Abstract
Background
The supercapsular percutaneously-assisted total hip (SuperPATH®) approach for total hip arthroplasty (THA) was developed to promote early mobilization and greater range of motion, physiologic gait kinematics and improved pain control. The superpath approach is a hybrid of the Superior Capsulotomy (SuperCap®) approach and the percutaneously assisted total hip (PATH®) technique.
Methods
Postoperative radiographs of 66 consecutive patients from the first 100 patients who underwent the SuperPATH approach were analysed by an independent third party for component position and seating, femoral offset and leg length. A detailed description of preoperative and postoperative preparation, soft tissue dissection, preparation of the femoral canal and acetabulum, and implant positioning is also provided with figures to illustrate.
Results
All components in this case series were well seated and position deemed optimal. Leg lengths were measured to within 5 mm of the contralateral side and mean acetabular abduction angle was 40.13° (SD 6.30°).
Conclusions
Through preservation of the external rotators, hip capsule, and abductor integrity, the SuperPATH approach for THA maximally preserves the surrounding soft tissue envelope. Implant position was optimal within the ‘learning curve’ of the first 100 cases for described THA safe zones. Long term outcome data for the SuperPATH approach are being collected as part of an ongoing study to compare to favourable short and mid-term results.
Keywords: Arthroplasty, minimally invasive, radiographic outcomes, supercapsular percutaneously-assisted total hip (SuperPATH), technique, total hip replacement
Introduction
Several tissue-sparing minimally invasive approaches for total hip arthroplasty (THA) have been described over the past 15 years (1-4). These techniques have been developed to encourage early mobilization, preserve gait kinematics, decrease pain and therefore postoperative narcotic analgesia, as well as to promote unrestricted range of hip motion and facilitate expedited discharge home.
A novel tissue sparing surgical technique was described by Murphy in 2004 that allowed preparation of the femoral canal and implantation of the femoral stem through a superior capsulotomy incision (SuperCap®, MicroPort Orthopedics Inc., Arlington, TN, USA) (2). This technique described using minimally invasive angled instruments utilised the interval created by reflecting the gluteus medius and minimus muscles anteriorly and the piriformis tendon posteriorly. The result of which was preservation of the short external rotators, posterior capsule and avoidance of dislocation of the femoral head which is instead excised in pieces.
In 2004, a technique was developed by Penenberg et al. and subsequently described along with the first 250 patients who underwent a percutaneously-assisted total hip (PATH®, MicroPort Orthopedics Inc., Arlington, TN, USA) (3). This approach allowed acetabular preparation through a 1 cm distally located incision “portal” without requiring release of the iliotibial band or short external rotators. A single incision modification of this approach was described more recently in 2012 by Roger et al. using specialised instrumentation and retractors to expose and prepare the acetabulum (4). This direct superior approach required dislocation of the femoral head, also required routine release of the conjoined tendon and sometimes the piriformis tendon.
In 2011, the surgical technique and initial experience of the supercapsular percutaneously assisted total hip (PATH®) (SuperPath®, MicroPort Orthopedics Inc., Arlington, TN, USA) was published (1). This technique was created by combining the percutaneous preparation of the acetabulum through a portal of the PATH approach and the femoral reaming and broaching of the SuperCap approach. This technique reported a low complication rate, excellent gait kinematics, low transfusion rate, a shortened length of hospital stay and high proportion of discharge to home rather than an inpatient physical therapy facility.
A recent multicentre study describing outcome characteristics for the SuperPath technique in over 450 patients reported a 30-day all cause readmission rate of 2.3% and a transfusion rate of 3.3% (5). The average length of stay was 1.6 days with 91% of patients discharged home, 4.1% to skilled nursing facilities, 3.8% to home care and 0.6% to inpatient physical therapy facilities. The rate of deep vein thrombosis was 0.2%, periprosthetic fracture 0.8% and dislocation 0.8%. Cost analysis of patients undergoing SuperPATH has been reported with savings to the hospital for the index procedure of over 28% (6).
This study describes an independent radiographic assessment of an early consecutive series of patients who underwent SuperPATH arthroplasty and a detailed description of the surgical technique.
