Abstract
Purpose
Asthmatics have unique characteristics that may influence cardiovascular morbidity. We tested the association of lower airway caliber, obstructive sleep apnea (OSA) and other asthma-related factors, with systemic hypertension (HTN).
Methods
Asthma individuals at specialty clinics completed the Sleep Apnea Scale of the Sleep Disorders Questionnaire (SA-SDQ). Medical records were reviewed for diagnosed HTN, OSA and comorbidities, spirometry and current medications. FEV1% predicted was categorized as ≥80 (reference), 70-79, 60-69 and <60. SA-SDQ ≥36 for men and ≥32 for women defined high OSA risk.
Results
Among 812 asthmatics (mean age±standard deviation: 46±14 years), HTN was diagnosed in 191 (24%), OSA in 65 (8%), and OSA or high OSA risk (combined OSA variable) in 239 (29%). HTN was more prevalent in lower FEV1% categories (p<0.0001), in subjects with OSA, and those with combined OSA variable (55% vs. 21% and 46% vs. 14%, respectively, both p<0.0001). With adjustment for covariates, associations with HTN remained significant for some FEV1% categories (70-79% odds ratio=1.60 [95% CI: 0.90-2.87]; 60-69% 2.73 [1.28-5.79]; <60% 0.96 [0.43-2.14]), and for OSA (2.20 [1.16-4.19]). The combined OSA variable in comparison to OSA alone demonstrated a stronger association with HTN (3.17 [1.99-5.04]) in a reiteration of this model. Inhaled corticosteroids (ICS) at lowest doses, in comparison to no ICS use had an independent “protective” association with HTN (0.44 [0.22-0.90]).
Conclusions
In this young population, lower airways obstruction and OSA were positively associated with HTN. In contrast, lower ICS doses attenuated likelihood for HTN. Adequate control of airway inflammation at appropriate ICS doses, and screening for OSA may reduce the burden of HTN in asthma.
Keywords: Asthma, airway obstruction, hypertension, lung, sleep apnea, obstructive
INTRODUCTION
Asthma, obstructive sleep apnea (OSA) and systemic hypertension (HTN) are among most common chronic illnesses. Asthma and OSA affect approximately 8% and 2-4% of middle-aged adults [3, 41]. Based on NHANES, about 16% of adults 20-75 years old have stage 1 and 2 (clinical) HTN [30]. These diseases impose substantial economic burden [1, 3, 24].
In a large population-based study, asthmatics were more likely to have HTN than non-asthmatics, independent of traditional risk factors [13]. This suggests that asthma individual has a unique set of predisposing characteristics for HTN. Reduced lung function (FEV1) and accompanying inflammation may be one such characteristic. The occurrence of inflammation as systemic rather than simply confined to the airways in asthma is a concept growing in acceptance [6]. Reduced lung function is linked to cardiovascular mortality [33], and systemic inflammation has been proposed as one underlying mechanism [33]. Indeed, an inverse relationship between FEV1% and systemic levels of inflammatory markers was shown [31, 36], and systemic inflammation in the setting of chronic lung disease is a strong and consistent marker of future cardiovascular events [23]. Asthma comorbidities, such as rhinitis and atopy—likewise associated with low-grade systemic inflammation—may also contribute to HTN risk [6]. Asthma medications may have their own effects, since long acting bronchodilators have been associated with mortality in asthma subsets [26]. Inhaled corticosteroids at higher doses are absorbed systemically [25], and certainly, systemic corticosteroids have been shown to have undesirable effects, such as hypertension and other cardiovascular outcomes [16, 37]. These may be due, in part, to mineralocorticosteroid effects, with retention of sodium and fluid [16, 37].
OSA—a recognized cause for HTN [10]— is more prevalent in asthma [4, 21, 34]. OSA propagates an inflammatory cascade that has been implicated in the pathogenesis of cardiovascular disease [24, 32]. Asthma and OSA, however, feature a bidirectional relationship, such that OSA worsens asthma [9, 11, 17]. Given this interaction, it is possible that these disorders exacerbate each other’s systemic inflammatory milieu, giving rise to an enhanced predisposition for cardiovascular disease.
To date, no studies have addressed the role of lower airway obstruction, OSA, co-morbidities and medications as risk factors for HTN in asthma patients. We therefore hypothesized that airways obstruction, OSA and higher doses of ICS will be associated with HTN in these patients. Elucidating these relationships would allow physicians to address causal factors for HTN and reduce its burden in asthma. Preliminary results were published in abstract form [35].
METHODS
Study Design
This was a cross-sectional study conducted at the University of Michigan (UM)- Ann Arbor (May 2004 - April 2006) and University of Wisconsin (UW)- Madison (July 2007- December 2009). Subjects were enrolled as part of a study examining the relationship between OSA and asthma which received approvals from both Institutional Review Boards. Written informed consent was obtained from each participant.
Study Subjects
Patients with asthma, aged 18-75, at routine follow-up at Allergy and Pulmonary clinics were enrolled. Those in for urgent visits and pregnant women were excluded. Subjects had asthma diagnosed (based upon ATS criteria [2]) and managed by an academic specialist. Standard of care at visits includes history, physical exam, asthma control assessment and spirometry.
Data Collection
A self-administered survey included the Sleep Apnea scale of the Sleep Disorders Questionnaire (SA-SDQ), a validated tool to identify patients at high risk for OSA [14]. SA-SDQ contains 8 symptom-items (loud snoring disruptive to the bed partner; breathing pauses during sleep; sudden gasping arousals; worsening of snoring while supine or after alcohol; nocturnal nasal congestion and sweating; and a history of hypertension), as well as demographics. Scores ≥36 for men and ≥32 for women define high risk for OSA, as validated with polysomnography (PSG) [14].
