007/s00213-005-0183-4. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Peartree NA, Sanabria F, Thiel KJ, Weber SM, Cheung TH, Neisewander JL. A new criterion for acquisition of nicotine self-administration in rats. Drug and Alcohol Dependence. 2012 doi: 10.1016/j.drugalcdep.2011.12.011. doi: 10.1016/j.drugalcdep.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 1997;129(1):35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Smith TT, Levin ME, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Gradual and immediate nicotine reduction result in similar low-dose nicotine self-administration. Nicotine and Tobacco Research. 2013;15(11):1918–1925. doi: 10.1093/ntr/ntt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF. Science and Human Behavior. Macmillan; New York: 1953. [Google Scholar]
- Sorge RE, Clarke PB. Rats self-administer intravenous nicotine delivered in a novel smoking-relevant procedure: effects of dopamine antagonists. Journal of Pharmacology and Experimental Therapeutics. 2009;330(2):633–640. doi: 10.1124/jpet.109.154641. doi: 10.1124/jpet.109.154641. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117(1):2–10. doi: 10.1007/BF02245088. discussion 14-20. [DOI] [PubMed] [Google Scholar]
- U.S. Congress . Family Smoking Prevention and Tobacco Control Act. U.S. Government Printing Office; 2009. Retrieved from http://www.gpo.gov/ [Google Scholar]
- U.S. Department of Health and Human Serves . U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2012. Preventing Tobacco Use Among Youth and Youth Adults: A Report of the Surgeon General. [Google Scholar]
- Villegier AS, Lotfipour S, McQuown SC, Belluzzi JD, Leslie FM. Tranylcypromine enhancement of nicotine self-administration. Neuropharmacology. 2007;52(6):1415–1425. doi: 10.1016/j.neuropharm.2007.02.001. doi: 10.1016/j.neuropharm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Wing VC, Shoaib M. Contextual stimuli modulate extinction and reinstatement in rodents self-administering intravenous nicotine. Psychopharmacology (Berl) 2008;200(3):357–365. doi: 10.1007/s00213-008-1211-y. doi: 10.1007/s00213-008-1211-y. [DOI] [PubMed] [Google Scholar]