Abstract
Background
Neoadjuvant chemoradiotherapy followed by surgery is the primary treatment option for patients with locally advanced esophageal cancer. This multicenter phase I trial examined intratumoral injection of TNFerade biologic, an adenoviral vector that expresses the human tumor necrosis factor-α gene, with chemoradiotherapy in locally advanced esophageal cancer.
Objectives
To assess pathologic complete response (pCR), time to disease progression, progression-free survival, survival, and safety and tolerance in patients treated with preoperative chemoradiation combined with endoscopy or EUS-guided intratumoral injection of TNFerade biologic.
Design/Intervention
Five weekly injections of TNFerade biologic, dose-escalated logarithmically from 4 × 108 to 4 × 1011 particle units (PU), were given in combination with cisplatin 75 mg/m2 and intravenous 5-fluorouracil 1000 mg/m2/d for 96 hours on days 1 and 29, and concurrent radiation therapy to 45 Gy. Surgery was performed 9 to 15 weeks after treatment.
Setting
U.S. multicenter study.
Patients
Patients with stage II and III esophageal cancer were enrolled.
Main Outcome Measurements
Primary outcome measures were safety, feasibility, tolerability, and rate of pCR. Secondary outcome measures were overall survival (OS) and disease-free survival.
Results
Twenty-four patients with a median age of 61 years were enrolled; 88% of the patients were men, 21% were stage II, and 79% were stage III. Six (29%) had a pCR, observed among 21 patients (20 who underwent esophagectomy and 1 at autopsy). Dose-limiting toxicities were not observed. The most frequent potentially related adverse events were fatigue (54%), fever (38%), nausea (29%), vomiting (21%), esophagitis (21%), and chills (21%). At the top dose of 4 × 1011 PU, thromboembolic events developed in 5 of 8 patients. The median OS was 47.8 months. The 3-and 5-year OS rates and disease-free survival rates were 54% and 41% and 38% and 38%, respectively.
Limitations
We included primarily adenocarcinoma.
Conclusions
Preoperative TNFerade, in combination with chemoradiotherapy, is active and safe at doses up to 4 × 1010 PU and is associated with long survival. This regimen warrants additional studies. (Clinical trial registration number: NCT00051480.)
In 2010, approximately 16,640 patients received a diagnosis of esophageal cancer in the United States. The prognosis is poor because only approximately 13% of all patients will survive at 5 years after the diagnosis, despite modern medical treatment.1 The high lethality of esophageal cancer, including those that are considered locally advanced, underscores the need for new, safe, and effective strategies for its prevention and treatment.
Tumor necrosis factor-α (TNF-α) is a soluble cytokine and mediator of the cellular immune response with potent anticancer activities.2 Its anticancer activities occur through a number of mechanisms including apoptosis, necrosis, antiangiogenesis, immunomodulation, and direct antitumor toxicity. TNF-α has potent cytotoxic properties, predominantly through the production of hydroxyl radicals, which, in turn, lead to DNA damage.3,4 Because radiation therapy (RT) also produces cell damage by free radical formation, a synergistic interaction between TNF-α and RT is conceivable. However, TNF-α cannot be infused systemically because it causes severe toxic effects. Thus, targeted administration of TNF-α to cancer cells is necessary.
The use of a replication-deficient adenovirus that has TNF-α complementary DNA ligated to a radiation-inducible promoter gene incorporated into its genome holds promise as a new way to selectively deliver TNF-α into cancer cells (Online Fig. 1, available online at www.giejournal.org). Direct injection of the adenovirus into solid tumor and subsequent activation of the TNF-α gene by RT provides spatial and temporal control of the expression of TNF-α in cancer cells.5 TNFerade biologic has been tested, with and without RT, against tumor xeno-grafts of Seg-1 human esophageal adenocarcinoma cells.6 Radiation leads to a fivefold increase in TNF-α levels. It also has been studied in combination with chemoradiotherapy to treat patients with a variety of solid tumors. An important property of adenovirus-based delivery of TNF-α is the observation that adenovectors traffic through the lymphatics.7,8 The transfer of the TNF-α gene to regional lymph nodes may serve to eliminate or control metastases to regional lymph nodes. Regional lymph node metastases are common in esophageal cancer and thus are typically inside the radiation port. We hypothesized that preoperative TNFerade biologic plus chemoradiotherapy could be an active regimen against esophageal cancer. Herein, we report on a multicenter phase I trial in patients with locally advanced cancer of the esophagus or the gastroesophageal junction.
