Abstract
We report the design, testing and in vivo application of pH sensitive contrast agents designed specifically for Cerenkov imaging. Radioisotopes used for positron emission tomography (PET) emit photons via Cerenkov radiation. The multispectral emission of Cerenkov radiation allows for selective bandwidth quenching, where a band of photons are quenched by absorption by a functional dye. Under acidic conditions, 18F-labeled derivatives emit the full spectrum of Cerenkov light. Under basic conditions, the dyes change color and a wavelength-dependent quenching of Cerenkov emission is observed.
METHODS
Mono and di-18F-labeled derivatives of phenolsulfonphthalein (phenol red) and meta-cresolsulfonphthalein (cresol purple) were synthesized by electrophilic fluorination. Cerenkov emission was measured at different wavelengths as a function of pH in vitro. Intramolecular response was measured in fluorinated probes; intermolecular quenching by mixing phenolphthalein with 18F FDG. Monofluorocresol purple (MFCP) was tested in mice treated with acetazolamide to cause urinary alkalinization and Cerenkov images compared to PET images.
RESULTS
Fluorinated pH indicators were produced with radiochemical yields of 4-11% at >90% purity. Selective Cerenkov quenching was observed intramolecularly with difluorophenol red or MFCP, and intermolecularly in phenolphthalein 18F-FDG mixtures. The probes were selectively quenched in the bandwidth closest to the indicator’s absorption maximum (λmax) at pHs above the indicator pKa. Addition of acid or base to the probes resulted in reversible switching from unquenched to quenched emission. In vivo, the bladders of acetazolamide-treated mice exhibited a wavelength-dependent quenching in Cerenkov emission, with the greatest reduction occurring near the λmax. Ratiometric imaging at two wavelengths showed significant decreases in Cerenkov emission at basic pH and allowed the estimation of absolute pH in vivo.
CONCLUSIONS
We have created contrast agents that selectively quench photons emitted during Cerenkov radiation within a given bandwidth. In the presence of a functional dye, such as a pH indicator, this selective quenching allows for a functional determination of pH in vitro and in vivo. This method can be used to obtain functional information from radiolabeled proves using multimodal imaging. It allows for the imaging of non-fluorescent chromophores and is generalizable to any functional dye that absorbs at suitable wavelengths.
Keywords: Cerenkov imaging, ratiometric imaging, pH measurement, electrophilic fluorination, metacresol purple, phenolphthalein
INTRODUCTION
Cerenkov imaging (CI) is a recently described imaging modality that detects photons emitted by radioactive particles during beta decay. Cerenkov radiation (CR) arises when charged particles exceed the speed of light when traveling through a dielectric medium and is the source of the blue light emitted by nuclear reactors. CR was described by Pavel Cerenkov, for which he won the 1958 Nobel Prize with Frank and Tamm (1, 2). However, the potential for CI was not recognized until 2009 when the first small animal study was published (3).
CI can be accomplished using standard optical imaging equipment, detecting the photons using a cooled, charge-couple device (CCD) camera. This allows for high throughput (3-5 animals per scan in optical scanners) with scan times that are often less than 5 minutes (4, 5). In addition, any β-emitter, positron or electron, can be imaged, allowing for CI of nuclei such as 32P (6).
Most Cerenkov photons are in the visible range, and as a result are subject to tissue absorption and scattering, limiting the depth of detection. While more suited for small animal applications, CI also has the potential for human imaging. Cerenkov studies of 131I in human thyroid (7) and 18F-fluorodeoxyglucose (18F-FDG) in positive lymph nodes from cancer patients (8) have recently been published. Future human use will most likely be restricted to surface tumors or tumors accessible endoscopically (9, 10). Many breast tumors present close to the surface and the breast is both compressible and has good optical properties, making this a potential target (11-14). Intraoperative use is also possible, and other groups are pursuing this approach (15-17).
Importantly, β+-decay leads to the generation of 2 types of photons, the high-energy 511 keV gamma rays released after positron annihilation and detectable by PET, and the visible photons released as CR. These properties allow for the development of multimodal PET-CI tracers (18-21). Current radiotracer methods detect primarily location of the radioisotope, it is difficult to assess probe metabolism directly. However, CI is an optical technique and the photons can be selectively quenched or transferred to higher wavelengths (22-24). This allows for the creation of contrast agents where function can be measured optically while binding, kinetics and location can be followed with PET.
This paper describes Cerenkov contrast agents (25-28) based on the concept of selective bandwidth quenching (28). Cerenkov light is emitted continuously across the visible spectrum with intensity proportional to the inverse square of the wavelength, 1/λ2. A change in absorption by a functional chromophore, such as a fluorinated pH indicator becoming basic, reduces Cerenkov emission across a selective bandwidth, resulting in an attenuated signal.
