Abstract
Peru struggles to prevent cervical cancer (CC). In the jungle, prevention programs suffer from significant barriers although technology exists to detect CC precursors. This study used community based participatory research (CBPR) methods to overcome barriers. The objective was to evaluate the utility of CBPR techniques in a mother–child screen/treat and vaccinate program for CC prevention in the Peruvian jungle. The CC prevention program used self-sampling for human papillomavirus (HPV) for screening, cryotherapy for treatment and the HPV vaccine Gardasil for vaccination. Community health leaders (HL) from around Iquitos participated in a two half day educational course. The HLs then decided how to implement interventions in their villages or urban sectors. The success of the program was measured by: (1) ability of the HLs to determine an implementation plan, (2) proper use of research forms, (3) participation and retention rates, and (4) participants’ satisfaction. HLs successfully registered 320 women at soup kitchens, schools, and health posts. Screening, treatment, and vaccination were successfully carried out using forms for registration, consent, and results with minimum error. In the screen/treat intervention 100 % of participants gave an HPV sample and 99.7 % reported high satisfaction; 81 % of HPV + women were treated, and 57 % returned for 6-month followup. Vaccine intervention: 98 % of girls received the 1st vaccine, 88 % of those received the 2nd, and 65 % the 3rd. CBPR techniques successfully helped implement a screen/treat and vaccinate CC prevention program around Iquitos, Peru. These techniques may be appropriate for large-scale preventive health-care interventions.
Keywords: Human papillomavirus, Cervical cancer screening, Community based participatory, Research techniques, Peru
Introduction
World-wide cervical cancer is the third most common cancer in women, accounting for 13 % of all female cancers [1].1 According to the WHO, Peru’s age adjusted incidence of cervical cancer at 34.5 per 100,000 women is remarkably higher than the rest of South America at 24.1 [2]. Many factors account for this difference. Peru’s national guidelines for cervical cancer detection, in place since 2000, include Pap smears for women age 25–59. However, from 2000 to 2003, only 40.3 % of women in this age range underwent screening, with significantly higher rates of screening in coastal areas than in the central regions of the country [3]. While the high rate of cervical cancer is partly due to lack of screening, it is also due to poor follow up with only 25 % of women with abnormal Pap tests receiving appropriate referral. Furthermore, there is also evidence that misclassification of Pap smears is also a significant problem with one study of 5,435 Peruvian women showing that the sensitivity for carcinoma in situ of cytology was only 42.5 %, thereby missing a significant amount of high grade disease [4]. Finally, the high rates of cervical cancer in Peru may also be due to a high prevalence of human papillomavirus (HPV). In a case–control study conducted in Lima, HPV prevalence was 17.7 % among cytological normal women, which is significantly higher than the global prevalence (10 %) [5].
Primary screening for cervical cancer by detection of high-risk types of the HPV (HR-HPV) from a self-collected vaginal swab is both a sensitive and effective alternative to cytology based programs. A study of 10,000 Chinese women reported the sensitivity of a self-collected sample for HR-HPV equal to a physician obtained endocervical sample (dependent on the type of assay used), and considerably more sensitive than cytology [6]. Furthermore, multiple studies among Hispanic women in the USA and Mexico, as well as among women in Africa and Asia have shown self-sampling to be acceptable [7–11]. There is also evidence that screening and treatment with HPV testing is cost effective, with a potential in Peru of a 60 % reduction in cervical cancer at only $591 per year of life saved [12]. This analysis was not based on self-collected specimens, which may significantly reduce this cost even further.
For primary prevention, the HPV vaccine has been shown to be very effective and was, at one time, incorporated into Peru’s national health system. Thus, the technology for both primary and secondary prevention of cervical cancer exists; however, prevention programs focused on high-risk, medically underserved women continue to face significant barriers in effectively reaching the women most in need.
