Abstract
Congenital hyperinsulinism (CHI) is a rare disorder of hypoglycaemia in children due to excessive and dysregulated insulin secretion. Octreotide, a somatostatin analogue, is used in the treatment of hypoglycaemia in Diazoxide unresponsive CHI, but is associated with side effects such as gastrointestinal dysmotility and rarely, necrotising enterocolitis. It would be important to recognise rare but serious side effects from Octreotide therapy, particularly with long-term use. In this report, we have described drug-induced hepatitis with moderately high doses of Octreotide in a child with diffuse CHI. While serum alanine transaminase levels rose significantly with Octreotide therapy (maximum dose 30 μg/kg/day), hepatitis resolved following discontinuation of medical treatment. Liver enzymes should be monitored routinely in children with CHI using long-term Octreotide treatment, particularly with high doses. The presence of drug-induced hepatitis should prompt discontinuation of Octreotide treatment with likely subsequent resolution.
Background
Congenital hyperinsulinism (CHI) is a rare condition of dysregulated insulin secretion causing severe hypoglycaemia in infancy and childhood.1 At diagnosis of CHI, oral Diazoxide is used as first-line therapy to achieve euglycaemia. In those who are Diazoxide unresponsive, Octreotide is commonly used as second-line treatment. If Octreotide fails to achieve euglycaemia, or if treatment is complicated by serious adverse effects such as necrotising enterocolitis,2 pancreatectomy is usually required.
Octreotide is a somatostatin analogue that stabilises the pancreatic beta cell membrane and reduces insulin exocytosis.3 The dose range usually varies between 5 and 30 mcg/kg/day,4 although higher doses have been used to achieve glycaemic stability.5 Side effects of Octreotide include gastric dysmotility and abdominal distension in the short term and biliary sludging and growth stasis in the longer term. Tachyphylaxis may also occur, whereby larger doses of Octreotide are required to achieve euglycaemia.5 Recently, fatal necrotising enterocolitis in neonates has been reported,2 suggesting that the use of Octreotide in the medical treatment of CHI may be associated with serious adverse effects. Additionally, we now report the presence of Octreotide-induced hepatitis with high-dose octreotide therapy that resolved after the drug was discontinued and euglycaemia was achieved by pancreatectomy.
Case presentation
A baby girl born at 42 weeks of gestation with a birth weight of 4.56 kg (2.02 standard deviation score) presented to the local hospital with hypoglycaemic seizures on day 3 of life. Initial blood glucose at point-of-care testing was <1 mmol/l, with confirmation of hypoglycaemia with a laboratory sample. The diagnosis of CHI was made by the presence of detectable insulin at the time of hypoglycaemia, absent ketogenesis and a high requirement for dextrose (12.7 mg/kg/min).
Diazoxide was commenced soon after diagnosis of CHI, but the response was only partial. Octreotide was commenced as subcutaneous bolus injections on day 23 of life at a dose 5 μg/kg/day with satisfactory initial clinical response. Genetic testing revealed a compound heterozygous ABCC8 mutation suggestive of diffuse CHI. Although initially responsive to Octreotide, hypoglycaemia recurred in spite of dose escalation (maximum dose 20 mcg/kg/day) and subtotal pancreatectomy was required to maintain euglycaemia at 11 months of age. Histology confirmed the diagnosis of diffuse CHI.
Investigations
In the postoperative period, the patient was euglycaemic for 2 months. However, hypoglycaemia recurred as a result of hyperinsulinism (glucose 2.4 mmol/l, insulin 5.8 mU/l). At this time, second pancreatectomy was considered but postponed on account of the likelihood of fibrous adhesions following pancreatitis at initial surgery. Octreotide was recommenced as subcutaneous bolus injections with escalation to 20 μg/kg/day. Unfortunately, satisfactory response was not achieved and therefore Octreotide was further escalated to 30 μg/kg/day administered by a subcutaneous continuous pump.
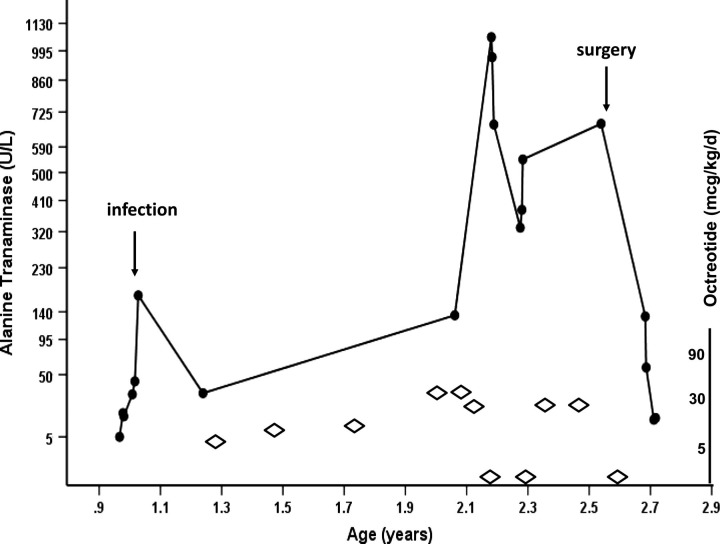
The patient's serum alanine transaminase (ALT) levels were measured as part of routine investigations. While levels were normal prior to Octreotide treatment, ALT increased transiently following an episode of candida central line sepsis, treated with Fluconazole for 10 days (figure 1). Thereafter, ALT increased with escalating doses of Octreotide (30 μg/kg/day) to reach a peak level of 1061 U/l. Octreotide was maintained in the same high dose, during which time ALT levels remained significantly high, although lower than prior peak levels (figure 1). No other drugs were used in our patient over this period of time.