We previously reported the synthesis of fluoroderivatives of the pH indicators phenolsulfonphthalein (Phenol Red, PR) (26), meta-cresolsulfonphthalein (Cresol Purple, CP) (27) and phenolphthalein (PP) (25). Here we show that the 18F-isotopomers can serve as noninvasive pH probes for CI. We report 18F-radiolabeling of CP and PR derivatives (Figure 1), in vitro testing and in vivo measurement of pH in a mouse model of urinary alkalinization. The principle of selective bandwidth quenching is demonstrated intermolecularly using PP mixed with 18F-FDG and intramolecularly using fluorinated PR and CP. The quenching is shown to be reversible and pH dependent. Finally 18F-labeled CP is used to estimate pH in the bladders of mice treated with acetazolamide, a carbonic anhydrase inhibitor. Ratiometric imaging is employed at different wavelengths to determine absolute pH values in vivo. These results provide methods for multispectral optical imaging of nonfluorescent molecules with the potential for dual measurement of function and location using radiolabeled probes.
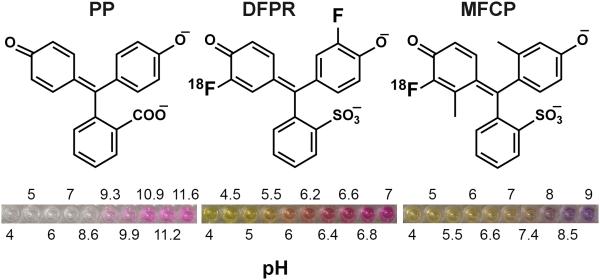
Figure 1. Chemical structures of the pH indicators.
Left: phenolphthalein (PP); Middle: difluorophenol red (DFPR); Right: monofluorocresol purple (MFCP). The pH dependent changes in the indicator color is shown underneath.
MATERIALS AND METHODS
All reagents were purchased from Sigma-Aldrich unless otherwise stated. 18F-FDG and 18F-NaF were obtained from the Cyclotron Facility at the University of Pennsylvania.
18F labeling
Labeling was performed using a custom-made electrophilic fluorination unit as previously described (25-27). 18F-F2 gas was prepared by the 20Ne(d,α)18F reaction using an IBA 18/9 Cyclone accelerator. 18F-F2 gas (0.1% in Ne, Matheson Tri-Gas Inc) with a total activity of 100-200 mCi in 35 µmoles of 19F2 carrier, was bubbled for 5 minutes through a freshly prepared solution of the equivalent amount of the monosodium salt of the indicator in glacial acetic acid (2 mg/mL). The mixture was evaporated under vacuum at 120 °C, redissolved in 2 mL of water, injected onto a semi-preparative HPLC column (Phenomenex, Synergi 4 µm Hydro-RP 80 Å 10x250 mm) and eluted with 25% ethanol-water buffer at 2 mL/min with detection of radioactivity and absorption at 430 nm. Fractions corresponding to fluorinated products were collected, evaporated to dryness, and redissolved in saline for further application. An average (n=5) radiochemical yield was 4±1% for monofluorinated (MFPR), 7±1% difluorinated (DFPR), and 1±0.5% trifluorinated (TFPR) derivatives. For CP (n=9) the radiochemical yields were 9.6±1% for monofluorinated (MFCP), 11±0.7% for difluorinated (DFCP), and 4.2±0.7% for trifluorinated (TFCP). In both cases we were able to detect small amounts of tetrafluorinated derivatives. The specific activity at the time of experiment was 1-2 Ci/mmol for monofluorinated and 2-4 Ci/mmol difluorinated derivatives respectively. The purity of the probes was >90% by analytical HPLC. Analytical HPLC for DFPR and NMR data for MFCP have been published [26,27].
In vitro Cerenkov imaging
Optical imaging was performed on a PerkinElmer IVIS Lumina II. For open filter measurements, an exposure time of 30 seconds was used with large binning and f/stop=1. For selective emission filter measurements, the exposure time was 60 seconds. The Lumina has 4 emission filters with wavelengths 515-575, 575-650, 695-770 and 810-875 nm. The heater plate was set to room temperature to minimize evaporation. Black walled 96-well plates (BD Biosciences) were filled with 200 μL of probe at the indicated activities and concentrations. For pH imaging, the pH of the solution was adjusted using 0.1 M acetate (pH 4-5.5), MES (pH 5.5-6.5), phosphate (pH 6.5-8), Trizma (pH 8-9), borate (pH 9-10) or carbonate (pH 10-11) buffers. For switching experiments, the pH was adjusted using 1M HCl or NaOH. For phantom experiments, a 1-cm3 acrylic phantom (Data Spectrum Corporation) was filled with 18F-FDG and imaged under the same conditions. Image quantification was performed using Living Image software (PerkinElmer) by drawing regions of interest (ROIs) around each well and determining the radiance. A radioactive decay constant was applied to all data except for pH switching experiments to account for the time between plating and imaging.