Medical records were reviewed by study physicians for established diagnoses of comorbid lung diseases (including chronic obstructive pulmonary disease), HTN and OSA and their treatment status, and related comorbidities (rhinitis, chronic sinusitis and nasal polyps). Spirometric data, asthma (inhaled corticosteroids [ICS], systemic corticosteroid, long-acting beta agonist) and antihypertensive medications at the current visit were also extracted.
Statistical Analysis
The outcome was a clinical diagnosis of HTN, as extracted from the records. FEV1% was categorized as: ≥80% (normal), 70-79% (mildly reduced), 60-69% (moderately reduced), <60% (severely reduced); for each, a dummy variable was created and referred to the normal (≥80%) category. Owing to reduced clinical recognition [40], OSA was defined as: (1) OSA (history of clinically-diagnosed and untreated OSA); and (2) combined OSA variable (high OSA risk on SA-SDQ or a history of clinically-diagnosed and untreated OSA). Doses of ICS were classified as: low (category 1), medium (category 2) and high (category 3), per NAEPP guidelines [22]. A dummy variable was created for each ICS category and compared with “no ICS use (category 0)”, as the reference. HTN subjects treated with ≥2 classes of antihypertensives were classified as having severe HTN.
Baseline variables were summarized as mean±SD for continuous and percentages for categorical variables. Two-sample t-tests, chi-squared or Fisher exact tests were used, as appropriate, to analyze group differences in continuous and categorical variables, respectively. Logistic regression was used to test for univariate relationships of HTN with FEV1% categories, OSA or combined OSA variable, non-modifiable (age and sex) and modifiable (smoking, BMI) demographic variables, comorbidities (rhinitis, chronic sinusitis and nasal polyps), and asthma medications. Multivariate logistic regression models were then fitted with HTN as the dependent variable and categories of FEV1% predicted, OSA or combined OSA variable, with stepwise adjustment for the aforementioned covariates, regardless of their univariate associations with HTN. Two-sided p-values <0.05 indicated statistical significance. Analyses were performed using SAS 9.2 software, (SAS institute; Cary, NC).
RESULTS
Of the 1,012 subjects invited to participate, 951 (94%) consented. Of those, 64 were excluded for co-morbid lung disease. Among the remaining 887 subjects, 140 had been diagnosed with OSA of whom 75 were on treatment with continuous positive airway treatment (CPAP) and were excluded from further analyses, due to potential beneficial effects of CPAP treatment on lung function.[9] Thus, a total of 812 subjects (244 from UM and 568 from UW) were analyzed.
Sample characteristics are presented in Table 1. The majority were females, and the sample was rather young on average (46±14 yo). The mean FEV1 was 92 ±16% predicted, with the majority of subjects having values ≥60% predicted. Eight percent of our subjects had a clinical diagnosis of OSA, and 27% had either high OSA risk or a history of OSA. Over three quarters (78%) were on ICS, 9% on chronic oral glucocorticoids and 59% on LABA.
Table 1.
Demographic, physiologic and clinical characteristics of n=812 subjects with asthma
| Characteristic | Mean±SD or Number (%) |
|---|---|
| Age (years) | 46±14 |
| Gender (female) | 541 (67%) |
| BMI (kg/m2) | 29.0±6.8 |
| Current smoking | 37 (5%) |
| FEV1 (% predicted) | 92±19 |
| ≥80% | 621 (77%) |
| 70-79% | 89 (11%) |
| 60-69% | 49 (6%) |
| 50-59% | 32 (4%) |
| 40-49% | 9 (1%) |
| <40% | 5 (1%) |
| FVC (% predicted) | 92±16 |
| FEV1/FVC | 76±9 |
| FEF25-75 (% predicted) | 69±31 |
| History of rhinitis | 734 (90%) |
| History of chronic sinusitis | 246 (30%) |
| History of nasal polyps | 120 (15%) |
| ICS use | 631 (78%) |
| Low dose | 189 (23%) |
| Medium dose | 235 (29%) |
| High dose | 207 (25%) |
| Oral corticosteroids | 70 (9%) |
| LABA | 475 (59%) |
| SA-SDQ | 28±7 |
| High OSA risk on SA-SDQ | 221 (27%) |
| History of OSA | 65 (8%) |
| History of OSA or high OSA risk on SA-SDQ* | 239 (29%) |
defined as scores on Sleep Apnea scale of the Sleep Disorders Questionnaire (SA-SDQ) ≥36 for men and ≥32 for females.27
Abbreviations: s.d.= standard deviation; BMI= body mass index; FEV1%= forced expiratory volume in first second; FVC%=forced vital capacity; FEF 25-75%= forced expiratory flow between 25% and 75% of vital capacity (all these physiologic variables are expressed as percentages of predicted values); ICS= inhaled corticosteroid; LABA=Long acting β-agonist; OSA=obstructive sleep apnea (diagnosed and untreated); SA-SDQ= Sleep Apnea scale of the Sleep Disorders Questionnaire.
The prevalence of HTN was 191 (24%) and was similar between the two centers (UM vs. UW: 27% vs. 22%, p=0.12). Among subjects with HTN, 181 (95%) were on antihypertensive medications, the rest were following diet/life-style modification measures. The prevalence of HTN increased with lower FEV1% almost in a linear fashion, except for the severely reduced category (chi-square statistic=28, p-value<0.0001) (Figure 1). HTN was more common in individuals with vs. those without OSA (chi-square statistic=40, p-value<0.0001), and in those with the combined OSA variable vs. those without (chi-square statistic=95, p-value<0.0001) (Figure 2).
Figure 1.
