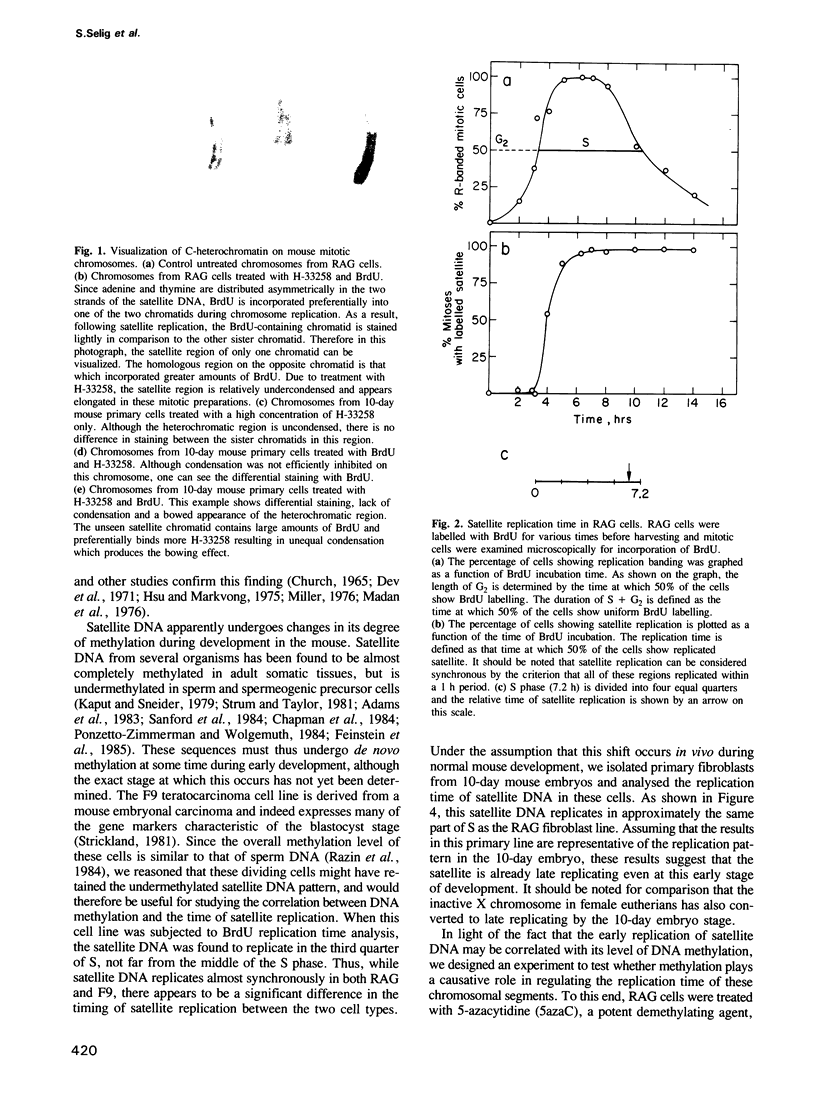
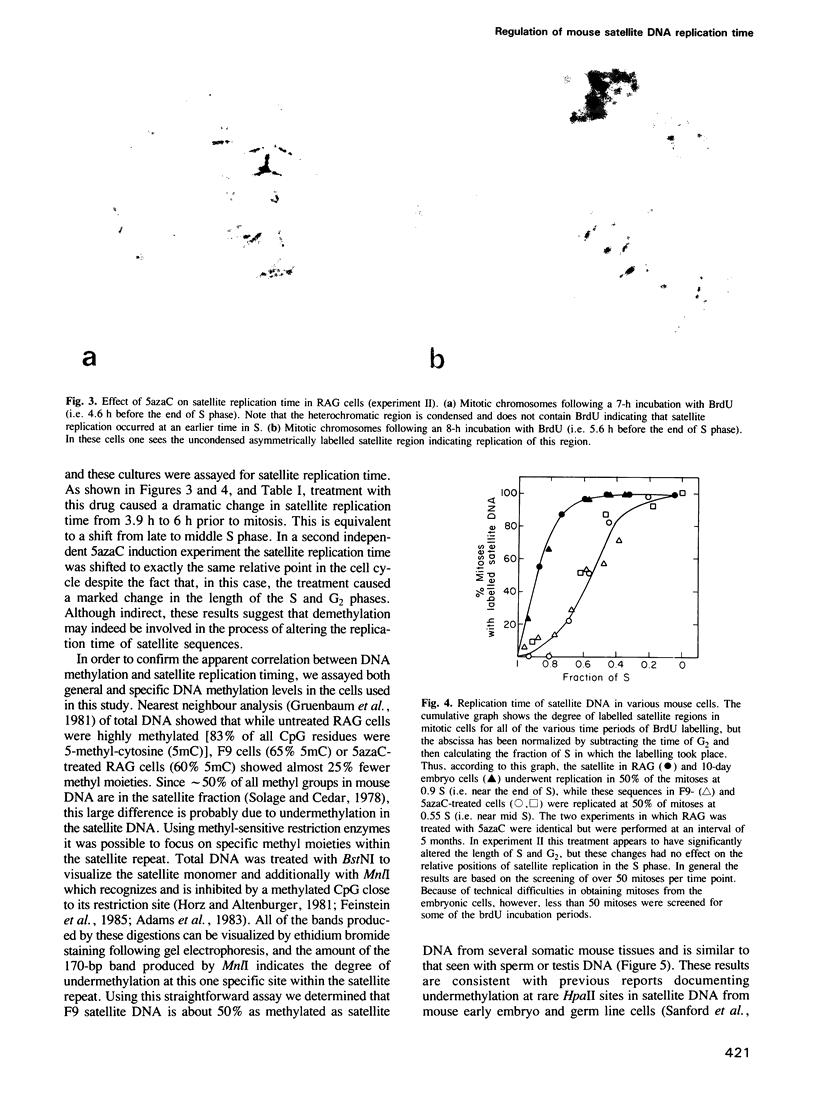
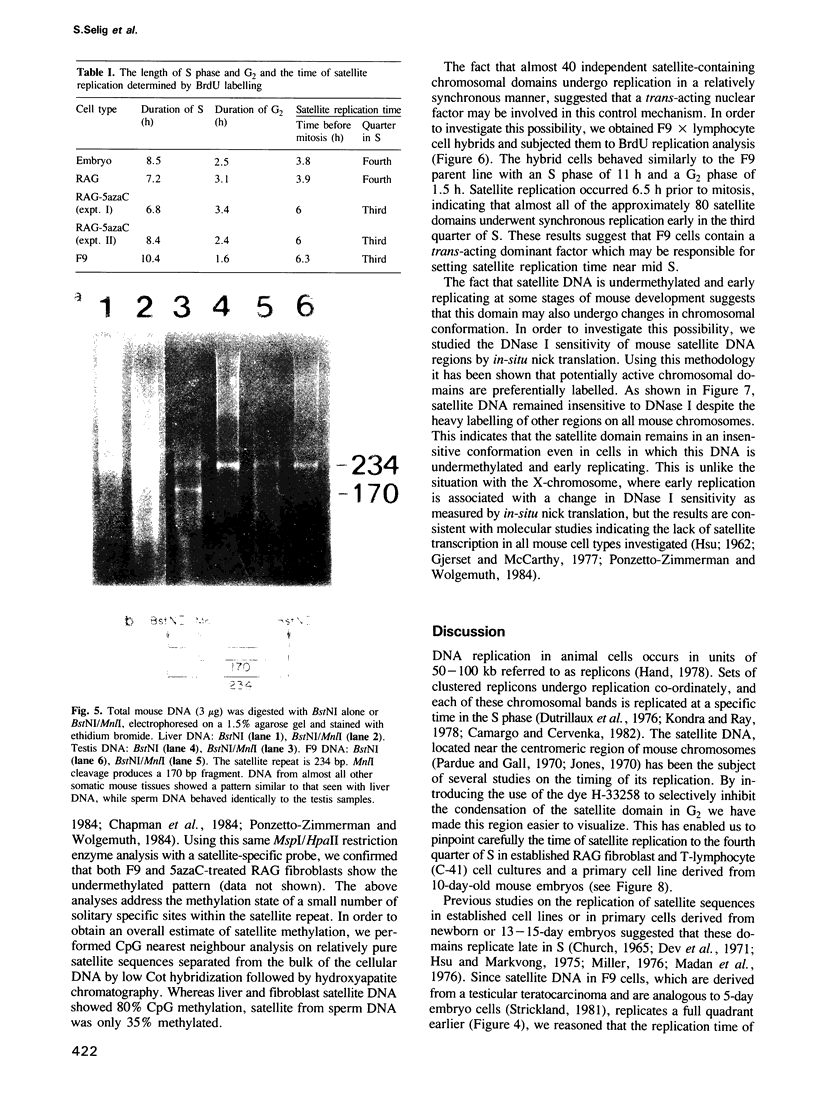
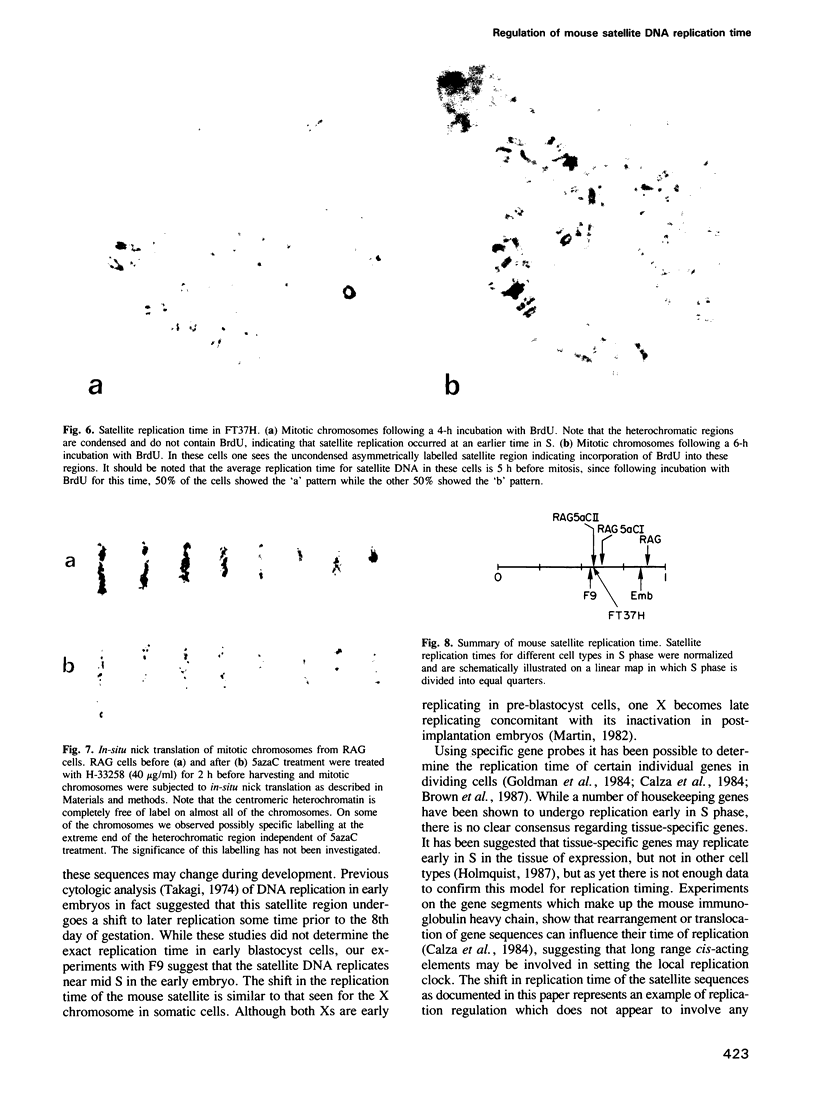
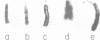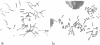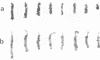Abstract
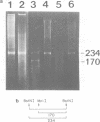
The satellite DNA sequences located near the centromeric regions of mouse chromosomes replicate very late in S in both fibroblast and lymphocyte cells and are heavily methylated at CpG residues. F9 teratocarcinoma cells, on the other hand, contain satellite sequences which are undermethylated and replicate much earlier in S. DNA methylation probably plays some role in the control of satellite replication time since 5-azacytidine treatment of RAG fibroblasts causes a dramatic temporal shift of replication to mid S. In contrast to similar changes accompanying the inactivation of the X-chromosome, early replication of satellite DNA is not associated with an increase in local chromosomal DNase I sensitivity. Fusion of F9 with mouse lymphocytes caused a dramatic early shift in the timing of the normally late replicating lymphocyte satellite heterochromatin, suggesting that trans-activating factors may be responsible for the regulation of replication timing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L., Burdon R. H., Fulton J. Methylation of satellite DNA. Biochem Biophys Res Commun. 1983 Jun 15;113(2):695–702. doi: 10.1016/0006-291x(83)91782-5. [DOI] [PubMed] [Google Scholar]
- Brown E. H., Iqbal M. A., Stuart S., Hatton K. S., Valinsky J., Schildkraut C. L. Rate of replication of the murine immunoglobulin heavy-chain locus: evidence that the region is part of a single replicon. Mol Cell Biol. 1987 Jan;7(1):450–457. doi: 10.1128/mcb.7.1.