METHODS
Eligibility
We initiated a multicenter phase I trial to evaluate pre-operative chemoradiation plus TNFerade biologic before esophagectomy for locally advanced but resectable esophageal cancer (cancers of the junction were classified as esophageal). The study was a dose escalation phase I study designed to identify the maximum tolerated dose of TNFerade biologic, suitable to take into a larger phase II clinical trial.
The eligibility criteria included previously untreated patients, aged 18 to 75 years, who had stage II or III, resectable, histologically confirmed adenocarcinoma or squamous cell carcinoma of the esophagus. Patients were required to have a Karnofsky performance status score of 70 or higher and a life expectancy longer than 6 months. Patients were ineligible for this study if there was suspicion of aortic, tracheal, or major vessel involvement, including tumor abutting or extending into these structures, and extensive locoregional disease. In addition to standard radiology studies, all patients underwent EUS to determine local staging. Patients were excluded if they had a history of malignancies within the past 2 years except carcinoma in situ, esophageal cancer extending beyond 2 cm into the stomach, liver function test results greater than 2 times the upper limit of normal, coagulopathy with an international normalized ratio >1.5, renal insufficiency, significant anemia, thrombocytopenia or leucopenia, a contraindication to endoscopy including obstructive lesions that could not be dilated to pass the endoscope, or clinical evidence of active infection of any type or were pregnant or lactating, were taking experimental medications within the 4 weeks before day 1 of treatment, were on long-term systemic corticosteroid use, or had significant concurrent medical or psychiatric illness. All patients were required to provide written, informed consent according to institutional and federal guidelines.
Pretreatment evaluation
Before treatment, patients were evaluated by their medical history, physical examination, and laboratory testing. Baseline tumor staging was performed by using CT of the chest and abdomen and EUS with FNA of suspicious periesophageal lymph nodes when feasible. Lymph node biopsy was required for any suspected nodes outside the radiation field. Bronchoscopy was required to exclude tracheoesophageal fistula or invasion if the cancer was located less than 26 cm from the incisors. A bone scan was considered if the serum alkaline phosphatase level was 1.5 or more times the upper limit of normal. In addition to standard laboratory studies, blood tests to determine infection with hepatitis B and C virus were done. Throat swabs and urine samples were obtained for adenovirus cultures. Neutralizing antibody titer for adenovirus was also drawn.
Treatment plan
TNFerade biologic injections were started concurrently with chemoradiotherapy with 5-fluorouracil (5-FU) and cisplatin administered according to the schedule (Online Fig. 2, available online at www.giejournal.org). Based on previous animal and early phase I data, the starting dose of TNFerade biologic was 4 × 108 particle units (PU), and dose escalation was performed in 1-log increments until the maximum tolerated dose or 4 × 1011 PU dose was reached. Successive dosing cohorts received increasing doses of TNFerade biologic. There was no intrapatient dose escalation. Five intratumoral injections of TNFerade biologic were administered weekly by either EUS-guided fine needle injection (FNI) or direct endoscopic injection, per study site preference, during a 5-1/2–week course of chemoradiotherapy. The injection technique was performed as follows.
For endoscopic injection, a standard sclerotherapy needle was used. For EUS-guided FNI, a standard 22-gauge FNA needle (EchoTip; Cook Medical, Bloomington, Ind) was used. A total of 2-mL volume of TNFerade biologic was administered during each session, with the allowance of dividing the dose into as many as 4 separate sites of injection at the discretion of the investigator based on the anatomy of the tumor. For cicatricial lesions, injections were placed at the middle of the tumor at the point of maximal lumen obstruction. Injections were placed in the transverse plane at 12-, 3-, 6-, and 9-o’clock positions. Attempts were made to deliver the vector deep within the tumor, which was more precisely accomplished under EUS guidance. Patients were given prophylactic antibiotics (250–500 mg of ciprofloxacin orally or by intravenous piggyback 2 to 3 hours before the procedure and 12 and 24 hours after the procedure). With each subsequent injection, the investigator attempted to treat different areas of the tumor.