The PERU cervical cancer prevention study (PER-CAPS) is a community based participatory research (CBPR) project with a goal to design a cancer-screening model for underserved populations, and then implement this model using current cervical cancer screening technology. We hypothesized that HPV vaccination and screening, a costly preventative health measure, could be more efficiently done by the community, allowing health providers to focus available resources on the women who test positive. We chose cryotherapy for treatment since it was used in Peru, with reported efficacy from 70 to 95.5 % [13], and minimal side effects. The first phase of this work was completed in Manchay, Peru, a shanty town outside the capitol, Lima [14]. We now report the Iquitos phase of this project as we built on the lessons learned and applied them to the more remote and geographically challenging Peruvian jungle.
Materials and Methods
The study was approved by institutional review boards of the Cleveland Clinic, Merck USA, Merck Peru, the Ethics Committee of the National Institute of Neoplastic Disease (INEN) in Peru, and the Peru Instituto Nacional de Salud. The study was funded through the investigator initiated studies program of Merck, Inc., Preventive Oncology International, Inc. (POI), and the Fogarty International Clinical Research Fellows Program; and it was registered at Clinical Trials.Gov, number 01338051. The primary objective of this study was to develop our model for community based cervical cancer prevention (screening and vaccination) using the concepts founded in CBPR.
After making an estimate of the target population and considering limitations in access during the dry season, a combination of rural and urban communities along the Amazon was selected. Then an education program for the community health leaders (HLs) was planned based on PERCAPS Manchay. Finally, the HLs collaboratively developed a strategy for advertisement, registration, self-sampling, delivery of samples, reporting of results, and vaccination. A low literacy instructional sheet was piloted to explain the technique of self-collection using a brush designed for self-sampling (“Just for Me”, Preventive Oncology International, Cleveland Heights, Ohio, USA) and solid transport media cards (iFTA-elute GE Health-care, Piscadaway, NJ, USA). The self-collected HPV samples were sent to BGI Shenzhen (Shenzhen, China) for processing using the PCR based MALDI-TOF assay (Mass Array Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight mass spectrometry system) [15]. Women who tested positive for HR HPV were located and brought to the local health clinic for evaluation and treatment with cryotherapy (the details of the medical management and the new technologies employed have been previously reported [16]). After 6 months, the women treated with cryotherapy were relocated for a follow-up examination. For vaccination, HLs used the quadrivalent Merck vaccine for types 16/18/6 and 11 with doses according to the manufacturer’s instructions at time 0, 2, and 6 months. Following all cryotherapy treatments, as well as the 1st or 2nd doses of vaccine, a Spanish speaking physician (au-CA) conducted evaluations with the participants using questionnaires piloted in the prior Manchay project.
Women, ages 30–45 were eligible for the study. Exclusions were a current pregnancy, a history of hysterectomy, and a history of pelvic radiation. Girls ages 10–13 years were eligible to participate and receive HPV vaccination. Exclusions were the presence of an acute febrile illness, previous vaccination for HPV, and allergy to yeast.
The HL from each community attended the training. This included a midwife, a physician, a nurse, and three health technicians. The training took two half-days with the 1st day focused on education, including cervical cancer, HPV, screening, self-sampling, and the HPV vaccine. On the 2nd day, the design phase, the HLs developed recruitment strategies for enrolling 320 women, matched with girls to receive the HPV vaccine. They also developed a plan for facilitating the evaluation and treatment of the positives and the logistics of the HPV vaccination campaign.
The success of the program was measured by: (1) the ability of the HLs to design a workable model, (2) the successful use of research forms, (3) participation (goal 85 % of target) and retention rates (goal 90 % return of the positives), and (4) satisfaction of the participants (goal 90 %).
Results
Recruitment
During the training, the participants revealed that mother daughter pairs were not the functional reality in their villages. Therefore, recruitment was broadened to include any parous woman 30–45 paired with a daughter, granddaughter, niece, or “child of the community” aged 10–13.
In sum, 320 women meeting criteria were recruited: 175 in rural areas and 145 in the city. Participation was 100 % of the registered women, with all subjects returning a sample. All samples contained adequate DNA. The research forms were successfully managed with few exceptions, including missing signatures of mothers, daughters, or technicians which were later obtained within a few days of recruitment. In all villages, most participants immediately did the self-collection at the central clinics but a few brought the kits home and returned them the following day.