Methods
Ethics approval was covered by the Western Institutional Review Board. Patients included in this study presented with degenerative osteoarthritis for elective primary total hip replacement having failed non operative management.
SuperPATH arthroplasty was performed by a single surgeon (J.C.C.) between 2008 and 2009. A total of 66 consecutive patients’ postoperative radiographs were analysed by an independent American Board certified orthopaedic surgeon. This series of patients were collated from the first 100 cases performed by the senior author (J.C.C.).
Measurements were performed in a standard manner including acetabular component abduction, femoral shaft offset and presence of any leg length discrepancy. Implanted components were also assessed for alignment, position and appropriate seating. Predetermined radiographic zones were used to assess to evaluate implant seating and varus/valgus of femoral implant.
Surgical technique description
Preoperative planning—template/positioning
Preoperative digital templating was performed from a standardized anteroposterior radiograph of the pelvis with a radio-opaque size marker. Offset and the presence of any leg length discrepancy were taken into account with templated component placement. The distance from the tip of the greater trochanter to the shoulder of the femoral component was measured as a reference to plan intraoperative placement.
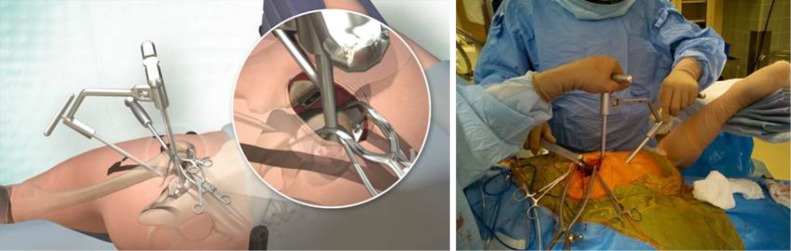
The patient was positioned in standard lateral decubitus with the operative leg in approximately 60° of flexion, and 20 to 30° of internal rotation to position the greater trochanter upward and open a window in the hip musculature (Figure 1). The foot was elevated on a padded Mayo stand so the leg rested in a slightly adducted position—this was considered the “home position” of the technique. While not required, the implanting surgeon (J.C.C.) recommends positioning the patient with a peg board and radiolucent positioning pegs to stabilise the patient on the operating table.
Figure 1.
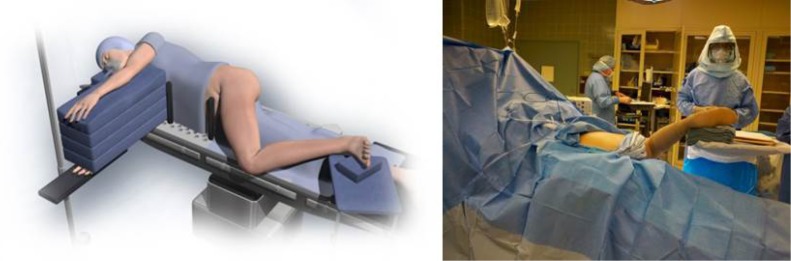
A patient was positioned on a standard peg-board in the “home position” with a foot on a padded Mayo stand.
Approach and soft tissue preservation
Following standard aseptic preparation and draping of the operative site, a skin incision was made from the tip of the greater trochanter to a length of 6 to 8 cm proximally in line with the femur in the “home position” (Figure 2). If anatomic landmarks were difficult to palpate, a shorter incision was made and extended proximally or distally as required. Dissection was continued sharply with haemostasis and the aid of a self retaining retractor to the investing fascia of the gluteus maximus muscle. The fascia was sharply incised with electrocautery in line with the skin incision and the gluteus maximus muscle was carefully split by blunt dissection in line with the fibres with wing tip elevators to expose the underlying bursa of the gluteus medius muscle. The bursa was incised and a Cobb elevator was placed anteriorly under the gluteus medius and then subsequently replaced with a blunt Hohmann retractor to protect the muscle.
Figure 2.
Following the initial incision, two wing-tipped elevators were used to split the gluteus maximus muscle and expose the underlying gluteus medius muscle.