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza R. E., Eckhardt L. A., DelGiudice T., Schildkraut C. L. Changes in gene position are accompanied by a change in time of replication. Cell. 1984 Mar;36(3):689–696. doi: 10.1016/0092-8674(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Camargo M., Cervenka J. Patterns of DNA replication of human chromosomes. II. Replication map and replication model. Am J Hum Genet. 1982 Sep;34(5):757–780. [PMC free article] [PubMed] [Google Scholar]
- Chapman V., Forrester L., Sanford J., Hastie N., Rossant J. Cell lineage-specific undermethylation of mouse repetitive DNA. Nature. 1984 Jan 19;307(5948):284–286. doi: 10.1038/307284a0. [DOI] [PubMed] [Google Scholar]
- Church K. Replication of chromatin in mouse mammary epithelial cells grown in vitro. Genetics. 1965 Oct;52(4):843–849. doi: 10.1093/genetics/52.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev V. G., Grewal M. S., Miller D. A., Kouri R. E., Hutton J. J., Miller O. J. The quinacrine fluorescence karyotype of Mus musculus and demonstration of strain differences in secondary constrictions. Cytogenetics. 1971;10(6):436–451. doi: 10.1159/000130164. [DOI] [PubMed] [Google Scholar]
- Dutrillaux B., Couturier J., Richer C. L., Viegas-Péquignot E. Sequence of DNA replication in 277 R- and Q-bands of human chromosomes using a BrdU treatment. Chromosoma. 1976 Oct 12;58(1):51–61. doi: 10.1007/BF00293440. [DOI] [PubMed] [Google Scholar]
- Feinstein S. I., Racaniello V. R., Ehrlich M., Gehrke C. W., Miller D. A., Miller O. J. Pattern of undermethylation of the major satellite DNA of mouse sperm. Nucleic Acids Res. 1985 Jun 11;13(11):3969–3978. doi: 10.1093/nar/13.11.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm W. G., Walker P. M., McCallum M. Some properties of the single strands isolated from the DNA of the nuclear satellite of the mouse (Mus musculus). J Mol Biol. 1969 Mar 28;40(3):423–443. doi: 10.1016/0022-2836(69)90163-6. [DOI] [PubMed] [Google Scholar]