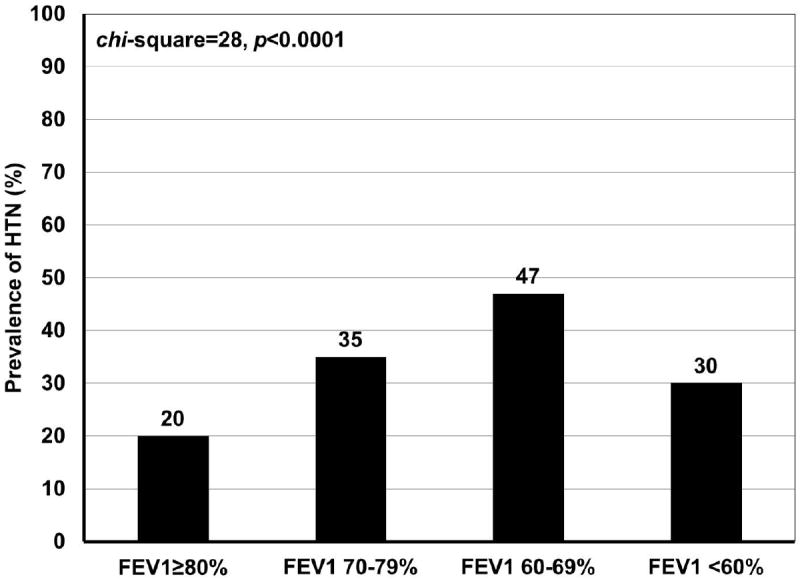
Prevalence of systemic hypertension by categories of FEV1% predicted in n=812 subjects with asthma
Abbreviations: FEV1%= forced expiratory volume in first second; HTN=systemic hypertension.
Figure 2.
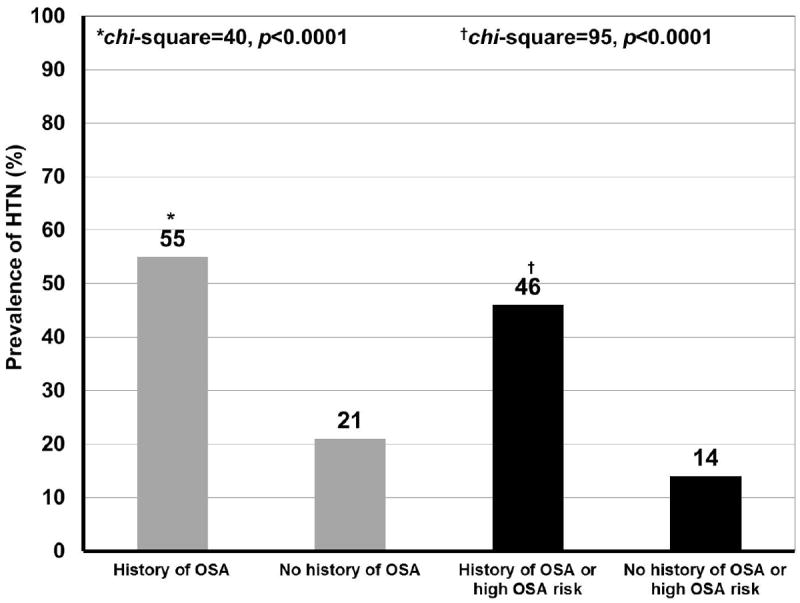
Prevalence of systemic hypertension in asthma subjects with and without OSA, and with or without OSA or high OSA risk on SA-SDQ*
Abbreviations: HTN=systemic hypertension; OSA= obstructive sleep apnea (diagnosed and untreated).
*defined as scores on Sleep Apnea scale of the Sleep Disorders Questionnaire (SA-SDQ) ≥36 for men and ≥32 for females.27
Univariate associations of HTN are shown in Table 2. With decreasing FEV1% the odds for HTN increased, though no further in the lowest category. A significant association was observed for OSA, which strengthened when the combined OSA variable was analyzed. Significant positive associations with HTN were observed for high ICS doses, oral corticosteroid and LABA. Among demographics, age and BMI demonstrated significant associations with HTN.
Table 2.
Univariate associations of systemic hypertension in n=812 subjects with asthma.
| Variable | Odds Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
|
| |||
| FEV1 (% predicted) categories: | |||
|
| |||
| ≥80% | Reference | - | - |
|
| |||
| 70-79% | 2.19 | 1.35-3.53 | 0.001 |
|
| |||
| 60-69% | 3.62 | 2.00-6.56 | <.0001 |
|
| |||
| <60% | 1.79 | 0.93-3.46 | 0.08 |
|
| |||
| History of OSA | 4.74 | 2.82-7.98 | <.0001 |
|
| |||
| History of OSA or high OSA risk on SA-SDQ* | 5.18 | 3.66-7.32 | <.0001 |
|
| |||
| Age | 1.10 | 1.08-1.11 | <.0001 |
|
| |||
| Gender | 0.92 | 0.65-1.30 | 0.63 |
|
| |||
| BMI | 1.09 | 1.06-1.11 | <0.0001 |
|
| |||
| Smoking | 0.62 | 0.25-1.50 | 0.29 |
|
| |||
| Rhinitis | 0.66 | 0.40-1.10 | 0.11 |
|
| |||
| Chronic sinusitis | 1.65 | 1.18-2.32 | 0.004 |
|
| |||
| Nasal polyps | 1.96 | 1.29-2.98 | 0.002 |
|
| |||
| ICS dose: | |||
| Low dose | 0.86 | 0.50-1.45 | 0.56 |
| Medium dose | 1.21 | 0.75-1.95 | 0.44 |
| High dose | 2.18 | 1.37-3.48 | 0.001 |
|
| |||
| Oral corticosteroids | 2.07 | 1.24-3.46 | 0.006 |
|
| |||
| LABA | 1.60 | 1.14-2.26 | 0.007 |
defined as scores on Sleep Apnea scale of the Sleep Disorders Questionnaire (SA-SDQ) ≥36 for men and ≥32 for females.27
Abbreviations: FEV1%= forced expiratory volume in first second; OSA= obstructive sleep apnea (diagnosed and untreated); BMI= body mass index; ICS= inhaled corticosteroid; LABA=long acting β-agonist.