An assistant abducted and externally rotated the leg to a more neutral position to decrease tension on the external rotators. A second Cobb elevator was placed posteriorly between the piriformis tendon and the gluteus minimus muscle then also replaced with a blunt Hohmann retractor to protect the sciatic nerve. The leg was returned to the “home position”, only minimal retraction was necessary to visualise the capsular interval at this point.
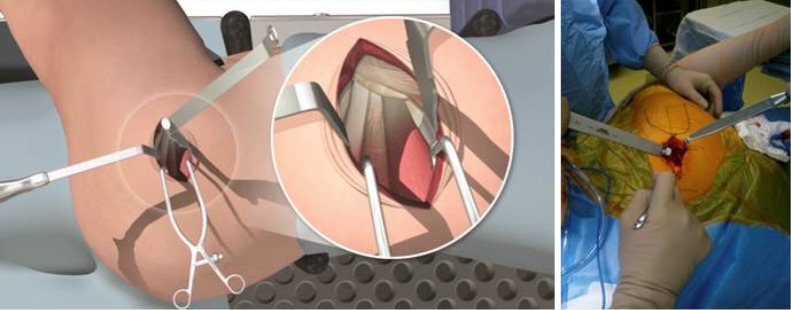
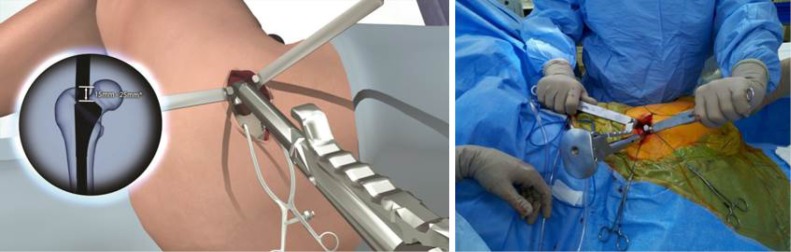
Hip capsule exposure was achieved by gently pushing the posterior border of the gluteus minimus muscle anteriorly using a Cobb elevator. The gluteus medius muscle was then retracted with a Zelpi retractor, without dissecting the muscle from the underlying capsule. This manoeuvre gave access the capsule at approximately 11 o’clock position. The capsule was then incised along the path of the skin incision with electrocautery (Figure 3), with attention to the trochanteric fossa to ensure haemostasis at the base of the femoral neck. The capsular incision was extended from the saddle of the femoral neck to 1 cm proximal to the acetabular rim. The assistant lifted the knee to neutral and a Cobb elevator is placed between the posterior capsule and posterior femoral neck, this was then replaced with a blunt Hohmann retractor. The leg was returned to the “home position” and an anterior blunt Hohmann retractor repositioned between the anterior capsule and anterior femoral neck in a similar manner. A small segment of labrum was typically then resected in line with the incision to facilitate exposure of the femoral head.
Figure 3.
With the leg in the “home position”, the capsule was incised along the path of the main incision using electrocautery.
Femoral preparation
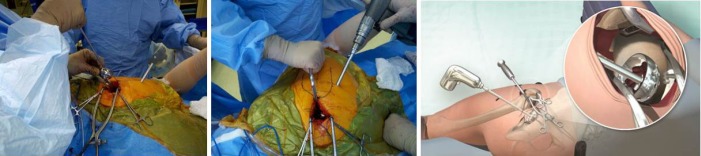
The femoral canal was entered at its zenith with a sharp starter reamer (Figure 4) aiming slightly anterior and laterally to follow the bow of the femur. Intramedullary reaming and cancellous resistance was confirmed with a cortical feeler gauge beyond the level reamed. A blunt metaphyseal reamer was then used to expand the proximal femoral opening in this same line and this was then followed with a lateralising reamer against the medial wall of the greater trochanter. The greater trochanter was supported with a finger placed on the iliotibial band to help align the lateralising reamer with the femoral canal. With the ream and broach system used, the femoral metaphysis was sequentially reamed until resistance met with the isthmus of the femoral canal.
Figure 4.
The femoral canal was prepared for broaching using starter, metaphyseal and lateralizing reamers.
An appropriately sized round calcar punch was then used to remove a channel of cortical bone with a small amount of cancellous bone from the centre of the femoral head to the reamed femoral canal. Medial calcar cancellous bone may be curetted to prevent undersizing of the implant and subsequent subsidence.