- Gjerset R. A., McCarthy B. J. Limited accessibility of chromatin satellite DNA to RNA polymerase from Escherichia coli. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4337–4340. doi: 10.1073/pnas.74.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goitein R., Hirschberg J., Marcus M., Sperling K. Patterns of heterochromatin replication and condensation correlate in rat kangaroo PtK2 cells. Cytogenet Cell Genet. 1984;38(2):116–121. doi: 10.1159/000132042. [DOI] [PubMed] [Google Scholar]
- Goldman M. A., Holmquist G. P., Gray M. C., Caston L. A., Nag A. Replication timing of genes and middle repetitive sequences. Science. 1984 May 18;224(4650):686–692. doi: 10.1126/science.6719109. [DOI] [PubMed] [Google Scholar]
- Goto K., Akematsu T., Shimazu H., Sugiyama T. Simple differential Giemsa staining of sister chromatids after treatment with photosensitive dyes and exposure to light and the mechanism of staining. Chromosoma. 1975 Dec 10;53(3):223–230. doi: 10.1007/BF00329173. [DOI] [PubMed] [Google Scholar]
- Gregory P., Greene C., Shapira E., Wang N. Alterations in the time of X chromosome replication induced by 5-azacytidine in a patient with 48,XXXY/47,XXY. Cytogenet Cell Genet. 1985;39(3):234–236. doi: 10.1159/000132142. [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y., Stein R., Cedar H., Razin A. Methylation of CpG sequences in eukaryotic DNA. FEBS Lett. 1981 Feb 9;124(1):67–71. doi: 10.1016/0014-5793(81)80055-5. [DOI] [PubMed] [Google Scholar]
- HSU T. C. Differential rate in RNA synthesis between euchromatin and heterochromatin. Exp Cell Res. 1962 Aug;27:332–334. doi: 10.1016/0014-4827(62)90238-0. [DOI] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978 Oct;15(2):317–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Holmquist G. P. Role of replication time in the control of tissue-specific gene expression. Am J Hum Genet. 1987 Feb;40(2):151–173. [PMC free article] [PubMed] [Google Scholar]
- Hors-Cayla M. C., Heuertz S., Frezal J. Coreactivation of four inactive X genes in a hamster x human hybrid and persistence of late replication of reactivated X chromosome. Somatic Cell Genet. 1983 Nov;9(6):645–657. doi: 10.1007/BF01539470. [DOI] [PubMed] [Google Scholar]