RT was administered in 1.8-Gy daily fractions for a total dose of 45 Gy. Chemotherapy consisted of a cisplatin/5-FU regimen with cisplatin 75 mg/m2 on days 1 and 29 and 5-FU 1000 mg/m2/d via continuous intravenous infusion for 96 hours on days 1 and 29. Both 5-FU and cisplatin were initiated within 24 hours of RT on days 1 and 29.
Patients were restaged with chest and abdominal CT scans and liver function tests 7 to 14 days before esophagectomy, which were scheduled within 4 to 10 weeks after completion of radiation. Patients who were identified to have local invasion rendering the lesion unresectable or metastatic disease preoperatively were excluded from surgery.
Determination of the maximum tolerated dose
The National Cancer Institute Common Toxicity Criteria were used. The dose-limiting toxicity (DLT) was defined by the following criteria. (1) Any grade 3 or higher non-hematologic toxicity (except alopecia, nausea, vomiting, and diarrhea) possibly, probably, or definitely related to the combination of TNFerade biologic and chemoradiotherapy occurring during the combined modality treatment period through 2 weeks after the last administration of TNFerade biologic. Esophagitis was not considered a DLT unless grade 4. (2) The occurrence of grade 4 thrombocytopenia, neutropenia, or anemia during the combined modality treatment period through 2 weeks after the last administration of TNFerade biologic. (3) The occurrence of grade 3 or higher nausea, vomiting, or diarrhea despite maximal antiemetic and/or antidiarrheal agents in the combined modality treatment period through 2 weeks after the last administration of TNFerade biologic.
Surgery
Four to 10 weeks after chemoradiotherapy was completed, patients underwent esophagectomy. Patients needed adequate bone marrow recovery, defined as a white blood cell count of WBC 3000/μL or more, neutrophil count of 1500/μL or more, and platelet count of 100,000/μL or more for surgery to be performed. Surgery could be delayed beyond 10 weeks if the clinician thought that the patient’s physiologic status would not tolerate surgery immediately after chemoradiotherapy. Patients were restaged with chest and abdominal CT scans and repeat liver function tests 7 to 14 days before esophagectomy.
Follow-up of patients
A follow-up visit was required quarterly after enrollment for the first year and then semiannually. Standard clinical, laboratory function tests and CT scans (at 6 and 12 months after enrollment) were performed. Telephone calls were made semiannually for 5 years and will be made annually for a total of 15 years. The U.S. Food and Drug Administration requires follow-up of patients receiving a gene vector for 5 years.
Statistical considerations
Appropriate descriptive statistics were used for patient demographics, baseline variables, and all on-study safety and efficacy variables. Patient data were summarized by counts and percentages for categorical data and by medians and ranges for quantitative data. Additional analyses were performed to evaluate other potential differences. The Kaplan-Meier product-moment estimation technique was used to estimate the survival distributions with the Brookmeyer-Crowley method used to estimate the 95% confidence intervals for the medians (SAS version 9.2; SAS Institute, Cary, NC).
The primary outcome measures of the study were safety, feasibility, tolerability, and pCR at time of esophagectomy. pCR was defined as a total lack of tumor tissue after resection in the resected specimen. The secondary outcome measures were overall survival (OS) and disease-free survival (DFS). The following definitions were used. For all patients, OS was calculated from the day of enrollment to the time of death. Patients who did not die were censored at the date that they were last known to be alive. DFS was calculated as the difference between the date of enrollment and the date of objective tumor progression or death. Median survival and percentage of survival at 1, 2, 3, and 5 years were assessed for both OS and DFS. Because it was not possible to conduct this phase of the trial according to its intended experimental design, all statistical results should be taken as descriptive.