Results of multivariate analyses are depicted in Table 3. With progressive adjustment for covariates, although the incremental association of FEV1% predicted categories was attenuated, moderately reduced FEV1% maintained its statistical significance: when compared with the individuals with normal FEV1% predicted, those in this category were on average 173% more likely to have a diagnosis of HTN (odds ratio=2.73, 95% Confidence Interval [1.28-5.79], p=0.009); the association of mildly reduced FEV1% predicted was reduced to a trend (1.60 [0.90-2.87], p=0.11), whereas no association was seen for the severely reduced category. OSA history remained significantly associated with HTN, such that, when compared to individuals without, in those with OSA, the odds for HTN were on average 120% higher (2.20 [1.16-4.19], p=0.02), independent of all covariates. Interestingly, while not significantly associated in univariate analyses, in the multivariate model, a “protective” association of ICS doses emerged which gradually attenuated with higher doses: as compared to asthmatics not on ICS, the low dose users were 56% less likely (0.44 [0.22-0.90], p=0.02) to have HTN. The increased odds for HTN seen with high dose were lost in multivariate analysis. None of the comorbidities or other asthma medications retained independent significant associations with HTN. Reiteration of these models replacing the OSA with the combined OSA variable (Table 4) demonstrated its stronger association with HTN (3.17 [1.99-5.04], p<.0001), while the other relationships did not appreciably change.
Table 3.
Associations of systemic hypertension with FEV1% predicted, with adjustment for history of OSA (diagnosed and untreated), demographic and other asthma-related variables.
| ODDS RATIO ADJUSTED FOR HISTORY OF OSA | ODDS RATIO ADJUSTED FOR HISTORY OF OSA, NON-MODIFIABLE RISK FACTORS (AGE, SEX) | ODDS RATIO ADJUSTED FOR HISTORY OF OSA, NON-MODIFIABLE AND MODIFIABLE RISK FACTORS (BMI, SMOKING) | ODDS RATIO ADJUSTED FOR HISTORY OF OSA, NON-MODIFIABLE AND MODIFIABLE RISK FACTORS, AND ASTHMA-RELATED VARIABLES | |||||
|---|---|---|---|---|---|---|---|---|
| ORs (95%CI) | p-value | ORs (95% CI) | p-value | ORs (95% CI) | p-value | ORs (95% CI) | p-value | |
| FEV1% categories | ||||||||
| 70-79% | 2.00 (1.22-3.28) | 0.006 | 1.78 (1.02-3.10) | 0.04 | 1.65 (0.94-2.92) | 0.08 | 1.60 (0.90-2.87) | 0.11 |
| 60-69% | 3.03 (1.63-5.64) | 0.0004 | 3.37 (1.66-6.84) | 0.0008 | 3.15 (1.50-6.59) | 0.002 | 2.73 (1.28-5.79) | 0.009 |
| <60% | 1.48 (0.75-2.95) | 0.26 | 1.42 (0.68-3.04) | 0.36 | 1.19 (0.55-2.57) | 0.66 | 0.96 (0.43-2.14) | 0.93 |
| History of OSA | 4.37 (2.54-7.54) | <.0001 | 3.49 (1.93-6.32) | <.0001 | 2.29 (1.23-4.27) | 0.009 | 2.20 (1.16-4.19) | 0.02 |
| Age | - | - | 1.10 (1.08-1.12) | <.0001 | 1.10 (1.08-1.12) | <.0001 | 1.10 (1.08-1.14) | <.0001 |
| Gender | - | - | 0.68 (0.45-1.02) | 0.06 | 0.76 (0.50-1.15) | 0.19 | 0.70 (0.46-1.08) | 0.11 |
| BMI | - | - | - | - | 1.08 (1.05-1.11) | <.0001 | 1.08 (1.04-1.11) | <.0001 |
| Smoking | - | - | - | - | 0.68 (0.23-2.02) | 0.49 | 0.64 (0.21-1.99) | 0.45 |
| Rhinitis | 1.04 (0.53-2.04) | 0.91 | ||||||
| Chronic sinusitis | - | - | - | - | - | - | 1.34 (0.85-2.09) | 0.21 |
| Polyps | - | - | - | - | - | - | 1.34 (0.76-2.39) | 0.32 |
| ICS Doses | ||||||||
| Low | - | - | - | - | - | - | 0.44 (0.22-0.90) | 0.02 |
| Medium | - | - | - | - | - | - | 0.62 (0.31-1.24) | 0.18 |
| High | - | - | - | - | - | - | 0.79 (0.38-1.67) | 0.54 |
| Oral corticosteroids | - | - | - | - | - | - | 1.50 (0.78-2.91) | 0.22 |
| LABA | - | - | - | - | - | - | 1.29 (0.76-2.20) | 0.34 |
Abbreviations: ORs=odds ratio; CI= confidence interval; FEV1%= forced expiratory volume in first second; OSA= obstructive sleep apnea (diagnosed and untreated); BMI= body mass index; ICS= inhaled corticosteroid; LABA=long acting β-agonist.
Table 4.
Associations of systemic hypertension with FEV1% predicted, with adjustment for combined OSA variable (history or high OSA risk on SA-SDQ*), demographic and other asthma-related variables.