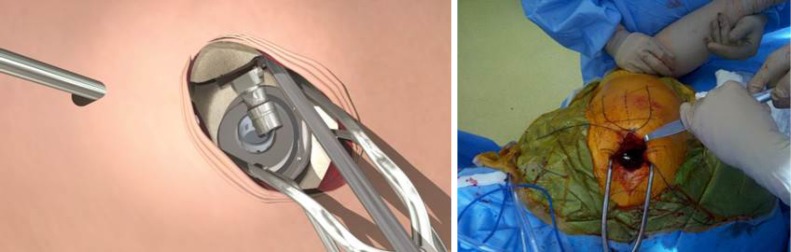
Sequential femoral broaches were then used to complete preparation and size the proximal femoral canal (Figure 5) while the femoral head and neck remained intact. The broach handle was then removed at the final size, leaving the broach in place to act as a femoral component trial. Broach trial position was compared to the preoperative template by measuring offset and distance from the tip of the greater trochanter to the shoulder of the broach trial.
Figure 5.
The femur was broached with the femoral head intact to minimize the risk of femoral neck fracture.
While an assistant lifted the leg into abduction, an oscillating saw was introduced and the femoral neck osteotomy performed level with the broach neck. A Schanz pin was inserted into the femoral head and used to rotate the head into maximal adduction to rupture the ligamentum teres, electrocautery may be used instead to divide the ligamentum. A second Schanz pin was inserted into the femoral head and the head removed from the main incision. Calcar integrity is then inspected.
Acetabular preparation
The leg was returned to the “home position” and spiked Hohmann retractors placed immediately extra-articular between the capsule and the labrum of the acetabulum anteriorly and posteriorly. The acetabulum was prepared by resecting calcified labrum and ensuring the transverse acetabular ligament (TAL) remained visible. The Hohmann retractors were removed at this point to facilitate acetabular preparation.
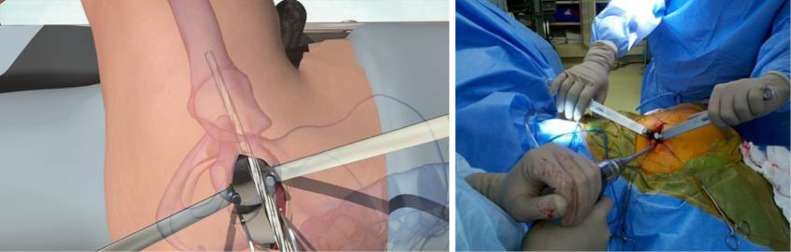
A Zelpi retractor was placed beneath the acetabular margin to retract the capsule in the proximal incision and a Romanelli retractor placed immediately distal. The femur was distracted by the assistant using a hook. The external portal placement guide was then used to pass a cannula proximally into the main incision through a 1 cm incision located 1 to 2 cm posterior to the femur (Figure 6). The external portal placement guide was removed leaving the cannula in place.
Figure 6.
The external portal placement guide was used to ensure accurate placement of a cannula through which acetabular reaming and component impaction was performed.
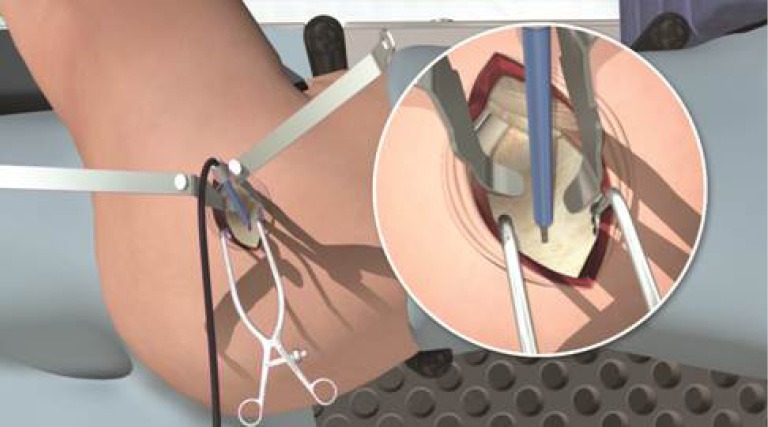
Hook distraction by the assistant was continued during acetabular reaming to provide translational control and was adjusted to concentrically ream the socket. An appropriately sized acetabular basket reamer was placed in the acetabulum through the main incision and connected to the reamer drive shaft inserted through the cannula (Figure 7). Following sequential reaming to size, the chosen acetabular implant was placed through the main incision and partially impacted using a T-handled angled impactor, then definitively impacted using a cup impactor through the cannula to line up with visual anatomic landmarks, namely the native acetabular version and TAL. Cup anteversion during final impaction was aided by femoral retraction by the assistant using the hook. Screw drilling with a sleeve inside the cannula and insertion with a straight ratchet screwdriver was also achieved through the cannula when necessary.