RESULTS
Demographic characteristics
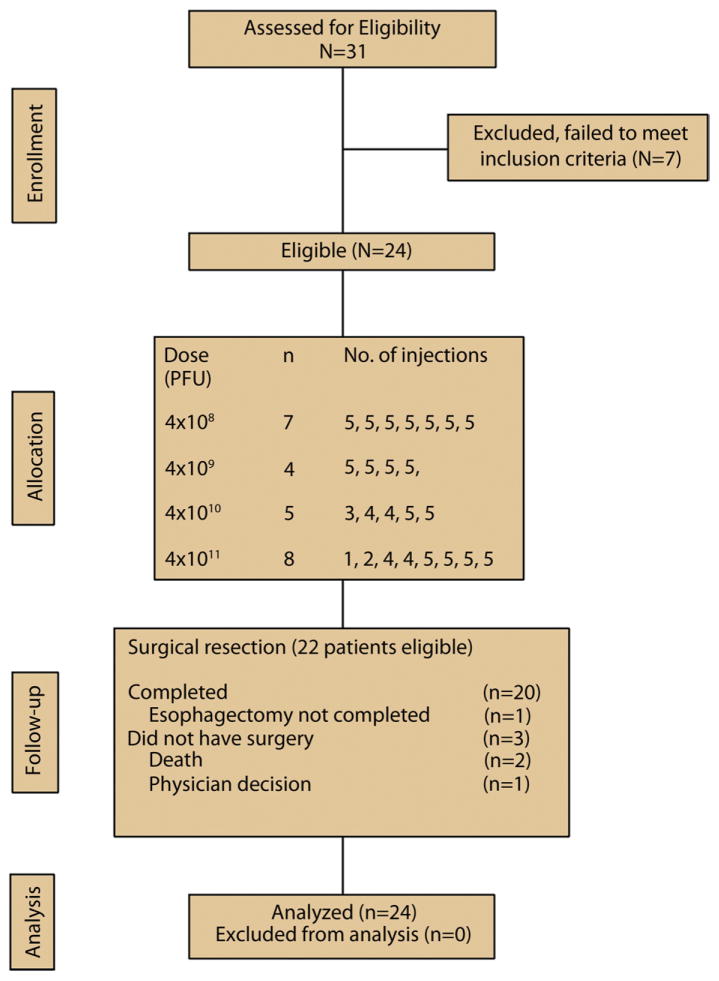
Twenty-four patients were enrolled from November 2002 to August 2004 at 7 centers across the United States (range 1–8 patients per center) (Fig. 1). The median age was 61 years (range 22–70 years) and 21 of 24 patients (88%) were men (Table 1). The median Karnofsky performance status score was 90 (range 80–100). Twenty patients (83%) had adenocarcinoma and 4 had squamous cell carcinoma. Twenty lesions (83%) were located in the gastroesophageal junction/distal esophagus, 3 (13%) were located in the mid part of the esophagus, and 1 (4%) was located in the proximal esophagus. All patients had either stage II (21%) or III (79%) disease.
Figure 1.
CONSORT diagram.
TABLE 1.
Patient characteristics (N = 24)
| Characteristic | No. of patients | % |
|---|---|---|
| Age, y | ||
| Median | 61 | |
| Range | 23–72 | |
| Sex | ||
| Male | 21 | 88 |
| Female | 3 | 12 |
| Karnofsky performance status score | ||
| Median | 90 | |
| Range | 80–100 | |
| Histology | ||
| Adenocarcinoma | 20 | 83 |
| Squamous cell carcinoma | 4 | 17 |
| Location of primary tumor | ||
| Distal esophagus/gastroesophageal junction | 20 | 83 |
| Middle esophagus | 3 | 13 |
| Proximal esophagus | 1 | 4 |
| Stage | ||
| II | 5 | 21 |
| III | 19 | 79 |
Treatment received
Injection of TNFerade biologic was put on hold in October 2004 because of concern over associated thromboembolic events. At the time when the hold was instituted, not all enrolled patients had completed the total planned number of TNFerade biologic injections. The study was closed to enrollment in May 2005.
The median follow-up was 43 months (range 1.4–77 months). Seventeen patients (71%) completed all of the planned 5 TNFerade biologic injections, 4 patients received 4 injections, and 1 patient each received 3, 2, and 1 injection(s). The reasons for incomplete dosing included the following. One patient each missed a single injection because of the clinical hold, inadequate volume, neutropenia, and no information given. One patient missed 2 injections because of fever. One patient each missed 3 and 4 injections, but the reasons were not provided. TNFerade biologic injections were administered via direct endoscopic visualization in 19 patients (Online Fig. 3, available online at www.giejournal.org), whereas the remaining 5 patients received TNFerade biologic via EUS-guided fine needle injection (Online Fig. 4, available online at www.giejournal.org).