The HLs developed a vaccine schedule coordinated with the school calendar to administer the first 2 doses prior to the summer holiday and the final dose 4 months later upon the girls’ return. In the recruitment of girls for vaccination, 2 girls were recruited twice reducing the number of girls to 318 instead of 320. Twelve girls who were not assented for the study also received the vaccine when HLs substituted them for registered girls they could not find during vaccination campaigns. Another error was the registration of 9 girls who had already initiated the state’s HPV vaccine series.
Notification of Test Results
Of the 320 women who completed self-sampling, 37 tested positive for HR HPV (11.5 %). In urban areas, notifications for positive women were delivered in writing to the subjects’ homes while participants with negative HPV were invited back to the soup kitchens to receive their results. In rural areas, nearly all results were delivered at the local health post and otherwise at their homes.
Treatment for the Positives
Of the 37 women who tested positive for HR HPV, 30 (81 %) presented for evaluation and cryotherapy at the Bellavista Nanay Health Post. Of the 7 women who did not present for evaluation, 1 chose treatment with traditional herbs, 2 were unavailable due to work, 1 was undergoing evaluation for an abdominal tumor, and the remaining 3 declined the procedure. Fifty-seven percent of the women (16) who had undergone cryotherapy returned for the 6 month follow up with colposcopy and biopsy (Fig. 1).
Fig. 1.
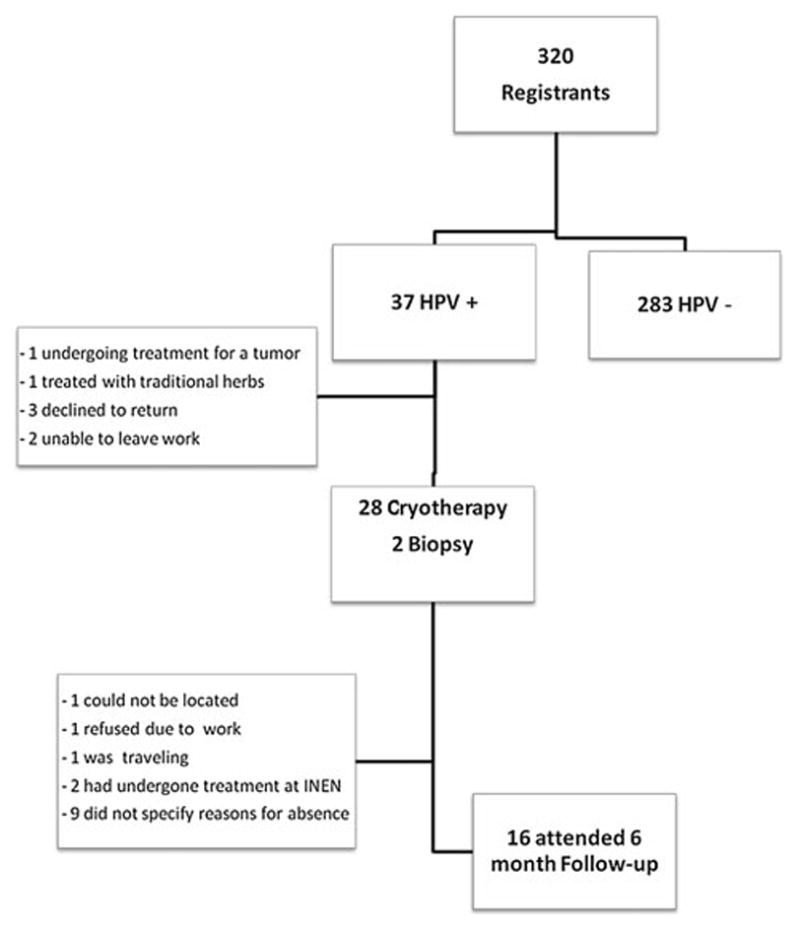
Human papillomavirus (HPV) self-sampling/cryotherapy participation rates
Vaccination
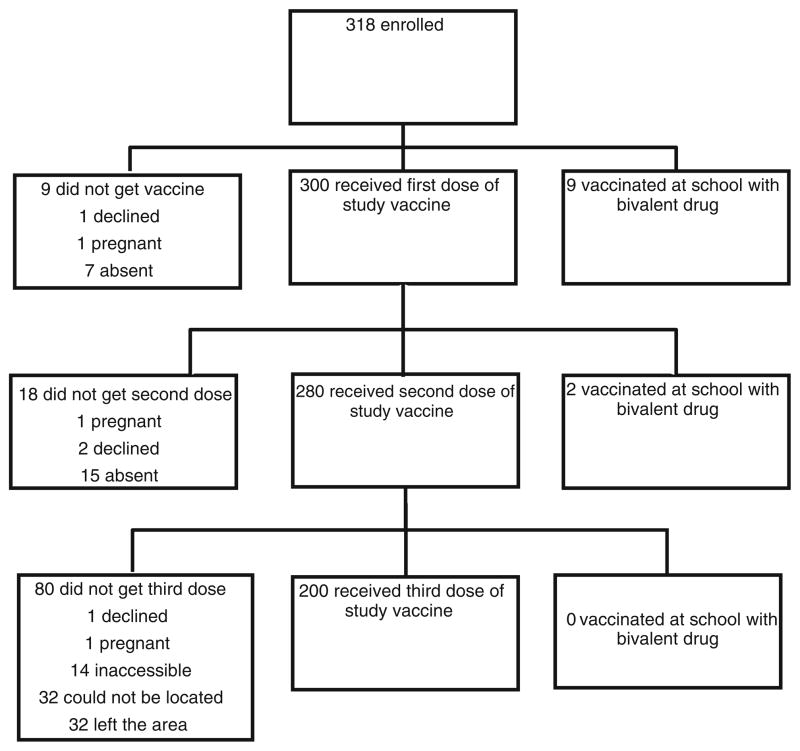
There were 318 girls assented for vaccination as noted above. Ultimately, 312 (98 %) girls received the first dose of vaccine (300 of those were originally registered and 12 girls who were not initially registered for the study). For those girls initially registered, reasons for not vaccinating included leaving the area (7), father declined (1), pregnant (1), and recruited in error since they had already initiated the series with the government campaign (9). In addition to the 1 girl who became pregnant and did not receive the 1st vaccine, 3 other girls became pregnant during the study. Two participants received the 1st vaccine prior to discovering they were pregnant and were reported for pharmaco-vigilance as adverse events. The other participant received the first dose of vaccine, subsequently became pregnant and withdrew from the study.