In vitro APET imaging
Following optical imaging, plates were centered in the FOV of the MOSAIC HP small animal APET scanner (29) (Philips Medical Systems) and imaged statically for 15 minutes. Images were reconstructed with a 3D Row Action Maximum Likelihood Algorithm (3D-RAMLA) (30). Corrections for decay, scatter, and attenuation were applied using a 137Cs source. Absolute quantification of activity from the dose calibrator was achieved with cross-calibration of phantom measurements to provide nCi/mL.
In vivo Cerenkov imaging
All experiments were approved by our Institutional Animal Care and Use Committee. For 18F-FDG, 4-6 week old nude mice (NCI) received ~435 µCi of 18F-FDG i.v. via tail vein. For pH experiments, mice received 160 mg/kg acetazolamide (X-Gen Pharmaceuticals) in saline or saline i.p. for 5 days. On day 5, urine was collected and pH determined using an Accumet pH meter. Four hours later, mice were injected i.v. with ~400 µCi 18F-MFCP. Thirty minutes later, mice were imaged using the IVIS Lumina II under 1.5% isoflurane/oxygen anesthesia using the following parameters: Open filter – Exposure time: 1 minute, Binning: Large, f/stop: 1; Selective Emission filters – Exposure time: 5 minutes. Image quantification was performed by drawing ROIs and determining the radiance. Optical data were corrected for radioactive decay to account for loss in activity and then normalized to the APET images by dividing the radiance by the PET counts in the same ROI to account for injection efficiency.
In vivo APET imaging
Subsequent to 18F-MFCP injection, each mouse was imaged statically in the MOSAIC HP small animal PET scanner for 15 minutes. The images were reconstructed as described above using a voxel size of 0.5 × 0.5 × 0.5 mm3. Decay, scatter, and attenuation correction was applied. All activity is assumed to be inside the mouse for purposes of calculation of activity concentration since we monitored the excreted activity from the mouse.
RESULTS
Initial experiments were performed to characterize Cerenkov emission in vitro and in vivo using different emission filters (Supplemental Information). A strong linear correlation was observed between optical and APET signals (Figure S1). Selective emission filters showed that the bulk of the photons were emitted at lower wavelengths (515-575 and 575-650 nm, Figure S2 and S3B) and the emission spectrum followed the expected 1/λ2 dependence (Figure S3B). Importantly, the emission spectrum exhibited different characteristics when CR was examined in vivo. Mice imaged following administration of 18F-FDG showed a marked decrease in Cerenkov intensity using the lowest wavelength (515-575 nm) filter, due to attenuation and scattering by tissue at these wavelengths (Figure S4).
To design Cerenkov specific contrast agents, intermolecular quenching of CR was first measured using PP, which is colorless when acidic (λacidic=276 nm) and fuchsia when basic (pKa=9.8, λbasic=553 nm, Figure 1). Mixing 100 µCi of 18F-FDG with 25 mM PP led to a pH-dependent quenching of CR when the open, 515-575 and 575-650 nm filters were used (Figure 2A). Quenching was less pronounced in the remaining filters and the signal was much lower in the 810-875 nm range reflecting the reduced photon emission at these wavelengths.
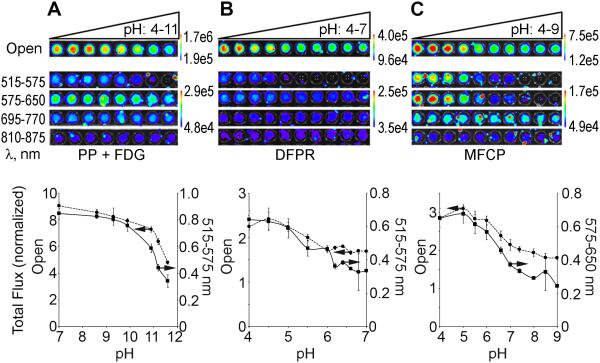
Figure 2. Inter- and intramolecular quenching of Cerenkov radiation.
(A) 25 mM PP was adjusted to pH 4-11, mixed with 100 µCi 18F-FDG and imaged using all filters on the IVIS Lumina II. The top panel shows images of the plate and the bottom panel quantifications of total flux normalized to APET counts for the open filter (dashed line) and optimal quenching 515-575 nm filter (solid line). (B) 100 µCi (25 nmol) 18F-DFPR adjusted to pH 4-7 with the open and 515-575 nm filters quantification shown (C) 140 µCi (70 nmol) 18F-MFCP adjusted to pH 4-9 with the open and 575-650 nm filters. For each pH indicator, the filtered images were set to the same scale. For all experiments, n=3.