- Hsu T. C., Markvong A. Chromosomes and DNA of Mus: terminal DNA synthetic sequences in three species. Chromosoma. 1975 Aug 11;51(4):311–322. doi: 10.1007/BF00326318. [DOI] [PubMed] [Google Scholar]
- Hörz W., Altenburger W. Nucleotide sequence of mouse satellite DNA. Nucleic Acids Res. 1981 Feb 11;9(3):683–696. doi: 10.1093/nar/9.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonka E., Goitein R., Marcus M., Cedar H. DNA hypomethylation causes an increase in DNase-I sensitivity and an advance in the time of replication of the entire inactive X chromosome. Chromosoma. 1985;93(2):152–156. doi: 10.1007/BF00293162. [DOI] [PubMed] [Google Scholar]
- Jablonka E., Goitein R., Sperling K., Cedar H., Marcus M. 5-aza-C-induced changes in the time of replication of the X chromosomes of Microtus agrestis are followed by non-random reversion to a late pattern of replication. Chromosoma. 1987;95(1):81–88. doi: 10.1007/BF00293846. [DOI] [PubMed] [Google Scholar]
- John B., Miklos G. L. Functional aspects of satellite DNA and heterochromatin. Int Rev Cytol. 1979;58:1–114. doi: 10.1016/s0074-7696(08)61473-4. [DOI] [PubMed] [Google Scholar]
- Jones K. W. Chromosomal and nuclear location of mouse satellite DNA in individual cells. Nature. 1970 Mar 7;225(5236):912–915. doi: 10.1038/225912a0. [DOI] [PubMed] [Google Scholar]
- Kaput J., Sneider T. W. Methylation of somatic vs germ cell DNAs analyzed by restriction endonuclease digestions. Nucleic Acids Res. 1979 Dec 20;7(8):2303–2322. doi: 10.1093/nar/7.8.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerem B. S., Goitein R., Diamond G., Cedar H., Marcus M. Mapping of DNAase I sensitive regions on mitotic chromosomes. Cell. 1984 Sep;38(2):493–499. doi: 10.1016/0092-8674(84)90504-x. [DOI] [PubMed] [Google Scholar]