The second dose of vaccine was administered to 280 girls (90.6 %). Reasons for not receiving the 2nd vaccination were: pregnant (1), unable to be located (15), parents declined (2), and vaccinated by the state (2).
The third dose was administered to 200 girls (71.5 %). For this final dose, 32 girls could not be located, 14 were inaccessible to the vaccine teams, 32 had left the area, 1 was pregnant and 1 parent refused (Fig. 2). The administration of this 3rd vaccine was significantly affected by severe flooding. Possibly all but 1 of the missed vaccinations for this 3rd dose was related to the flood that changed the landscape for many weeks.
Fig. 2.
Human papillomavirus (HPV) series vaccination results
Satisfaction Rates
Face to face evaluations, reaching 90 % of participants, started 12 weeks after registration. Acceptability was excellent, with 99.7 % of women stating they would participate in future screening and all but one able to do the self-sampling without assistance. While most women (97 %) reported no difficulty obtaining the sample, 8 reported mild pain, and 1 reported a discharge. Twenty percent reported that they would prefer obtaining a sample at a health post and 100 % were satisfied with the way they were informed of their results.
All women except 1 were able to state why they underwent cryotherapy, or colposcopy and biopsy. The most commonly reported side effects from cryotherapy were discharge, minor bleeding, and pain. No one stated that they would be afraid of the treatment or biopsies if they had to undergo the procedure in the future.
Discussion
We chose Iquitos and the villages along the Amazon to gain experience with the logistical and geographic barriers the jungle environment presented. The region more than delivered its share of challenges. However, by involving the communities in the design and implementation of the program, we achieved excellent recruitment, follow-up, and satisfaction for HPV screening/treatment and vaccination.