To demonstrate intramolecular quenching, 18F-labeled derivatives of a series of pH indicators were synthesized (25-27). The properties of 10 of these indicators are presented in Table 1. For further investigation we first selected difluorinated phenol red (DFPR, Figure 1), which exhibits a yellow (acidic) to red (basic) transition. 18F-DFPR (pKa=6.4, λacidic=428 nm, λbasic=570 nm) was examined in the pH range 4-7. Figure 2B shows selective CR quenching occurred at basic pH when the open, 515-575 and 575-650 nm filters were employed, with the greatest quenching observed near λbasic.
Table 1.
Properties of Parent and F-labeled pH indicators
| Parent Compound |
Mono- fluorinated |
Di- fluorinated |
||||
|---|---|---|---|---|---|---|
| Indicator name | pKa | λ max | pKa | λ max | pKa | λ max |
| Phenol Red | 7.9 | 558 | 7.3 | 564 | 6.4 | 570 |
| Cresol Red | 8.2 | 572 | 7.6 | 576 | 6.8 | 582 |
| Cresol Purple | 8.3 | 578 | 7.5 | 582 | 7.0 | 590 |
| Phenolphthalein | 9.7 | 553 | 9.3 | 558 | 8.7 | 564 |
| Naptholphthalein | 7.8 | 653 | 7.7 | 659 | 7.2 | 665 |
λmax is the absorbance maximum of the basic form in nm.
18F-DFPR exhibited less of a pH dependent effect than PP and the quenching plateaued at pH > 6, leading to little overall change in the Cerenkov emission. Thus, we examined 18F-labeled monofluorinated cresol purple (18F-MFCP, pKa=7.5, λacidic=440 nm, λbasic=582 nm, Figure 1), which is yellow when acidic and purple under basic conditions. As shown in Figure 2C, 18F-MFCP quenches Cerenkov emission in the open, 515-575 and 575-650 nm filters as pH increases. Quantification of the images is shown in Figure 2D-F. Only the open and 515-575 nm (PP+18F, 18F-DFPR) or 575-650 nm (18F-MFCP) filters are shown as they exhibited the greatest change in CR over the pH range. 18F-MFCP shows the greatest pH dependent changes in Cerenkov emission (2.7 as compared to 1.9 fold for 18F-DFPR and 2.5 fold for intermolecular PP) with a pKa relevant for in vivo studies. Quantification of the remaining filter sets is shown in Figure S5.
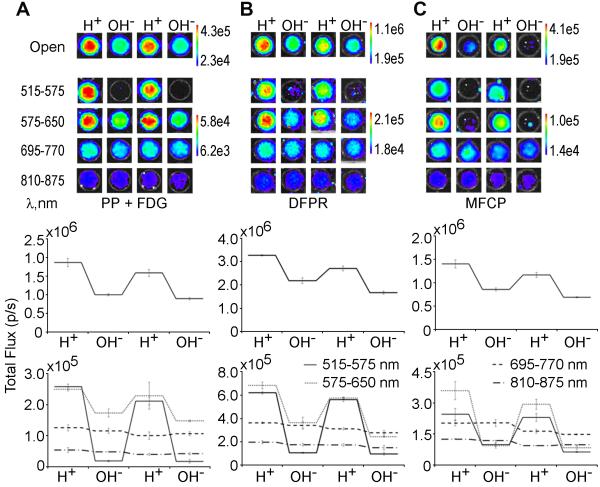
Intermolecular quenching of CR was shown to be switchable by adding acid or base to wells containing 25 mM PP and 100 µCi 18F-FDG (Figure 3A). Intramolecular switching was also observable with either of the fluorinated indicators, 18F-MFCP and 18F-DFPR (Figure 3B-C). Quantification of these images (Figure 3D-F) demonstrates that for PP, the greatest change was seen using the open filter or filters at wavelengths closest to the λmax (515-575 and 575-650 nm; Figure 3D). Both 18F-DFPR and 18F-MFCP exhibited similar characteristics (Figure 3E-F), with maximum switching observed near the λmax of the chromophores.
Figure 3. Optical switching of PP+18F-FDG, 18F-DFPR and 18F-MFCP.
10 µL of 1 M HCl (H+) or NaOH (OH-) was added to wells containing (A) 25 mM PP+200 μCi 18F-FDG. (B) 200 μCi (50 nmol) 18F-DFPR (λmax=570 nm), and (C) 100 µCi (50 nmol) 18F-MFCP (λmax=582 nm) and images acquired after acid or base addition. Images for open and selective filters are shown on top, quantification of each well is shown below.