- Kerem B. S., Goitein R., Richler C., Marcus M., Cedar H. In situ nick-translation distinguishes between active and inactive X chromosomes. Nature. 1983 Jul 7;304(5921):88–90. doi: 10.1038/304088a0. [DOI] [PubMed] [Google Scholar]
- Klebe R. J., Chen T., Ruddle F. H. Controlled production of proliferating somatic cell hybrids. J Cell Biol. 1970 Apr;45(1):74–82. doi: 10.1083/jcb.45.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondra P. M., Ray M. Analysis of DNA replication patterns of human fibroblast chromosomes: the replication map. Hum Genet. 1978 Aug 31;43(2):139–149. doi: 10.1007/BF00293591. [DOI] [PubMed] [Google Scholar]
- Lifschytz E., Hareven D., Azriel A., Brodsly H. DNA clones and RNA transcripts of four lampbrush loops from the Y chromosome of Drosophila hydei. Cell. 1983 Jan;32(1):191–199. doi: 10.1016/0092-8674(83)90509-3. [DOI] [PubMed] [Google Scholar]
- Madan K., Allen J. W., Gerald P. S., Latt S. A. Fluorescence analysis of late DNA replication in mouse metaphase chromosomes using BUdR and 33258 Hoechst. Exp Cell Res. 1976 May;99(2):438–444. doi: 10.1016/0014-4827(76)90604-2. [DOI] [PubMed] [Google Scholar]
- Marcus M., Nielsén K., Goitein R., Gropp A. Pattern of condensation of mouse and Chinese hamster chromosomes in G2 and mitosis of 33258-Hoechst-treated cells. Exp Cell Res. 1979 Aug;122(1):191–201. doi: 10.1016/0014-4827(79)90574-3. [DOI] [PubMed] [Google Scholar]
- Martin G. R. X-chromosome inactivation in mammals. Cell. 1982 Jul;29(3):721–724. doi: 10.1016/0092-8674(82)90432-9. [DOI] [PubMed] [Google Scholar]
- Matsukuma S., Utakoji T. Asymmetric decondensation of the L cell heterochromatin by Hoechst 33258. Exp Cell Res. 1978 May;113(2):453–455. doi: 10.1016/0014-4827(78)90390-7. [DOI] [PubMed] [Google Scholar]
- Miller O. J. Is the centromeric heterochromatin of Mus musculus late replicating? Chromosoma. 1976 Apr 21;55(2):165–170. doi: 10.1007/BF01798346. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Chromosomal localization of mouse satellite DNA. Science. 1970 Jun 12;168(3937):1356–1358. doi: 10.1126/science.168.3937.1356. [DOI] [PubMed] [Google Scholar]