| ODDS RATIO ADJUSTED FOR OSA (HISTORY OR HIGH RISK ON SA-SDQ*) | ODDS RATIO ADJUSTED FOR OSA, NON-MODIFIABLE RISK FACTORS (AGE, SEX) | ODDS RATIO ADJUSTED FOR OSA, NON-MODIFIABLE AND MODIFIABLE RISK FACTORS (BMI, SMOKING) | ODDS RATIO ADJUSTED FOR OSA, NON-MODIFIABLE AND MODIFIABLE RISK FACTORS AND ASTHMA-RELATED VARIABLES | |||||
|---|---|---|---|---|---|---|---|---|
| ORs (95%CI) | p-value | ORs (95% CI) | p-value | ORs (95% CI) | p-value | ORs (95% CI) | p-value | |
| FEV1% categories | ||||||||
| 70-79% | 1.77 (1.06-2.95) | 0.03 | 1.47 (0.83-2.61) | 0.18 | 1.49 (0.84-2.64) | 0.18 | 1.47 (0.82-2.64) | 0.20 |
| 60-69% | 2.60 (1.37-4.92) | 0.004 | 2.76 (1.34-5.69) | 0.006 | 2.88 (1.35-6.13 | 0.006 | 2.53 (1.18-5.46) | 0.02 |
| <60% | 1.41 (0.70-2.86) | 0.33 | 1.32 (0.61-2.87) | 0.49 | 1.24 (0.57-2.71) | 0.59 | 1.01 (0.45-2.29) | 0.97 |
| OSA (combined variable) | 4.75 (3.34-6.77) | <.0001 | 4.43 (2.97-6.62) | <.0001 | 3.30 (2.10-5.18) | <.0001 | 3.17 (1.99-5.04) | <.0001 |
| Age | - | - | 1.10 (1.08-1.12) | <.0001 | 1.10 (1.08-1.12) | <.0001 | 1.10 (1.08-1.12) | <.0001 |
| Gender | - | - | 0.85 (0.56-1.29) | 0.43 | 0.87 (0.57-1.34) | 0.53 | 0.82 (0.53-1.27) | 0.37 |
| BMI | - | - | - | - | 1.05 (1.02 -1.08) | 0.004 | 1.05 (1.01-1.08) | 0.006 |
| Smoking | - | - | - | - | 0.54 (0.18-1.59) | 0.26 | 0.51 (0.17-1.56) | 0.24 |
| Rhinitis | - | - | - | - | - | - | 0.96 (0.49-1.88) | 0.91 |
| Chronic sinusitis | - | - | - | - | - | - | 1.32 (0.84-2.09) | 0.24 |
| Polyps | - | - | - | - | - | - | 1.21 (0.67-2.19) | 0.53 |
| ICS Doses | ||||||||
| Low | - | - | - | - | - | - | 0.44 (0.21-0.89) | 0.02 |
| Medium | - | - | - | - | - | - | 0.59 (0.29-1.18) | 0.13 |
| High | - | - | - | - | - | - | 0.77 (0.36-1.65) | 0.50 |
| Oral corticosteroids | - | - | - | - | - | - | 1.43 (0.73-2.78) | 0.30 |
| LABA | - | - | - | - | - | - | 1.17 (0.68-2.00) | 0.57 |
defined as scores on Sleep Apnea scale of the Sleep Disorders Questionnaire (SA-SDQ) ≥36 for men and ≥32 for females.27
Abbreviations: ORs=odds ratio; CI= confidence interval; FEV1%= forced expiratory volume in first second; OSA= obstructive sleep apnea (diagnosed and untreated); BMI= body mass index; ICS= inhaled corticosteroid; LABA=long acting β-agonist.
DISCUSSION
This study of a large sample of asthma patients is the first to report relationships that may help explain unique risk factors for HTN. We found that decreased FEV1% is associated with HTN almost in a linear fashion (Table 3 and 4). Also, use of ICS appeared to have a dual perhaps counterbalancing relationship with HTN: at lower doses ICS had a “protective” association which was lost at higher doses (Table 3 and 4). Concomitant OSA was also associated with HTN, independent of traditional confounders and those more specific to this patient population (Table 3). When scores on a validated screen for OSA were included within a composite OSA variable, the strength of this association increased even further (Table 4).
Independent of other factors, decreasing FEV1% was associated nearly in a linear fashion with clinically-established diagnosis of HTN (Table 3 and 4): a trend toward statistical significance was noted with mild decreases in FEV1, a significant association was seen for moderately decreased FEV1, and while a significant univariate association was observed for the severely reduced category (Table 2) this wasn’t maintained in the final models (Table 3 and 4). These results are generally consistent with reports from population-based studies which found similar associations between FEV1 and some cardiovascular outcomes and mortality, though with significant associations even for the lowest FEV1 quintile category [33].
We also found a significant association of OSA with HTN (Table 3 and 4) of a greater magnitude than that observed in general populations where it has been well-recognized [27]. Evidence is mounting that patients with asthma have an increased predisposition for OSA [4, 21, 34] and that untreated OSA is an independent risk factor for poor asthma control [9, 11, 17]. Both asthma and OSA are increasingly recognized as systemic pro-inflammatory disorders, with shared similarities that may underlie the expression of HTN—in itself, an inflammatory process [28]—in this population. Inflammation is the key pathology in asthma. The complex milieu in the lower airways cross-talks with the bone marrow, causing a sustained “spillover” inflammation [6, 12], characterized by elevations in TNF-α and IL-1β [18], and IL-6 [5, 39], their levels correlate with airway reactivity [18], increase further during attacks and with allergen bronchoprovocation [39]. Additionally, asthma is associated with C-reactive protein (CRP), a sensitive marker of systemic inflammation [20]. These mediators have pleiotropic vascular properties, promoting wall stiffness, impairing nitric oxide-dependent vasodilation, and are elevated in patients with HTN [8, 28]. Furthermore, mast cells produce lipoxins and hydroxyeicosatetraenoic acids (HETEs), which interact with renal receptors producing vascular and/or glomerular vasoconstriction affecting renal hemodynamics, predisposing to HTN [7, 29]. A possible explanation of the observed FEV1% associations could be that with its mild to moderate decreases, gradually enhanced levels of mediators occur and predispose to HTN. We did not find an association with HTN for the severely reduced FEV1 category, which is likely due to the small number of subjects in this subset. This observation was in contrast to our expectation, and to the observation that this was the subset most frequently using highest ICS doses (chi-square statistic=73, p<0.0001) and oral corticosteroids (Fisher exact, p<0.0001), both of which have been linked to adverse cardiovascular outcomes [37, 38]. In OSA, the related systemic inflammation is playing a central role in its cardiovascular consequences [24, 32]. Intermittent hypoxia preferentially activates NF-kB-mediated inflammatory pathways and inflammatory cells which release inflammatory mediators such as TNF-α, IL-6, CRP, leading to vascular pathology. Treatment with CPAP attenuates levels of some of these markers [24, 32].