Treatment tolerance and toxicity
There were no protocol-defined DLTs. TNFerade biologic in combination with chemoradiation was generally well tolerated at the lower dose levels of 4 × 108 to 4 × 1010 PU. The most frequently reported adverse events were nausea (83%), fatigue (75%), vomiting (63%), mucosal inflammation (58%), diarrhea (54%), fever (50%), dehydration (46%), anorexia (42%), and dysphagia (42%) (Online Table 1, available online at www.giejournal.org). The most frequent adverse events judged to be potentially related to TNFerade biologic were fatigue (54%), fever (38%), nausea (29%), esophagitis (21%), vomiting (21%), and chills (21%).
In total, thrombotic/thromboembolic events (TEs) developed in 8 of 24 patients (Table 2). Two patients experienced TEs during active treatment, whereas the TEs in the remaining 6 patients occurred after the last injection of TNFerade (range 2–213 weeks). At the top dose of 4 × 1011 PU, TEs were noted in 5 of 8 patients (63%) compared with only 3 of 16 patients (19%) at the lower doses of 4 × 108 to 4 × 1010 PU. Although the majority of patients recovered from the TEs, 2 patients died as an outcome. One of these TE-related deaths occurred 213 weeks after treatment, whereas the other occurred only 2 weeks after last injection.
TABLE 2.
Summary of thrombotic/thromboembolic events
| Patient | Cohort | Event | Timing of event | Outcome of event |
|---|---|---|---|---|
| 1 | 4 × 108 | Subclavian-jugular (related to central line) and PE (post-resection; surgical complications | Post-Rx wk 6 | Recovered |
| 2 | 4 × 1010 | Clotted port/lower extremity DVT | Post-Rx wk 29 | Recovered |
| 3 | 4 × 1010 | Bilateral PE | Post-Rx wk 27 | Recovered |
| 4 | 4 × 1011 | PE | Post-Rx wk 213 | Death |
| 5 | 4 × 1011 | Multiple PEs | Post-Rx wk 6 | Ongoing at time of death |
| 6 | 4 × 1011 | Lower extremity DVT/multiple PE’s | During Rx wk 6 | Recovered |
| 7 | 4 × 1011 | PE | During Rx wk 2 | Recovered |
| 8 | 4 × 1011 | Clotted port/PE | Post Rx wk 2 | Death |
PE, Pulmonary embolism; Post-Rx, after last injection of TNFerade; DVT, deep venous thrombosis; During Rx, during active treatment.
There were 3 deaths during the course and follow-up of the study caused by adverse events. In addition to the 2 TE-related deaths, 1 patient died of exsanguination caused by an esophageal fistula 7 weeks after completion of treatment.
Twenty-two patients were eligible for esophagectomy. One patient did not have surgery because of a clinician decision. Of the 21 patients who underwent esophagectomy, the surgery in 1 patient had to be terminated because the tumor was not fully resectable. In this patient, the pathologic response rate could not be determined.
Efficacy: tumor response
We assessed the pathologic response of 21 patients: 20 patients who had esophagectomy and 1 autopsy of a patient who died of a pulmonary embolism before surgery. A pCR was observed in 6 patients (29%) including the patient who died. Of the 6 pCR tumors, 2 were squamous cell carcinoma and 4 were adenocarcinoma (P = not significant). All 6 were staged as T3N1 at baseline. In addition to these pCRs, there were 2 other patients who did not have surgical resection, but were still alive at 68 and 73 months after enrollment.