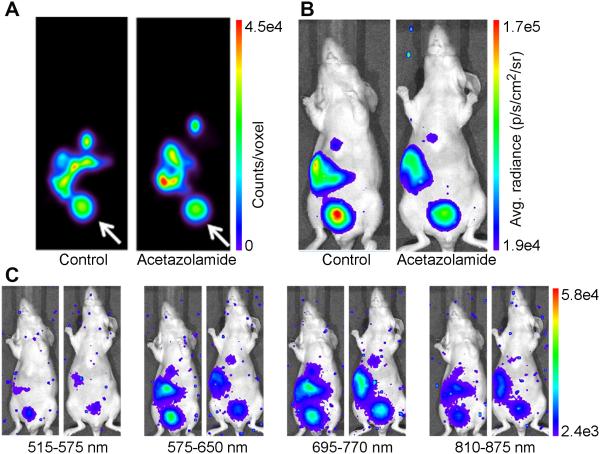
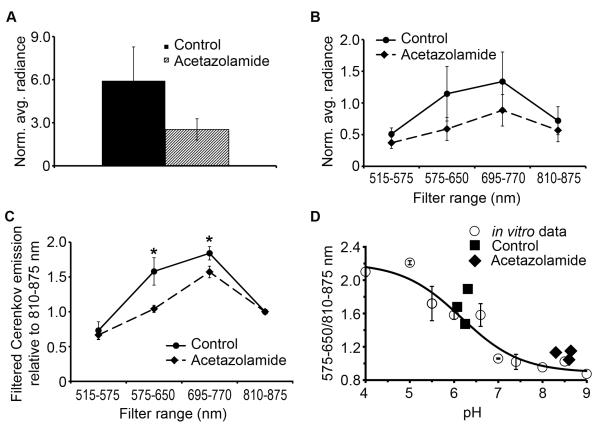
To test 18F-MFCP in vivo, nude mice were treated with acetazolamide (160 mg/kg, i.p.) or PBS for 5 days. On day 6, mouse urine was collected and pH measured. The urine of PBS-treated mice had a pH of 6.2±0.1 (n=3), while acetazolamide-treated mice had a urine pH of 8.5±0.2 (n=3). Mice were administered 18F-MFCP and imaged optically 30 minutes later followed by APET imaging. The APET images show probe accumulation in gall bladder, liver, intestine and bladder (Figure 4A). When normalized to dose injected per animal, a difference of less than 5% was observed in the average SUVs from bladders of PBS- and acetazolamide-treated mice (1.443±0.405 vs 1.444±0.471 respectively). Optical images taken using the open filter show a brighter signal in the PBS-treated mouse bladder compared to the acetazolamide-treated animal (Figure 4B). When the remaining filters were employed, the greatest difference between PBS- and acetazolamide-treated animals occurred using the 575-650 nm filter (Figure 4C) consistent with the λbasic=582 nm for 18F-MFCP. The images were quantified by correcting the average radiance for decay to the injection time of the tracer and normalizing to mean PET counts. Open filter images showed a decrease in acetazolamide-treated mice just outside the level of significance (Figure 5A, p=0.07). Quantification of the selective filter sets (Figure 5B) showed non-significant decreases with the greatest difference observed again using the 575-650 nm filter. Emissions at wavelengths outside the absorbance range of MFCP were not affected by acetazolamide treatment.
Figure 4. In vivo imaging of Cerenkov quenching.
Mice were treated with either saline or 160 mg/kg acetazolamide for 5 days prior to imaging with 18F-MFCP. (A) APET images of a single coronal slice 0.5 mm thick through the center of the mouse in control and acetazolamide-treated mice show 18F-MFCP accumulation in bladder (white arrow), gall bladder, liver, intestine. Optical images of the same mice were acquired using (B) open filter and (C) the four emission filters.
Figure 5. Quantification of in vivo Cerenkov images.
Average radiance from the bladder using (A) open filter, (B) the 4 selective filters, (C) the ratio of emission at each filter relative to 810-875 nm. * significant, p<0.05, Students t-test, (D) Ratiometric pH determination. Open circles depict the ratio of Cerenkov emission at (575-650 nm)/(810-875 nm) for in vitro 18F-MFCP (data from Figure 2C). The solid line is a fit to a sigmoidal curve and gives a pKa of 6.25, similar to 6.4 determined by the open filter data. Black diamonds and squares represent Cerenkov emission from control and acetazolamide-treated mice plotted against actual urine pH.
Ratiometric calculations were performed by taking emission for each filter relative to 810-875 nm, which was invariant to pH changes and where the greatest light penetration is expected. This resulted in a significant difference between acetazolamide and PBS-treated mice for the 575-650 (p=0.01, Students t-test) and 695-770 (p=0.02), but not for 515-575 nm filters (p=0.47, Figure 5C). A ratiometric pH response curve was created from in vitro 18F-MFCP data using Cerenkov emission at 575-650 nm, where the greatest pH response was observed, relative to 810-875 nm (Figure 5D). A fit to a sigmoidal curve gave a pKa=6.25, R2=0.956. The in vivo ratios from control and acetazolamide treated mice were plotted against ex vivo urine pH. The in vivo ratios predicted absolute pH with reasonable accuracy, although the values tended to lie above the curve (Figure 5D).