Our study, for the first time, suggests a “double-edged sword” relationship of ICS with HTN (Table 3 and 4): lower doses of ICS may confer a “protective” association while the opposite may be applicable for higher doses. In COPD, low dose ICS therapy reduced the likelihood of acute myocardial infarction, when controlling for other known risks [19]. This may be explained by control of inflammation, since circulating levels of CRP significantly decreased with ICS [15]. On the other hand, high dose corticosteroid users have an increased risk of hospitalization due to cardiovascular disease [38]. High corticosteroid doses were associated with increased risk for atrial fibrillation, explained in part by mineralocorticosteroid effects [37] with retention of sodium and water, thereby predisposing to HTN and atrial fibrillation [16, 37]. Doses in between (medium ICS dose, which represented over a third –37% – of our steroid users), may or may not heighten risk for HTN, as ICS differ in their potency and systemic absorption from the airways [25]. Nonetheless, corroborating the existent literature with our data renders our speculation intriguing and worthy to be prospectively tested.
Taken together, our data suggest complex interactions modulating HTN risk in asthma (Figure 3). The result of reciprocally interactive pathways of asthma with OSA, in one individual, may be an augmented systemic inflammatory response, leading to a heightened risk for HTN. Furthermore, the ICS dose alters the balance of systemic anti-inflammatory vs. mineralocorticoid effects, such that at higher doses mineralocorticoid effects take more of a role and may further augment the HTN risk (Figure 3).
Figure 3.
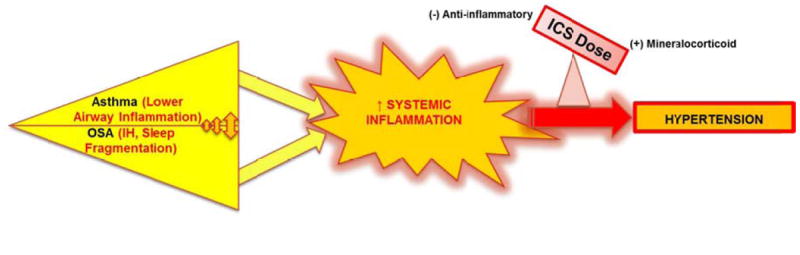
Proposed interaction between asthma, OSA and inhaled corticosteroid in modulating risk for systemic hypertension in patients with asthma
Both asthma and OSA (via intermittent hypoxia—IH, sleep fragmentation) are associated with sustained inflammatory states, which share similarities and could predispose to cardiovascular morbidity, such as HTN. Since asthma and OSA feature a bidirectional interaction, it is possible that in one individual, these disorders exacerbate each other’s systemic inflammatory state, giving rise to an augmented systemic inflammatory response, leading to a heightened risk for HTN. The ICS dose, by altering the balance of anti-inflammatory vs. mineralocorticoid effects may further modulate the HTN risk. See Discussion for details.
Abbreviations: OSA= obstructive sleep apnea; IH=intermittent hypoxia caused by obstructive sleep apnea; ICS= inhaled corticosteroid.
The strengths of this study rely on the high participation rate (94%), yielding one of the largest samples of asthma patients. Second, these subjects were well-characterized with concomitant assessment of multiple relevant variables from medical records, objective measures (ie, spirometry) and surveys. Furthermore, the vast majority of patients with a HTN diagnosis were either using antihypertensive medications (95%) or had documentation of following diet/life-style measures (5%), enhancing the confidence in the HTN diagnosis. The prevalence of diagnosed HTN in our sample was higher than that estimated by NHANES (14%) (HTN stage 1 and 2) for adults aged 40-49 [30], the age group most heavily represented in our study; thus, such high prevalence of HTN makes this population most suitable for testing the associations of interest. There are limitations to our study. First, this is a cross-sectional study and thus it cannot prove cause and effect relationships. However, the data brought forth, in the setting of these biologically putative pathways, are provocative and merit future prospective investigation. Second, the assigned diagnosis of HTN from the chart may lead to an underestimation of the prevalence of HTN, if for example patients may not have had primary care or anti-HTN medications recorded in the system, as the specialists may be less likely to assign a diagnosis of HTN despite observations of high blood pressure readings in their office visits and more likely to defer this task to primary care. Furthermore, we did not conduct objective measures of blood pressure, to assess its control. Third, the SA-SDQ has not been validated in asthma populations. However, the observed associations for clinically-diagnosed (and untreated) OSA offer robust credence overall to the data. Additionally, the OSA in comparison to no OSA subjects, on average, scored much higher on the SA-SDQ scale (37 vs. 27, p<0.001). Likewise, 75% of OSA vs. only 23% of no OSA subjects (chi-square statistic=78, p<0.0001) fulfilled the criteria for high OSA risk, as validated with PSG in other patient populations [14]. Last, the SA-SDQ does contain a question assessing HTN. We know of no work that used the SA-SDQ questionnaire without any of its individual questions, and thus have no validated cut-point to use in defining our combined OSA variable, if the HTN question is excluded. Nonetheless, the associations observed when using the clinically-diagnosed OSA variable lays credence to the data observed when using the combined OSA variable. Furthermore, as is in the general population [40], OSA is likely underdiagnosed also in asthmatics, yielding further support for the analysis using the combined OSA variable. Until studies on this topic are done, this possibility cannot be refuted. Further prospective studies overcoming all these limitations are necessary.
In summary, in this large sample of asthma patients, moderately decreased FEV1% and prevalent comorbidity with OSA were associated with diagnosed-HTN, independent of traditional confounders. Additionally, ICS may have a dose-related counterbalancing effect on the presence of HTN. Our data suggest that adequate control of lower airway inflammation at the most appropriate dose of ICS may attenuate cardiovascular risk, and earlier screening for OSA may allow new opportunities to reduce the burden of HTN and its consequences in asthma. The interacting and modulating pathways that emerged in this study warrant prospective studies with objective methods and more detailed cardiovascular outcomes, to better clarify their role in cardiovascular risk in asthma.
