Abstract
We present a 17-year-old boy, diagnosed with tyrosinaemia type I at an age of 7 months, with new complaints of severe intermittent photophobia and burning eyes. His tyrosinaemia type I is treated with nitisinone and a protein-restricted diet. Dietary compliance is low since he entered puberty. His ocular complaints are attributable to subepithelial corneal deposits, resembling the common corneal phenotype of tyrosinaemia type II. Serum tyrosine levels were markedly elevated. Tyrosinaemia is a metabolic disease of tyrosine metabolism, subdivided into two types. Corneal deposits and photophobia are cardinal features of untreated tyrosinaemia type II, but not of type I. Novel treatment strategies (with nitisinone) for type I tyrosinaemia lead to a phenotype comparable with type II, including these corneal deposits. At follow-up visits his ocular complaints unfortunately remained unchanged, though he states his dietary compliance improved through the years.
Background
We present a case where uncommon corneal deposits in tyrosinaemia type I, treated with nitisinone, develop due to low therapeutic compliance in puberty. Corneal deposits are seldom reported in tyrosinaemia type I.
To our knowledge, only one case of corneal deposits in type I tyrosinaemia is described in the literature. We think this case report, combined with quality images, provides an insight, since the ophthalmologist plays an important role in tyrosinaemia diagnosis. It furthermore illustrates the consequences of dietary non-compliance, leading to these debilitating ocular problems.
Case presentation
A 17-year-old boy, known with tyrosinaemia type I since the age of 7 months, was referred with severe photophobia and burning eyes. The symptoms appeared to have an intermittent relapsing course over 2 years. Ophthalmic examination revealed a visual acuity of 25/20, evident epiphora and discrete linear branching subepithelial corneal deposits. Fluorescein application revealed no staining (see figure 1). His tyrosinaemia is treated with nitisinone (Orfadin, NTBC: 2-(2-nitro-4-fluoromethylbenzoyl)-1,3-cyclohexanedione), a diet restricted in natural protein and supplements of amino acids. Serum tyrosine levels were 600 µmol/l (range 35–107). Since puberty his dietary compliance is low.
Figure 1.
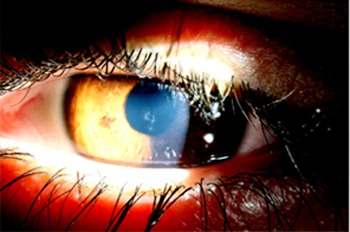
Subepithelial corneal deposits in tyrosinaemia type I, right eye.
His medical history shows a persistently high α-fetoprotein and severe obstipation in times of low dietary compliance. Routine ultrasound showed a homogenous liver of normal dimensions and a stable splenomegaly of 14.6×6.0 cm.
Treatment
Ocular pain relief with bandage contact lenses (PureVision plano contact lens, Bausch and Lomb) and preservative-free artificial tears (Oculotect s.c. eight times daily).
Nitisinone (Orfadin) 40 mg in the morning and 30 mg in the evening.
Diet restricted in natural protein (45 g daily) and supplements of amino acids (30 g daily). Vitaflow 135 ml three times daily.
Outcome and follow-up
Symptoms remain intermittent at best. Contact lens wear is continued. Serum tyrosine levels remain elevated. Patient is routinely seen for metabolic and ophthalmic evaluation.
Discussion
Tyrosinaemia is a rare metabolic disorder of tyrosine metabolism, leading to elevated levels of toxic metabolites. Tyrosinaemia is subdivided into three types. Corneal deposits and photophobia are cardinal features of untreated tyrosinaemia type II (oculocutaneous tyrosinaemia), but not of type I (figures 2 and 3). Untreated tyrosinaemia type I is characterised by acute liver failure or chronic liver dysfunction and renal Fanconi syndrome. Dietary treatment improves liver function but does not prevent the development of hepatocellular carcinoma. Nitisinone prevents the production of carcinogenic metabolites, thereby reducing cancer risk and the need for subsequent liver transplantation. It revolutionised the management of type I tyrosinaemia,1 although corneal deposits have been described in animals after long-term administration. These deposits mimic the common corneal phenotype of tyrosinaemia type II.2
Figure 2.
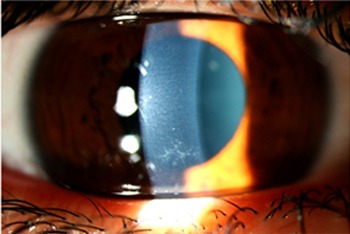
Closeup of subepithelial deposits leading to severe photophobia while maintaining good visual acuity, left eye.
Figure 3.
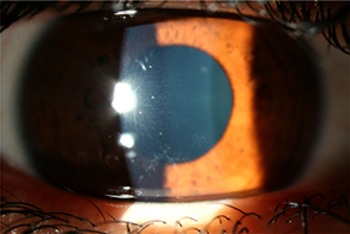
Symmetrical distribution of corneal deposits, left eye.
Only one case of corneal deposits after nitisinone treatment is described, showing a relationship between ocular complaints and serum tyrosine levels.3 Several recent long-term follow-up studies do not report on ocular symptoms or signs after nitisinone treatment for tyrosinaemia type I.4 5
Learning points.
Nitisinone treatment for tyrosinaemia type I led to an altered phenotype, resembling tyrosinemia type II.
The ophthalmologist plays an important role for supportive therapy and pain relief.
It is of utmost importance that patients and parents are aware of the consequences of low dietary compliance; not only for cancer risk, but also to prevent debilitating corneal problems.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.McKiernan PJ. Nitisinone in the treatment of hereditary tyrosinaemia type 1. Drugs 2006;66:743–50. [DOI] [PubMed] [Google Scholar]
- 2.Lock EA, Gaskin P, Ellis MK, et al. The effect of a low-protein diet and dietary supplementation of threonine on corneal lesions, the extent of tyrosinemia, and the activity of enzymes involved in tyrosine catabolism in the rat. Toxicol Appl Pharmacol 1998;150:125–32. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad S, Teckman JH, Lueder GT. Corneal opacities associated with NTBC treatment. Am J Ophthalmol 2002;134:266–8. [DOI] [PubMed] [Google Scholar]
- 4.Masurel-Paulet A, Poggi-Bach J, Rolland MO, et al. NTBC treatment in tyrosinaemia type I: long-term outcome in French patients. J Inherit Metab Dis 2008;31:81–7. [DOI] [PubMed] [Google Scholar]
- 5.Gissen P, Preece MA, Willshaw HA, et al. Ophthalmic follow-up of patients with tyrosinaemia type I on NTBC. J Inherit Metab Dis 2003;26:13–16. [DOI] [PubMed] [Google Scholar]