There were several differences between the prior study in Manchay and this study in Iquitos. This study shows the progression of the model even in a more challenging environment, as we benefitted from the lessons learned from Manchay. In Iquitos, as opposed to our prior study, the recruitment was done by individuals with healthcare training; however the burden on the healthcare system was still minimal. The model does not need the HLs to have healthcare training, but it should be able to use existing community based systems if they are already efficiently reaching the people.
Another important change was loosening the criteria for “daughters” of the participating mothers. This occurred once HLs observed that mother–daughter pairs consisted of women with their own children as well as nieces or neighbors.
In Iquitos, the training was two half days while in the earlier Manchay study, the training occurred over two and a half days. The abbreviated training makes the intervention more scalable and did not adversely affect results. Some of the reduction was due to their healthcare background, but most was purposeful, based on the experiences in Manchay. Overall, the training and design meeting reflected a successful step in the evolution of our model.
Challenges in recruitment depended on both cultural issues and geography. The urban HLs experienced logistical and psychosocial challenges with recruitment for both women and girls, in contrast to rural HLs, better known in their communities. While there was some awareness of the HPV vaccine due in large part to the government vaccination program, attendance in urban areas at parental meetings regarding the vaccine was poor, likely due to parents working during the day. We concluded that this scheduling plan by the urban HL was unsuccessful. Logistical challenges with flooding and impassable bridges also affected participation in urban areas.
In contrast, HLs from the villages reported that many women were easily recruited following meetings convened by the HLs in their communities. Others were informed by word of mouth and presented to the health centers for registration. Of note, only the urban women were recruited house to house. This made rural recruitment much more efficient, a major difference from our experience in Manchay.
Using the instruction sheet, the HLs spent less than 10 min was per subject on recruitment, mainly on completion of study forms. This suggests a non-study screening would be even more efficient since research informed consent forms would not be required.
Regarding vaccination, the CBPR design was successful in recruitment although also less efficient in urban areas. Peru has a history of vaccination in schools, so it was surprising that the school based uptake in the urban centers was not more successful. According to the technician who used this approach for enrollment, the interest varied according to the teachers’ interest and ability to motivate parents.
Our study used a modified CBPR approach where the researchers determined the intervention a priori and the community designed the implementation. Both design features and the very high satisfaction rates achieved proved the benefit of using CBPR techniques. Another strength of the model was the potential to select appropriate timing for both the vaccine initiative and follow up strategy. The HLs envisioned that first 2 doses could be administered prior to school dismissal, followed by a 4 month interval during which the girls would be on vacation. Although this did not result as originally planned due to delay in vaccine arrival, these timing logistics should be most effectively determined by the communities.
Despite our successes, there were several challenges and limitations affecting follow-up for both the screen/treat intervention and the vaccine intervention. The evaluation and/or treatment of 81 % of women who screened positive did not reach our goal of 95 %. In explanation, 2 of the key HLs who had worked in their rural communities for years had moved to new jobs. Therefore, the task of ensuring attendance to an unknown procedure in a distant setting was in new hands, likely impacting adherence to protocol. This reinforced our belief in the significance of the CBPR approach, suggesting the importance of being anchored at the village level.
Regarding vaccination, a school based system is most effective around the world, including Peru. Our study tried to capitalize on the school based system by using the same sites, but had the disadvantage of not being integrated into and organized by the school system. Additionally, complications with both flooding and vaccine delivery greatly impacted vaccination schedules, and HLs were forced to resort to door to door strategies for many girls. Access was virtually impossible in some areas due to the severe flooding and some girls had fled their homes. Despite these challenges, the technicians still vaccinated 90 % (288/318) of the girls. Although the concept of a “mother–daughter program” is appealing, the logistics clash, undermining the overall efficiency of the program. Therefore we believe a school based vaccination program that is not linked to screening is most efficient.