Acknowledgments
The authors are grateful to the study subjects at both institutions, for their participation. As well, to student assistants for administering the questionnaires in clinics, and for data entry. We recognize the help from the providers and staff at the Pulmonary Clinics and Briarwood Asthma-Airways Center at the University of Michigan- Ann Arbor, and Allergy and Pulmonary Clinics at the University of Wisconsin-Madison in recruiting subjects for this study.
Funding support: This study was funded by the University of Michigan General Clinical Research Center (MO1 RR00042) and Neurology Department Training Grant 5T32NS007222; University of Wisconsin School of Medicine and Public Health, Department of Medicine, and Medical Education and Research Committee - New Investigator Award; 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health; and additional resources from William S. Middleton Memorial VA Hospital, Madison, Wisconsin.
Dr. Mihaela Teodorescu received funding from the University of Wisconsin School of Medicine and Public Health, Department of Medicine and Medical Education and Research Committee- New Investigator Award, and National Institutes of Health, National Center for Research Resources (MO1 RR00042 and 1UL1RR025011 Clinical and Translational Science Award [CTSA] program), for asthma-sleep apnea research.
Dr. RD Chervin has received research support from the National Institutes of Health and Fox Foundation; has served on advisory boards for Pavad Medical, not-for-profit Sweet Dreamzzz, and the NHLBI (Sleep Disorders Research Advisory Board); is a section editor for UpToDate, Inc.; received support for educational purposes from Philips Respironics, Inc. and Fisher Paykel, Inc.; has consulted for Arena Pharmaceuticals, Proctor & Gamble, and Zansors; serves on boards of directors for the American Academy of Sleep Medicine and the International Pediatric Sleep Association; and is named in University of Michigan patents for algorithms and devices to facilitate diagnosis and treatment of sleep disorders.
The content of this article is solely the responsibility of the authors and does not represent the views of the US Department of Veterans Affairs, NIH or the United States Government.
Abbreviations List
- AHI
Apnea-Hypopnea Index
- BMI
Body Mass Index (in kilograms per meter squared)
- CI
Confidence Interval
- CPAP
Continuous Positive Airway Pressure
- COPD
Chronic Obstructive Pulmonary Disease
- CRP
C-reactive protein
- FEF25-75
Forced Expiratory Flow between 25% and 75% of vital capacity
- FEV1
Forced Expiratory Volume in first second of the vital capacity
- FVC
Forced Vital Capacity
- GERD
Gastroesophageal Reflux Disease
- HTN
Systemic hypertension
- IH
Intermittent Hypoxia related to obstructive sleep apnea
- ICS
Inhaled Corticosteroid
- LABA
Long Acting β2-Agonist
- LTM
Leukotriene Modifiers
- NAEPP
National Asthma Education and Prevention Program
- NHANES
National Health and Nutrition Examination Survey
- OR
Odds Ratio
- OSA
Obstructive Sleep Apnea
- PEFR
Peak Expiratory Flow Rate
- PSG
Polysomnography (laboratory-based sleep study)
- SA-SDQ
Sleep Apnea scale of the Sleep Disorders Questionnaire
- s.d
standard deviation of the mean
Footnotes
Financial disclosures: Drs. S Ferguson, RE Gangnon, FB Consens, MC Teodorescu, and Ms. AG Peterson have no relationships to disclose.
References
- 1.Center for Disease Control and Prevention: National Center for Health Statistics. US Department of Health and Human Services. [April 28, 2013];FastStats. Hypertension 2009-2010. Available at http://www.cdc.gov/nchs/fastats/hyprtens.htm.
- 2.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 136(1):225–244. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services. National Institute of Health; National Heart, Lung and Blood Institute. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3) [April 15, 2013];Guidelines for the Diagnosis and Management of Asthma. Full Report 2007. Available at http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf.
- 4.Auckley D, Moallem M, Shaman Z, et al. Findings of a Berlin Questionnaire survey: comparison between patients seen in an asthma clinic versus internal medicine clinic. Sleep Med. 2008;9:494–499. doi: 10.1016/j.sleep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Bettiol J, Bartsch P, Louis R, et al. Cytokine production from peripheral whole blood in atopic and nonatopic asthmatics: relationship with blood and sputum eosinophilia and serum IgE levels. Allergy. 2000;55:1134–1141. doi: 10.1034/j.1398-9995.2000.00711.x. [DOI] [PubMed] [Google Scholar]
- 6.Bjermer L. Time for a paradigm shift in asthma treatment: from relieving bronchospasm to controlling systemic inflammation. J Allergy Clin Immunol. 2007;120:1269–1275. doi: 10.1016/j.jaci.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Capra V, Back M, Barbieri SS, et al. Eicosanoids and Their Drugs in Cardiovascular Diseases: Focus on Atherosclerosis and Stroke. Med Res Rev. 2012 Mar 20; doi: 10.1002/med.21251. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Chae CU, Lee RT, Rifai N, et al. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 9.Chan CS, Woolcock AJ, Sullivan CE. Nocturnal asthma: role of snoring and obstructive sleep apnea. Am Rev Respir Dis. 1988;137:1502–1504. doi: 10.1164/ajrccm/137.6.1502. [DOI] [PubMed] [Google Scholar]
- 10.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 11.Ciftci TU, Ciftci B, Guven SF, et al. Effect of nasal continuous positive airway pressure in uncontrolled nocturnal asthmatic patients with obstructive sleep apnea syndrome. Respir Med. 2005;99:529–534. doi: 10.1016/j.rmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Denburg JA, Sehmi R, Saito H, et al. Systemic aspects of allergic disease: bone marrow responses. J Allergy Clin Immunol. 2000;106:S242–246. doi: 10.1067/mai.2000.110156. [DOI] [PubMed] [Google Scholar]