OS and DFS
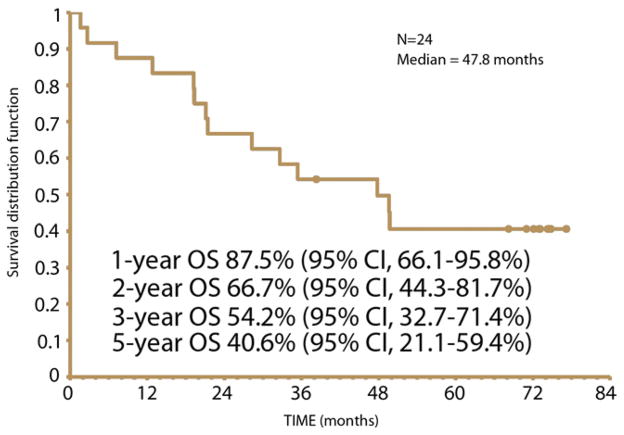
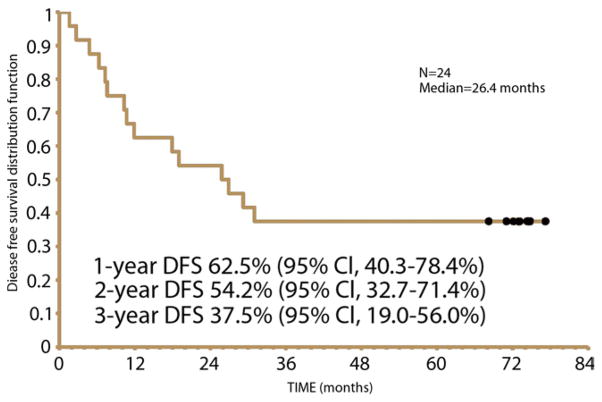
Survival analysis was conducted on all 24 patients. The median OS was 47.8 months (95% CI, 21.1 to nonestimatable months [the upper limit is not estimatable]). The 1-, 2-, 3-, and 5-year OS rates were 88%, 67%, 54%, and 41%, respectively (Fig. 2). The median DFS was 26.4 months (95% CI, 10.3 to nonestimatable months [the upper limit is not estimatable]). The 1-, 2-, and 3-year DFS rates were 63%, 54%, and 38%, respectively (Fig. 3).
Figure 2.
Overall survival (OS).
Figure 3.
Disease-free survival (DFS).
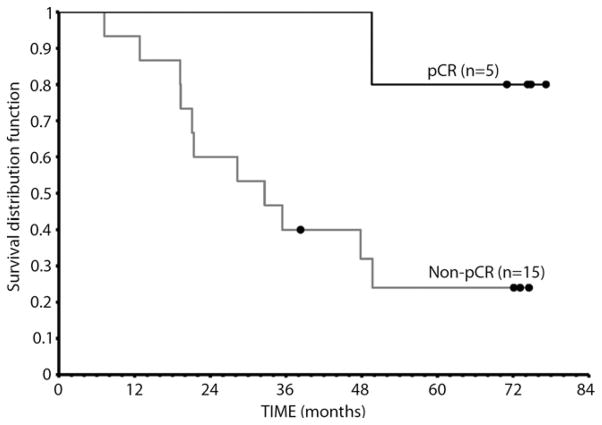
We performed 2 exploratory OS analyses stratified according to (1) dose of TNFerade biologic (Fig. 4) and (2) pathologic response rates among 20 patients who underwent esophagectomy (Fig. 5). All 5 patients who had a pCR at esophagectomy were long-term disease-free survivors.
Figure 4.
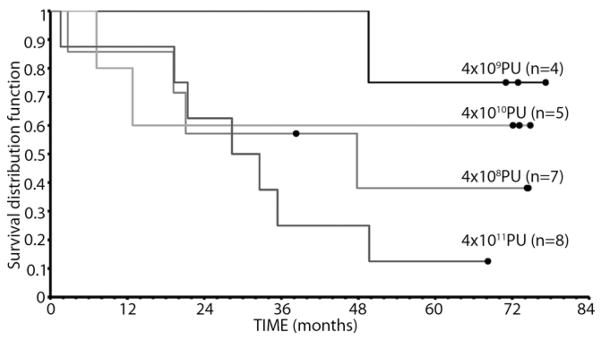
Overall survival, stratified according to dose of TNFerade biologic. PU, particle units.
Figure 5.
Overall survival, stratified according to complete pathologic response (cPR) among patients who underwent esophagectomy.
DISCUSSION
Our study provides supporting evidence that endoscopic or EUS-guided injection of as much as 4 × 1010 PU replication-deficient adenovirus serotype 5 vector (TNFerade biologic) to deliver TNF-α directly into esophageal cancer seems to be active and reasonably well tolerated. The top dose of 4 × 1011 PU was associated with thromboembolic complications and should probably be avoided in patients with esophageal cancer who receive concomitant treatment with chemoradiotherapy. The study also indicated that the long-term outcomes of patients treated with TNFerade biologic, followed by concurrent RT and systemic intravenous administration of 5-FU and cisplatin are quite promising.