Cultural issues presented additional challenges, with 4 young girls becoming pregnant, a contraindication to vaccination. The cases were reported to child welfare advocates but health workers were reluctant, feeling it undermined the clinician patient relationship in an area where nonconsensual intercourse and early sexual debut is not uncommon [17]. This also suggests that if girls are sexually active as early as 11, the opportunity to avoid infection by HPV vaccination may have been lost. Therefore, in this area vaccination should begin as early as possible which is 9 years according to the Gardasil package insert. However, the national guidelines of Peru’s ministry of health only permitted vaccination from 10 to 13.
This project represents a continuing effort of our research group to develop a screening model that separates the identification of who is positive from their management. It separates the work of the community from the healthcare system. To that end, we believe that using this model for cervical cancer screening based on self-collection, large populations can be screened which will support highly sensitive, high throughput/low cost per case, quality controlled, centralized processing. The feasibility of this concept was demonstrated quite well with the efficient transport and reporting of results from a distant lab in China. Clearly this concept is transferable to one or a few large referral labs in any country or possibly a collaborative agreement between countries.
We have learned that efficient modeling of a cervical cancer prevention program will ultimately be determined by the management of the positives. Facilities for diagnosis and treatment of high grade lesions in Iquitos are very limited. With the high burden of HPV infection (11.5 %) and a marginal infrastructure for detection of precursor lesions, the opportunity is ripe for expansion of a cervical cancer prevention program using a community based model in the Loreto region of Peru. The low and high jungle of Loreto and its capital of Iquitos represent 30 % of the land mass of Peru and 126,000 women between the ages of 30–50 in 2,500 villages. We have developed a plan for cervical cancer prevention based on our evolving community based preventive healthcare model, in the hopes of reaching the women in this extraordinarily challenging environment.
Footnotes
Jerome L. Belinson has received support in kind (reagents and testing) and funds for direct support and research, under the auspices of Preventive Oncology International Inc., from Hologic Inc., Qiagen, Gen-Probe, Merck Inc., BGI Shenzhen, and GE Healthcare. The other authors have no conflicts of interest to report. This work was supported by the National Institutes of Health Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood, and Lung Institute, National Institute of Dental and Craniofacial Research, National Institute On Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases, and National Institutes of Health Office of Women’s Health and Research through the Fogarty International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988) and the American Relief and Recovery Act, Merck Inc. “Investigator Initiated Studies Program”, and Preventive Oncology International.
Contributor Information
Carolina E. Abuelo, Email: cabuelo@partners.org, Charlestown HealthCare Center, Massachusetts General Hospital, 73 High St, Charlestown, MA 02445, USA
Kimberly L. Levinson, Women’s Health Institute, Cleveland Clinic, Cleveland, OH, USA
Jorge Salmeron, Instituto Mexicano del Seguro Social, Cuernavaca Morelos, Mexico.
Carlos Vallejos Sologuren, Oncosalud, Lima, Peru.
Maria Jose Vallejos Fernandez, Oncosalud, Lima, Peru.
Jerome L. Belinson, Women’s Health Institute, Cleveland Clinic, Cleveland, OH, USA. Preventive Oncology International, Cleveland Heights, OH, USA
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN v1.2, Cancer incidence and mortality worldwide: IARC cancerbase No. 10 [Internet] Lyon, France: International agency for research on cancer; 2010. [Accessed on 30 Oct 2011]. Available from: http://globocan.iarc.fr. [Google Scholar]
- 2.WHO/ICO information centre on HPV and cervical cancer (HPV Information Centre) Human papillomavirus and related cancers in Peru. Summary report. 2010 Available at www.who.int/hpvcentre.