Figure 1.
Pattern of rising alanine transaminase (ALT) enzyme levels (U/l) depicted by filled circles, with increasing doses of Octreotide (μg/kg/day) depicted by unfilled diamonds; age (years) has been plotted on the x-axis on a linear scale and ALT enzyme levels have been plotted on the y-axis on an exponential scale. ALT levels increased significantly to a peak of 1061 U/l (upper limit of normal 45 U/l). ALT levels decreased after Octreotide injections were stopped temporarily but increased after recommencing treatment. Following pancreatectomy and complete withdrawal of Octreotide, ALT levels returned to normal.
The presence of other causes of hepatitis was investigated thoroughly with a range of tests that included γ-glutamyl transferase activity, alkaline phosphatase levels, hepatitis virology screening, alpha one antitrypsin level, serum copper levels and ultrasound scanning, all of which were normal. A liver biopsy was performed later (after Octreotide was discontinued), which was reported as normal and excluded irreversible structural hepatic lesions.
Outcome and follow-up
Octreotide treatment was discontinued for 2 weeks during which glycaemic stability was achieved by a combination of glucagon and intravenous dextrose. ALT levels decreased following this procedure, although not completely down into the normal range (figure 1). ALT increased soon after recommencing Octreotide. At this point, a decision was made to discontinue Octreotide and proceed to second pancreatectomy. A second near total pancreatectomy was successfully performed, and Octreotide was not recommenced while glycaemic control remained satisfactory. In 1 week after surgery, ALT levels returned to normal and continue to remain so till date, implying resolution of hepatitis caused by Octreotide.
Discussion
Octreotide is a useful second-line treatment option in diffuse CHI, and aids the current management strategy to avoid surgery and thereby preserve pancreatic tissue. Although serious adverse effects such as necrotising enterocolitis2 are recognised to occur, low-to-moderate-dose Octreotide is generally well tolerated by most patients with CHI. It would be important to recognise hepatitis as a serious side effect of Octreotide in moderately high doses for the long-term medical treatment of diffuse CHI. There are occasional anecdotal reports of drug-induced hepatitis in adults treated with Octreotide.6 There are no reports of Octreotide-induced hepatitis in children and certainly not in those with CHI. It would be important to recognise that Octreotide-induced hepatitis resolves after stopping treatment.
The mechanism of high-dose Octreotide causing hepatitis is not known. It has been hypothesised that Octreotide may reduce splanchnic blood flow in a dose-dependent manner causing ischaemic changes to the bowel mucosa. Similarly, it is possible that Octreotide may adversely influence hepatic blood flow, thereby causing hepatitis. In this patient, the association of serum ALT levels increasing temporally with increasing doses of Octreotide, the exclusion of common causes of hepatitis and subsequent normalisation of ALT following complete cessation of the drug strongly suggest that hepatitis was induced by Octreotide. We would recommend serial monitoring of liver function in all children with CHI treated with Octreotide and stop treatment if serum ALT is persistently elevated.
Learning points.
Drug-induced hepatitis may occur with Octreotide used for the treatment of congenital hyperinsulinism in children.
Children on long-term Octreotide should have liver function monitoring, in particular serum alanine transaminase (ALT) levels.
Rising ALT levels in the absence of other causes of hepatitis is suggestive of Octreotide-induced hepatitis, which is reversible following discontinuation of treatment.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Aynsley-Green A, Hussain K, Hall J, et al. Practical management of hyperinsulinism in infancy. Arch Dis Child Fetal Neonatal Ed 2000;82:F98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laje P, Halaby L, Adzick NS, et al. Necrotizing enterocolitis in neonates receiving octreotide for the management of congenital hyperinsulinism. Pediatr Diabetes 2010;11:142–7. [DOI] [PubMed] [Google Scholar]
- 3.De Leon DD, Stanley CA. Mechanisms of disease: advances in diagnosis and treatment of hyperinsulinism in neonates. Nat Clin Pract Endocrinol Metab 2007;3:57–68. [DOI] [PubMed] [Google Scholar]
- 4.Kapoor RR, Flanagan SE, James C, et al. Hyperinsulinaemic hypoglycaemia. Arch Dis Child 2009;94:450–7. [DOI] [PubMed] [Google Scholar]
- 5.Arnoux JB, Verkarre V, Saint-Martin C, et al. Congenital hyperinsulinism: current trends in diagnosis and therapy. Orphanet J Rare Dis 2011;6:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arosio M, Bazzoni N, Ambrosi B, et al. Acute hepatitis after treatment of acromegaly with octreotide. Lancet 1988;2:1498. [DOI] [PubMed] [Google Scholar]