- Paterno G. D., Adra C. N., McBurney M. W. X chromosome reactivation in mouse embryonal carcinoma cells. Mol Cell Biol. 1985 Oct;5(10):2705–2712. doi: 10.1128/mcb.5.10.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzetto-Zimmerman C., Wolgemuth D. J. Methylation of satellite sequences in mouse spermatogenic and somatic DNAs. Nucleic Acids Res. 1984 Mar 26;12(6):2807–2822. doi: 10.1093/nar/12.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Webb C., Szyf M., Yisraeli J., Rosenthal A., Naveh-Many T., Sciaky-Gallili N., Cedar H. Variations in DNA methylation during mouse cell differentiation in vivo and in vitro. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2275–2279. doi: 10.1073/pnas.81.8.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford J., Forrester L., Chapman V., Chandley A., Hastie N. Methylation patterns of repetitive DNA sequences in germ cells of Mus musculus. Nucleic Acids Res. 1984 Mar 26;12(6):2823–2836. doi: 10.1093/nar/12.6.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer D. A., Priest J. H. Reversal of DNA methylation with 5-azacytidine alters chromosome replication patterns in human lymphocyte and fibroblast cultures. Am J Hum Genet. 1984 May;36(3):534–545. [PMC free article] [PubMed] [Google Scholar]
- Solage A., Cedar H. Organization of 5-methylcytosine in chromosomal DNA. Biochemistry. 1978 Jul 11;17(14):2934–2938. doi: 10.1021/bi00607a036. [DOI] [PubMed] [Google Scholar]
- Somssich I. E., Spira J., Hameister H., Klein G. Correlation between tumorigenicity and banding pattern of chromosome 15 in murine T-cell leukemia cells and hybrids of normal and malignant cells. Chromosoma. 1984;91(1):39–45. doi: 10.1007/BF00286483. [DOI] [PubMed] [Google Scholar]
- Sperling K., Kerem B. S., Goitein R., Kottusch V., Cedar H., Marcus M. DNase I sensitivity in facultative and constitutive heterochromatin. Chromosoma. 1985;93(1):38–42. doi: 10.1007/BF01259444. [DOI] [PubMed] [Google Scholar]