DISCUSSION
In this paper we describe the synthesis, in vitro characterization and in vivo testing of 18F-labeled pH indicators designed to exploit the characteristics of CR. The multispectral nature of Cerenkov emission allows for selective quenching of a band of photons by a functional chromophore that is used to report on pH.
The biological manifestations of pH modulation are numerous and significant. From reduced pH in lysosomes (31) to reduced pH in the extracellular space of tumors (32, 33), multimodal in vivo pH sensors could have considerable impact on the field of molecular imaging. Our approach differs from other methods of measuring pH (34) in that we are directly monitoring the absorption of colorimetric pH indicators, which is a standard method of pH determination. While optical imaging agents that emit in the near infrared range are preferred for in vivo imaging (35), the bulk of the Cerenkov signal is located near the blue end of the visible spectrum (3, 36). To create a balance between available signal and adequate penetration of light through tissue, pH-responsive CI probes were developed to exhibit selective bandwidth quenching in the 575-650 nm range. Our initial mouse studies showed that although we could detect 18F-FDG in nude mice using all of the emission filters, the Cerenkov emission using the 515-575 nm filter was markedly lower than the 575-650 nm filter, due to photon absorption and scattering at shorter wavelengths.
The common pH indicator, PP (λmax=553 nm) was used to demonstrate intermolecular CR quenching. Wavelength-dependent quenching of CR was observed to be the most effective at 515-575 nm in the pH range 4-11 with relatively little quenching seen outside the absorption range of PP. However, PP could not quench CR in our target bandwidth of 575-650 nm and the pH necessary for quenching (>9) was not biologically relevant.
Intermolecular quenching of CR has also been shown in vivo using nanoparticles (37). However, intramolecular quenching would ultimately be more useful as it would require a single contrast injection. As a result 18F-DFPR, a derivative of the pH indicator commonly used in cell culture, was synthesized. Quenching could be now observed in both the 515-575 nm and the target bandwidth of 575-650 nm. Although 18F-DFPR could effectively quench CR, a relatively small difference in emission was observed between acidic and basic forms. Thus we synthesized 18F-MFCP and found that it outperformed 18F-DFPR and PP in the magnitude of quenching in the target bandwidth.
In addition selectively quenching a targeted bandwidth, it was critical that the sensors be switchable in order to accurately reflect the surrounding environment. The sequential addition of base and acid to 18F-MFCP led to stepwise quenching and restoration of CI, respectively. While 18F-MFCP exhibited the highest potential as an in vivo probe, we also investigated PP+18F-FDG and 18F-DFPR and found that they were both capable of switching. These results indicate that it is possible to rapidly monitor pH changes dynamically in the extracellular tumor space of the tumor microenvironment.
In vivo experiments were carried out using 18F-MFCP in a mouse model of urinary alkalization. Acetazolamide is a carbonic anhydrase inhibitor that is used to treat metabolic and respiratory alkalosis. In doing so, it causes blood acidification and urinary alkalization. The latter property, along with the collection of small molecule radiopharmaceuticals in urine due to renal clearance, provided a model to test how well 18F-MFCP reports on in vivo pH. Using a pH meter, mouse urine pH was measured to be 6.2±0.1 in controls and 8.5±0.2 in acetazolamide treated animals. Optical images of the bladder obtained following 18F-MFCP administration showed a clear difference in CR output between the two experimental conditions whereas no difference in PET signal was seen. Control animals exhibited a brighter signal than acetazolamide treated animals, with the greatest difference seen in the open and 575-650 nm filter images. Following imaging, expelled urine from acetazolamide treated animals was dark purple (not shown), indicating further that the urine was basic as measured by 18F-MFCP.
When ROIs around the bladder were quantified, the differences were not significant unless ratios were taken relative to the invariant signal at 810-875 nm. Using the ratiometric method, it is possible for 18F-MFCP to report on absolute pH. We constructed a normalized pH response curve using in vitro 18F-MFCP data by taking the ratio of emission at 575-650 to 810-875 nm. When the same ratios from the control and acetazolamide treated mice were plotted on the pH response curve, the CR values correlated well, although they tended to lie above the curve and underestimate the actual pH. This effect may arise from scattering or absorption of light at different wavelengths, which would not contribute to the in vitro data. It is interesting to note that the pKa for 18F-MFCP as determined by Cerenkov emission in vitro (6.2) is lower than the pKa measured using absorption (7.2). The reason for this is uncertain, although similar effects were observed for 18F-DFCR and 18F-PP mixed with FDG.