A median OS and DFS of 47.8 months and 26.4 months, respectively, are remarkable in light of the current literature. A recent study by Spigel et al9 was reported in which preoperative oxaliplatin, docetaxel, and capecitabine with concurrent RT were used in localized esophageal or gastroesophageal junction carcinoma that showed median survivals of less than 24.1 months. Other previous studies have reported similar limited survival data.10
Data concerning the prevalence of and outcomes related to TEs led to the study being put on hold in 2004. In this study, we observed that there was a tendency toward a higher incidence of TEs at the top dose of TNFerade biologic at 4 × 1011 PU. It is conceivable that, in susceptible patients, this high dose of TNFerade biologic and chemoradiation, increases the risk of these events. Since that time, new information has been published to suggest that cisplatin is highly thrombogenic.11 A prospective, exploratory analysis of TEs in a randomized, controlled trial involving 964 patients with 4 different regimens identified cisplatin as an independent risk factor (hazard ratio 0.51; 95% CI, 0.34–0.76; P = .01).11
Other factors may also contribute to TEs in patients with esophageal cancer. In this study, patients underwent a weekly endoscopy, which might in some cases have caused dehydration. Dehydration is a known pathophysiologic mechanism underlying the hypercoagulability of malignancies.12 The standard practice for preparation for endoscopy at the time of this study was for patients to fast starting at midnight before the procedure. After endoscopy, many patients might have also reduced oral intake because of the sedation that they received during the endoscopic procedure. They also might have had decreased mobility. Most of the patients included in this study had adenocarcinoma, which predisposed them to mucin-related coagulopathy.12 Although there may be significant concern in prescribing low-molecular weight heparin for prophylaxis against thromboembolism in patients with esophageal cancers, improvement in hydration and mobility can be beneficial in future studies. Recent guidelines from the American Society of Anesthesiologists permit clear fluid intake up to 2 hours before an upper endoscopy instead of stopping fluid intake at midnight.13
Our multicenter study has limitations. By its nature, this phase I study included a limited number of patients. Therefore, many questions were left unanswered. The study, however, does suggest that endoscopically delivered TNFerade biologic, with concurrent RT and systemic intravenous administration of 5-FU and cisplatin, seems to be active and a relatively safe preoperative regimen for esophageal cancer at doses as high as 4 × 1010 PU. Given the poor prognosis of patients with locally advanced disease and the promising survival data, this regimen warrants additional randomized studies with comparison with standard neoadjuvant therapy.
Supplementary Material
Take-home Message.
TNFerade biologic injected directly into the tumor via endoscopy or EUS, with concurrent radiation therapy and systemic intravenous administration of 5-fluorouracil and cisplatin, seems to be active and a relatively safe preoperative regimen for esophageal cancer at doses as high as 4 × 1010 PU.
Given the poor prognosis of patients with locally advanced disease and the promising survival data, this regimen warrants additional studies.
Acknowledgments