- Strickland S. Mouse teratocarcinoma cells: prospects for the study of embryogenesis and neoplasia. Cell. 1981 May;24(2):277–278. doi: 10.1016/0092-8674(81)90313-5. [DOI] [PubMed] [Google Scholar]
- Sturm K. S., Taylor J. H. Distribution of 5-methylcytosine in the DNA of somatic and germline cells from bovine tissues. Nucleic Acids Res. 1981 Sep 25;9(18):4537–4546. doi: 10.1093/nar/9.18.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi N. Differentiation of X chromosomes in early female mouse embryos. Exp Cell Res. 1974 May;86(1):127–135. doi: 10.1016/0014-4827(74)90657-0. [DOI] [PubMed] [Google Scholar]
- Takagi N., Sugawara O., Sasaki M. Regional and temporal changes in the pattern of X-chromosome replication during the early post-implantation development of the female mouse. Chromosoma. 1982;85(2):275–286. doi: 10.1007/BF00294971. [DOI] [PubMed] [Google Scholar]
- Takagi N., Yoshida M. A., Sugawara O., Sasaki M. Reversal of X-inactivation in female mouse somatic cells hybridized with murine teratocarcinoma stem cells in vitro. Cell. 1983 Oct;34(3):1053–1062. doi: 10.1016/0092-8674(83)90563-9. [DOI] [PubMed] [Google Scholar]
- Tamame M., Antequera F., Villanueva J. R., Santos T. High-frequency conversion to a "fluffy" developmental phenotype in Aspergillus spp. by 5-azacytidine treatment: evidence for involvement of a single nuclear gene. Mol Cell Biol. 1983 Dec;3(12):2287–2297. doi: 10.1128/mcb.3.12.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varley J. M., Macgregor H. C., Erba H. P. Satellite DNA is transcribed on lampbrush chromosomes. Nature. 1980 Feb 14;283(5748):686–688. doi: 10.1038/283686a0. [DOI] [PubMed] [Google Scholar]
- Venolia L., Gartler S. M., Wassman E. R., Yen P., Mohandas T., Shapiro L. J. Transformation with DNA from 5-azacytidine-reactivated X chromosomes. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2352–2354. doi: 10.1073/pnas.79.7.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]