Figure 7.
An appropriately sized acetabular basket reamer was inserted through the main incision and the reamer drive shaft was introduced through the cannula.
Using the T-handle into the broach, the femur was translated by the surgeon posteriorly and then assistant provided internal rotation by lifting the foot and gentle telescoping of the femur into the operative field. A trial modular neck sized preoperatively from the template was selected and placed into the femoral broach. The trial acetabular liner and femoral head were placed in the acetabular component. The head and neck were then brought together by the surgeon controlling translation through the main incision by the T-handle and the assistant controlling rotation by raising or lowering the foot or knee (Figure 8). An intra-operative AP radiograph was taken to compare restoration of leg length, femoral shaft offset and trial component position.
Figure 8.
Trial components were introduced and assembled in-situ.
Trial reduction and implantation
Stability and range of motion were tested prior to the trial components being removed by distracting the neck off the broach. The trial implants were replaced with definitive components. Final stability testing was again performed, the wound thoroughly irrigated and the entire capsular incision repaired anatomically with a continuous suture. The wound was closed in layers in the standard fashion.
Postoperative management
An immediate postoperative radiograph was taken to confirm component seating and final alignment. Multimodal analgesia was used orally including acetaminophen, a GABA-agonist, a NSAID and a short acting opioid for breakthrough pain as required. Intravenous, regional and local narcotics were not usually used. There were no restrictions to postoperative movement, and no requirement for assistive care devices such as abduction pillows or raised toilet seating. Most patients left hospital for their home on the first postoperative day with instructions to cease using a walking frame or stick as soon as they are able. Patients were able to sleep on their operative side once the surgical site is comfortable.
Postoperative followup after discharge included an outpatient wound check at 7 days, repeat radiograph and progress check at 4 weeks then 6 months and 1 year postoperatively.
Results
Independent assessment of implants position showed all components to be well seated. Offset and leg lengths had been restored to within 5 mm of the contralateral hip in all cases. The mean acetabular component abduction angle was 40.13° (SD 6.30°) measured from a horizontal line derived from the ischial tuberosities and compared to the radiographic tear drop.
Discussion
The SuperPATH approach allows for maximal tissue sparing through preservation of external rotators, minimizing stretching of the gluteus medius and preservation of the hip capsule fibres through anatomic repair of superior incision. In complex cases or where access proves difficult, the incision may be extended into a standard posterolateral approach. A single assistant only is required for this technique, and the leg is not moved into unnatural positions, thus minimizing the stress on surrounding tissues. This technique is compatible with multiple implant designs and cemented or uncemented fixation types.
Traditionally, arthroplasty surgeons have aimed for cup abduction angles of 45° and anteversion of 10-15°. Thus aiming to achieve a combined anteversion of 25° to 45°, with a range between 25° to 35° for men and 30° to 45° for women (7-10). The safe zone described by Lewinnek et al. that have been shown to be associated with the lowest postoperative dislocation rate is a cup anteversion of 5° to 25° and abduction angle of 30° to 50° (7).
The SuperPATH technique involves reaming and broaching the femur in situ without dislocation and prior to performing a femoral neck osteotomy. This allows precise measurement and replication of the patient’s natural femoral offset and anteversion. The TAL and native acetabular version are used to align the acetabular components before trialling the implants through a range of motion to check for impingement.