- 3.Paz Soldan V, Lee F, Carcamo C, Garnett GP, Garcia P. Who is getting Pap smears in urban Peru? International Journal of Epidemiology. 2008;37:862–869. doi: 10.1093/ije/dyn118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almonte M, Ferreccio C, Winkler JL, Cuzick J, Tsu V, Robles S, et al. Cervical screening by visual inspection, HPV testing, liquid-based and conventional cytology in Amazonian Peru. International Journal of Cancer. 2007;121(4):796–802. doi: 10.1002/ijc.22757. [DOI] [PubMed] [Google Scholar]
- 5.Garcia F, Barker B, Santos C, Brown EM, Nuño T, Giuliano A, et al. Cross-sectional study of patient- and physician-collected cervical cytology and human papillomavirus. Obstetrics and Gynecology. 2003;102(2):266–272. doi: 10.1016/s0029-7844(03)00517-9. [DOI] [PubMed] [Google Scholar]
- 6.Belinson Jh, Duh H, Yang B, Wu R, Belinson SE, Qu X, et al. Improved sensitivity of vaginal self-collection and high-risk human papillomavirus testing. International Journal of Cancer. 2011;130(8):1855–1860. doi: 10.1002/ijc.26202. [DOI] [PubMed] [Google Scholar]
- 7.Anhang R, Nelson JA, Telerant R, Chiasson MA, Wright TC. Acceptability of self-collection of specimens for HPV DNA testing in an urban population. Journal of Women’s Health. 2005;14:721–728. doi: 10.1089/jwh.2005.14.721. [DOI] [PubMed] [Google Scholar]
- 8.Dzuba IG, Díaz EY, Allen B, Leonard YF, Lazcano Ponce EC, Shah KV, et al. The acceptability of self-collected samples for HPV testing versus the Pap test as alternatives in cervical cancer screening. Journal of Women’s Health and Gender-Based Medicine. 2002;11(3):265–275. doi: 10.1089/152460902753668466. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell S, Ogilvie G, Steinberg M, Sekikubo M, Biryabarema C, Money D. Assessing women’s willingness to collect their own cervical samples for HPV testing as part of the ASPIRE cervical cancer screening project in Uganda. International Journal of Gynaecology and Obstetrics. 2011;2:111–115. doi: 10.1016/j.ijgo.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Taylor S, Wang C, Wright TC, Denny L, Kuhn L. A comparison of human papillomavirus testing of clinician-collected and self-collected samples during follow-up after screen-and-treat. International Journal of Cancer. 2011;129(4):879–886. doi: 10.1002/ijc.25731. [DOI] [PubMed] [Google Scholar]
- 11.Tisci SM, Shen YHM, Fife DR, Huang J, Goycoolea J, Ma CP, et al. Patient acceptance of self-sampling for human papillomavirus in rural China. Journal of Lower Genital Tract Disease. 2003;7:107–116. doi: 10.1097/00128360-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahé C, et al. Cost-effectiveness of cervical-cancer screening in five developing countries. New England Journal of Medicine. 2005;353(20):2158–2168. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 13.McClung EC, Blumenthal PD. Efficacy, safety, acceptability and affordability of cryotherapy: A review of current literature. Minerva Ginecologica. 2012;64(2):149–172. [PubMed] [Google Scholar]
- 14.Levinson KL, Abuelo C, Chyung E, Salmeron J, Belinson SE, Sologuren CV, et al. The Peru cervical cancer prevention study (PERCAPS): Community-based participatory research in manchay. International Journal of Gynecological Cancer. 2013;1:141–147. doi: 10.1097/IGC.0b013e318275b007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du H, Yi J, Wu R, Belinson S, Qu X, Yang B, et al. A new PCR based mass spectrometry system for high-risk HPV Part II—clinical trial. American Journal of Clinical Pathology. 2011;136(6):920–923. doi: 10.1309/AJCPJDAORUY4EYR6. [DOI] [PubMed] [Google Scholar]
- 16.Levinson Kimberly L, Abuelo Carolina, Chyung Eunice, Salmeron Jorge, Belinson Suzanne E, Vallejos Sologuren Carlos, Santos Ortiz Carlos, Jose Vallejos Maria, Belinson Jerome L. The Peru cervical cancer prevention study (PERCAPS): The technology to make screening accessible. [Accepted Jan 2013];Gynecologic Oncology. 2013 doi: 10.1016/j.ygyno.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown A, Jejeebhoy J, Shah I, Yount K. Department of reproductive health and research family and community health. World Health Organization; Geneva: Sexual relations among young people in developing countries: evidence from WHO case studies. http://whqlibdoc.who.int/hq/2001/WHO_RHR_01.8.pdf. [Google Scholar]