- 13.Dogra S, Ardern CI, Baker J. The relationship between age of asthma onset and cardiovascular disease in Canadians. The Journal of asthma : official journal of the Association for the Care of Asthma. 2007;44:849–854. doi: 10.1080/02770900701752391. [DOI] [PubMed] [Google Scholar]
- 14.Douglass AB, Bornstein R, Nino-Murcia G, et al. The Sleep Disorders Questionnaire I: Creation and multivariate structure of SDQ. Sleep. 1994;17:160–167. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- 15.Girdhar A, Kumar V, Singh A, et al. Systemic inflammation and its response to treatment in patients with asthma. Respir Care. 2011;56:800–805. doi: 10.4187/respcare.00601. [DOI] [PubMed] [Google Scholar]
- 16.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. Jama. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 17.Guilleminault C, Quera-Salva MA, Powell N, et al. Nocturnal asthma: snoring, small pharynx and nasal CPAP. Eur Respir J. 1988;1:902–907. [PubMed] [Google Scholar]
- 18.Halasz A, Cserhati E, Kosa L, et al. Relationship between the tumor necrosis factor system and the serum interleukin-4, interleukin-5, interleukin-8, eosinophil cationic protein, and immunoglobulin E levels in the bronchial hyperreactivity of adults and their children. Allergy Asthma Proc. 2003;24:111–118. [PubMed] [Google Scholar]
- 19.Huiart L, Ernst P, Ranouil X, et al. Low-dose inhaled corticosteroids and the risk of acute myocardial infarction in COPD. Eur Respir J. 2005;25:634–639. doi: 10.1183/09031936.05.00079004. [DOI] [PubMed] [Google Scholar]
- 20.Jousilahti P, Salomaa V, Hakala K, et al. The association of sensitive systemic inflammation markers with bronchial asthma. Ann Allergy Asthma Immunol. 2002;89:381–385. doi: 10.1016/S1081-1206(10)62039-X. [DOI] [PubMed] [Google Scholar]
- 21.Julien JY, Martin JG, Ernst P, et al. Prevalence of obstructive sleep apnea-hypopnea in severe versus moderate asthma. J Allergy Clin Immunol. 2009;124:371–376. doi: 10.1016/j.jaci.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Kapa S, Sert Kuniyoshi FH, Somers VK. Sleep apnea and hypertension: interactions and implications for management. Hypertension. 2008;51:605–608. doi: 10.1161/HYPERTENSIONAHA.106.076190. [DOI] [PubMed] [Google Scholar]
- 23.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Jimenez F, Sert Kuniyoshi FH, Gami A, et al. Obstructive sleep apnea: implications for cardiac and vascular disease. Chest. 2008;133:793–804. doi: 10.1378/chest.07-0800. [DOI] [PubMed] [Google Scholar]
- 25.Martin RJ, Szefler SJ, Chinchilli VM, et al. Systemic effect comparisons of six inhaled corticosteroid preparations. American journal of respiratory and critical care medicine. 2002;165:1377–1383. doi: 10.1164/rccm.2105013. [DOI] [PubMed] [Google Scholar]
- 26.Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- 27.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA : the journal of the American Medical Association. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 28.Pauletto P, Rattazzi M. Inflammation and hypertension: the search for a link. Nephrol Dial Transplant. 2006;21:850–853. doi: 10.1093/ndt/gfl019. [DOI] [PubMed] [Google Scholar]
- 29.Poch E. Role of lipoxygenase metabolites in cardiovascular disease. Curr Hypertens Rep. 2003;5:1–2. doi: 10.1007/s11906-003-0001-5. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi AI, Suri MF, Kirmani JF, et al. Prevalence and trends of prehypertension and hypertension in United States: National Health and Nutrition Examination Surveys 1976 to 2000. Med Sci Monit. 2005;11:CR403–409. [PubMed] [Google Scholar]
- 31.Rasmussen F, Mikkelsen D, Hancox RJ, et al. High-sensitive C-reactive protein is associated with reduced lung function in young adults. Eur Respir J. 2009;33:382–388. doi: 10.1183/09031936.00040708. [DOI] [PubMed] [Google Scholar]
- 32.Ryan S, Taylor CT, Mcnicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax. 2009;64:631–636. doi: 10.1136/thx.2008.105577. [DOI] [PubMed] [Google Scholar]
- 33.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 34.Teodorescu M, Consens FB, Bria WF, et al. Correlates of daytime sleepiness in patients with asthma. Sleep Med. 2006;7:607–613. doi: 10.1016/j.sleep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Teodorescu M, Peterson RG, Consens FB, et al. Predictors of systemic hypertension in patients with asthma. American journal of respiratory and critical care medicine. 2008;177:A90. [Google Scholar]
- 36.Thorleifsson SJ, Margretardottir OB, Gudmundsson G, et al. Chronic airflow obstruction and markers of systemic inflammation: results from the BOLD study in Iceland. Respir Med. 2009;103:1548–1553. doi: 10.1016/j.rmed.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Der Hooft CS, Heeringa J, Brusselle GG, et al. Corticosteroids and the risk of atrial fibrillation. Arch Intern Med. 2006;166:1016–1020. doi: 10.1001/archinte.166.9.1016. [DOI] [PubMed] [Google Scholar]
- 38.Wei L, Macdonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med. 2004;141:764–770. doi: 10.7326/0003-4819-141-10-200411160-00007. [DOI] [PubMed] [Google Scholar]
- 39.Yokoyama A, Kohno N, Fujino S, et al. Circulating interleukin-6 levels in patients with bronchial asthma. American journal of respiratory and critical care medicine. 1995;151:1354–1358. doi: 10.1164/ajrccm.151.5.7735584. [DOI] [PubMed] [Google Scholar]
- 40.Young T, Evans L, Finn L, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 41.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]