CONCLUSIONS
We have demonstrated the ability to image pH using selective bandwidth quenching of Cerenkov specific contrast agents. In vivo results show that 18F-MFCP is capable of reporting pH in animal models. This opens a number of possibilities because it allows for direct imaging of visible chromophores. The concept of selective bandwidth quenching is generalizable to other dyes that alter their absorption in response to chemical or physiological stimuli and that absorb at wavelengths appropriate for CI. These concepts define a method for the functional imaging of non-fluorescent chromophores that could be used in conjunction with PET.
Supplementary Material
ACKNOWLEDGEMENTS
Supported by a Transdisciplinary Awards Program in Translational Medicine and Therapeutics-Translational Biomedical Imaging Core (TAPITMAT-TBIC) grant through UL1RR024134 and R01EB018645-01 (EJD).
REFERENCES
- 1.Tamm IE. General characteristics of Vavilov-Cherenkov radiation. Science. 1960;131:206–210. doi: 10.1126/science.131.3395.206. [DOI] [PubMed] [Google Scholar]
- 2.Cherenkov PA. Radiation from high-speed particles. Science. 1960;131:136–142. doi: 10.1126/science.131.3394.136. [DOI] [PubMed] [Google Scholar]
- 3.Robertson R, Germanos MS, Li C, Mitchell GS, Cherry SR, Silva MD. Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys Med Biol. 2009;54:N355–365. doi: 10.1088/0031-9155/54/16/N01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, Ren G, Miao Z, et al. Molecular optical imaging with radioactive probes. PLoS One. 2010;5:e9470. doi: 10.1371/journal.pone.0009470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aweda TA, Eskandari V, Kukis DL, et al. New covalent capture probes for imaging and therapy, based on a combination of binding affinity and disulfide bond formation. Bioconjug Chem. 2011;22:1479–1483. doi: 10.1021/bc2002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spinelli AE, Kuo C, Rice BW, et al. Multispectral Cerenkov luminescence tomography for small animal optical imaging. Opt Express. 2011;19:12605–12618. doi: 10.1364/OE.19.012605. [DOI] [PubMed] [Google Scholar]
- 7.Spinelli AE, Ferdeghini M, Cavedon C, et al. First human Cerenkography. J Biomed Opt. 2013;18:20502. doi: 10.1117/1.JBO.18.2.020502. [DOI] [PubMed] [Google Scholar]
- 8.Thorek DL, Riedl C, Grimm J. Clinical Cerenkov luminescence imaging of 18F-FDG. J Nucl Med. 2013;55:95–98. doi: 10.2967/jnumed.113.127266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang JG, Liu HF. Functional imaging and endoscopy. World J Gastroenterol. 2011;17:4277–4282. doi: 10.3748/wjg.v17.i38.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kothapalli SR, Liu H, Liao JC, Cheng Z, Gambhir SS. Endoscopic imaging of Cerenkov luminescence. Biomed Opt Express. 2012;3:1215–1225. doi: 10.1364/BOE.3.001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corlu A, Choe R, Durduran T, et al. Three-dimensional in vivo fluorescence diffuse optical tomography of breast cancer in humans. Opt Express. 2007;15:6696–6716. doi: 10.1364/oe.15.006696. [DOI] [PubMed] [Google Scholar]
- 12.Durduran T, Choe R, Yu G, et al. Diffuse optical measurement of blood flow in breast tumors. Opt Lett. 2005;30:2915–2917. doi: 10.1364/ol.30.002915. [DOI] [PubMed] [Google Scholar]
- 13.Tromberg BJ, Pogue BW, Paulsen KD, Yodh AG, Boas DA, Cerussi AE. Assessing the future of diffuse optical imaging technologies for breast cancer management. Med Phys. 2008;35:2443–2451. doi: 10.1118/1.2919078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boverman G, Fang Q, Carp SA, et al. Spatio-temporal imaging of the hemoglobin in the compressed breast with diffuse optical tomography. Phys Med Biol. 2007;52:3619–3641. doi: 10.1088/0031-9155/52/12/018. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Carpenter CM, Jiang H, et al. Intraoperative imaging of tumors using Cerenkov luminescence endoscopy: a feasibility experimental study. J Nucl Med. 2012;53:1579–1584. doi: 10.2967/jnumed.111.098541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland JP, Normand G, Ruggiero A, Lewis JS, Grimm J. Intraoperative imaging of positron emission tomographic radiotracers using Cerenkov luminescence emissions. Mol Imaging. 2011;10:177–186. 171-173. [PMC free article] [PubMed] [Google Scholar]