The study sample described was taken as a consecutive series during the first 100 cases, an often described learning curve benchmark (11-13). With reproducible radiographic outcomes, the SuperPATH technique shows reliability, even during the “learning curve”. This provides evidence contrary to some authors’ conclusions that minimally-invasive approaches may negatively influence component positioning. The authorship believes that such consistency in implant positioning may be the result of a lack of external soft-tissue forces during preparation and implantation, a hallmark of SuperPATH.
While long-term studies have not been completed for SuperPATH, short and mid-term studies have shown excellent and reproducible results, with complication and readmission rates lower than those published in other studies regarding alternative total hip approaches (1,5). The long-term outcomes are likely to be most greatly influenced by component positioning and implant design. With consistent implant positioning, long-term results for SuperPATH will likely be comparable to those reported previously for other approaches. Further work is necessary to confirm this, and data is currently being collected for this on-going study.
In summary, the results from this study compare favourably to safe zones described, furthermore due to soft tissue preservation there are no particular range of motion restrictions postoperatively allowing patients to achieve a high level of function with a very low dislocation risk. Preservation of soft tissue structures has also contributed to an overall low wound infection rate, low rate of transfusion and reduced inpatient stay.
Acknowledgements
None.
Footnotes
Conflicts of Interest: JC Chow is an active consultant for MicroPort Orthopedics Inc., receiving fees for educational purposes. DA Fitch is a paid employee of MicroPort Inc.
Financial Disclosure: Research funding and support was provided by Wright Medical Technology (now MicroPort Orthopedics Inc.).
References
- 1.Chow J, Penenberg B, Murphy S. Modified micro-superior percutaneously-assisted total hip: early experiences & case reports. Curr Rev Musculoskelet Med 2011;4:146-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy S. Technique of tissue-preserving, minimally-invasive total hip arthroplasty using a superior capsulotomy. Operative Techniques in Orthopaedics 2004;14:94. [Google Scholar]
- 3.Penenberg BL, Bolling WS, Riley M. Percutaneously assisted total hip arthroplasty (PATH): a preliminary report. J Bone Joint Surg Am 2008;90 Suppl 4:209-20. [DOI] [PubMed] [Google Scholar]
- 4.Roger DJ, Hill D. Minimally invasive total hip arthroplasty using a transpiriformis approach: a preliminary report. Clin Orthop Relat Res 2012;470:2227-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gofton W, Chow J, Olsen KD, et al. Thirty-day readmission rate and discharge status following total hip arthroplasty using the supercapsular percutaneously-assisted total hip surgical technique. Int Orthop 2015;39:847-51. [DOI] [PubMed] [Google Scholar]
- 6.Gofton W, Fitch DA. In-hospital cost comparison between the standard lateral and supercapsular percutaneously-assisted total hip surgical techniques for total hip replacement. Int Orthop 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.Lewinnek GE, Lewis JL, Tarr R, et al. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am 1978;60:217-20. [PubMed] [Google Scholar]
- 8.Ranawat CS, Maynard MJ. Modern techniques of cemented total hip arthroplasty. Tech Orthop 1991;6:17-25. [Google Scholar]
- 9.Widmer KH, Zurfluh B. Compliant positioning of total hip components for optimal range of motion. J Orthop Res 2004;22:815-21. [DOI] [PubMed] [Google Scholar]
- 10.Dorr LD. Hip arthroplasty: minimally invasive techniques and computer navigation, Philadelphia, PA: WB Saunders; 2006. [Google Scholar]
- 11.Losina E, Barrett J, Mahomed NN, et al. Early failures of total hip replacement: effect of surgeon volume. Arthritis Rheum 2004;50:1338-43. [DOI] [PubMed] [Google Scholar]
- 12.Sharkey PF, Shastri S, Teloken MA, et al. Relationship between surgical volume and early outcomes of total hip arthroplasty: do results continue to get better? J Arthroplasty 2004;19:694-9. [DOI] [PubMed] [Google Scholar]
- 13.Shervin N, Rubash HE, Katz JN. Orthopaedic procedure volume and patient outcomes: a systematic literature review. Clin Orthop Relat Res 2007;457:35-41. [DOI] [PubMed] [Google Scholar]