- 17.Thorek DL, Abou DS, Beattie BJ, et al. Positron lymphography: multimodal, high-resolution, dynamic mapping and resection of lymph nodes after intradermal injection of 18F-FDG. J Nucl Med. 2012;53:1438–1445. doi: 10.2967/jnumed.112.104349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson R, Germanos MS, Manfredi MG, Smith PG, Silva MD. Multimodal imaging with 18F-FDG PET and Cerenkov luminescence imaging after MLN4924 treatment in a human lymphoma xenograft model. J Nucl Med. 2011;52:1764–1769. doi: 10.2967/jnumed.111.091710. [DOI] [PubMed] [Google Scholar]
- 19.Ruggiero A, Holland JP, Lewis JS, Grimm J. Cerenkov luminescence imaging of medical isotopes. J Nucl Med. 2010;51:1123–1130. doi: 10.2967/jnumed.110.076521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Mitchell GS, Cherry SR. Cerenkov luminescence tomography for small-animal imaging. Opt Lett. 2010;35:1109–1111. doi: 10.1364/OL.35.001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natarajan A, Habte F, Liu H, et al. Evaluation of Zr-rituximab Tracer by Cerenkov luminescence imaging and correlation with PET in a humanized transgenic mouse model to image NHL. Mol Imaging Biol. 2013;15:468–475. doi: 10.1007/s11307-013-0624-0. [DOI] [PubMed] [Google Scholar]
- 22.Dothager RS, Goiffon RJ, Jackson E, Harpstrite S, Piwnica-Worms D. Cerenkov radiation energy transfer (CRET) imaging: a novel method for optical imaging of PET isotopes in biological systems. PLoS One. 2010;5:e13300. doi: 10.1371/journal.pone.0013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotagiri N, Niedzwiedzki DM, Ohara K, Achilefu S. Activatable probes based on distance-dependent luminescence associated with Cerenkov radiation. Angew Chem Int Ed Engl. 2013;52:7756–7760. doi: 10.1002/anie.201302564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Axelsson J, Davis SC, Gladstone DJ, Pogue BW. Cerenkov emission induced by external beam radiation stimulates molecular fluorescence. Med Phys. 2011;38:4127–4132. doi: 10.1118/1.3592646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kachur AV, Popov AA, Delikatny EJ, Karp JS, Popov AV. Synthesis of 18F-labeled phenolphthalein and naphtholphthalein. J Fluorine Chem. 2013;151:1–6. doi: 10.1016/j.jfluchem.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kachur AV, Popov AV, Karp JS, Delikatny EJ. Direct fluorination of phenolsulfonphthalein: a method for synthesis of positron-emitting indicators for in vivo pH measurement. Cell Biochem Biophys. 2013;66:1–5. doi: 10.1007/s12013-012-9390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kachur AV, Sardelis D, Bentzley C, Popov AV, Delikatny EJ, Karp JS. Synthesis and characterization of fluorinated derivatives of cresolsulfonphthalein. J Fluorine Chem. 2013;145:112–117. doi: 10.1016/j.jfluchem.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czupryna J, Kachur AV, Blankemeyer E, Popov AV, Karp JS, Delikatny EJ. In vivo pH detection using switchable 18F labeled Cerenkov probes [abstract] J Nucl Med. 2012;53(Supp 1):73. [Google Scholar]
- 29.Surti S, Karp JS, Perkins AE, et al. Imaging performance of A-PET: a small animal PET camera. IEEE Trans Med Imaging. 2005;24:844–852. doi: 10.1109/tmi.2005.844078. [DOI] [PubMed] [Google Scholar]
- 30.Daube-Witherspoon ME, Matej S, Karp JS, Lewitt RM. Application of the Row Action Maximum Likelihood Algorithm with spherical basis functions to clinical PET imaging. IEEE Trans Med Imaging. 2001;48:24–30. [Google Scholar]
- 31.Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74:69–86. doi: 10.1146/annurev-physiol-012110-142317. [DOI] [PubMed] [Google Scholar]
- 32.Robey IF, Baggett BK, Kirkpatrick ND, et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69:2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashim AI, Zhang X, Wojtkowiak JW, Martinez GV, Gillies RJ. Imaging pH and metastasis. NMR Biomed. 2011;24:582–591. doi: 10.1002/nbm.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Lin Y, Gillies RJ. Tumor pH and its measurement. J Nucl Med. 2010;51:1167–1170. doi: 10.2967/jnumed.109.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ntziachristos V, Bremer C, Weissleder R. Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol. 2003;13:195–208. doi: 10.1007/s00330-002-1524-x. [DOI] [PubMed] [Google Scholar]
- 36.Park JC, Il An G, Park SI, et al. Luminescence imaging using radionuclides: a potential application in molecular imaging. Nucl Med Biol. 2011;38:321–329. doi: 10.1016/j.nucmedbio.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Thorek DL, Das S, Grimm J. Molecular imaging using nanoparticle quenchers of Cerenkov luminescence. Small. 2014;10:3729–3734. doi: 10.1002/smll.201400733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.