We thank the following: Randall F. Holcombe, MD, Eric H. Radany, MD, Phuong T Nguyen, MD, John G. Lee, MD, Misagh Karimi, MD, Ninh Nguyen, MD, Jeffrey Kuo, MD, Saihong Ignatius Ou, MD from the UC Irvine; Tonya Kaltenbach, MD, Jon Kosek, MD, Robert Rouse, MD, Shai Friedland, MD and Albert C. Koong, MD, PhD, Lynne Dempsey, RN and Sherry Wren, MD from VA Palo Alto Health Care System and Stanford University; Jaffer Ajani, MD, Ritsuko Komaki, MD, Robert Scott Bresalier, MD, Joe Y. Chang, MD, PhD, Thomas M. Guerrero, MD, PhD, Linus Ho, MD, PhD, Melenda D. Jeter, MD, MPh, Zhonxing Liao, MD, Patrick M. Lynch, JD, MD, Joe B. Putnam, Jr, MD, David Christopher Rice, MD, Jack A. Roth, MD, Craig W. Stevens, MD, PhD, William Roy Smythe, MD, Ara A. Vaporciyan, MD, Garrett Lyndon Walsh, MD, James C. Yao, MD, Alexander A Dekovich, MD, Tsung-Teh Wu, MD, PhD from MD Anderson Cancer Center; Charles Casey Cunningham, MD William J. Hyman, MD Svetislava J. Vukelja, MD Frank T. Ward, MD, Richard Seidel, MD George A. DuVall, MD Damien Mallat, MD Robert F. Hebeler, Jr., MD John Kent Hamilton, MD Blair Conner, MD, John J. Nemunaitis, MD, Donald A. Richards, MD from US Oncology; Everett Vokes, MD, Mitchell Posner, MD, Irving Waxman, MD, Daniel Haraf, MD, Angela Bradbury, MD, Ok-Kyong Chaekal, MD, Philip Connell, MD, Nancy B. Davis, MD, Gregory Friberg, MD, Mark Ferguson, MD, Harvey Golomb, MD, Melinda Gordon, MD, Stacy Gray, MD, Supriya Gupta, MD, Michael Hall, MD, Philip Hoffman, MD, Stuart Krauss, MD, Charles Rudin, MD, Peter Tothy, MD, John Villano, MD, Jerome Winegarden, MD from the University of Chicago; Bernard Boulanger, MD, Paul Kearney, MD, Richard Schwartz, MD, Andrew Bernard, MD, Stephen Barnes, MD from the University of Kentucky; Ananya Das, MD, Afshin Dowlati, MD Julie A. Clayman, MD, John Greskovich, Jr., MD, Gerard Ashton Isenberg, MD, Timothy James Kinsella, MD, Nathan Levitan, MD, Michael V. Sivak, Jr., MD, Richard C. K. Wong, MD from the University Hospitals of Cleveland/Case University for their participation.
Abbreviations
- DFS
disease-free survival
- DLT
dose-limiting toxicity
- 5-FU
5-fluorouracil
- OS
overall survival
- pCR
pathologic complete response
- PU
particle units
- RT
radiation therapy
- TE
thrombotic/thromboembolic event
- TNF-α
tumor necrosis factor-α
Footnotes
DISCLOSURE: The following authors disclosed financial relationships relevant to this publication: Dr. Chang, consultant to GenVec, Inc; Dr. Pinto, grant from and consultant to Bristol-Myers Squibb and Genentech; grant from Amgen; paid lecturer for Imclone; consultant to Boehringer Ingelheim and TIBA Oncology. All other authors disclosed no financial relationships relevant to this publication. All financial and material support for this clinical trial was provided by GenVec, Inc, who was responsible for the design and conduct of the study and collection, management, review, and approval of the manuscript.
References
- 1.Kim T, Grobmyer SR, Smith R, et al. Esophageal cancer-the five year survivors. J Surg Oncol. 2011;103:179–83. doi: 10.1002/jso.21784. [DOI] [PubMed] [Google Scholar]
- 2.Old LJ. Tumor necrosis factor (TNF) Science. 1985;230:630–2. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- 3.Yamauchi N, Kuriyama H, Watanabe N, et al. Intracellular hydroxyl radical production induced by recombinant human tumor necrosis factor and its implication in the killing of tumor cells in vitro. Cancer Res. 1989;49:1671–5. [PubMed] [Google Scholar]
- 4.Larrick JW, Wright SC. Cytotoxic mechanism of tumor necrosis factor-alpha. FASEB J. 1990;4:3215–23. doi: 10.1096/fasebj.4.14.2172061. [DOI] [PubMed] [Google Scholar]
- 5.Hallahan DE, Mauceri HJ, Seung LP, et al. Spatial and temporal control of gene therapy using ionizing radiation. Nat Med. 1995;1:786–91. doi: 10.1038/nm0895-786. [DOI] [PubMed] [Google Scholar]
- 6.Gupta VK, Park JO, Jaskowiak NT, et al. Combined gene therapy and ionizing radiation is a novel approach to treat human esophageal adenocarcinoma. Ann Surg Oncol. 2002;9:500–4. doi: 10.1007/BF02557275. [DOI] [PubMed] [Google Scholar]
- 7.Korst RJ, Ailawadi M, Lee JM, et al. Adenovirus gene transfer vectors inhibit growth of lymphatic tumor metastases independent of a therapeutic transgene. Hum Gene Ther. 2001;12:1639–49. doi: 10.1089/10430340152528138. [DOI] [PubMed] [Google Scholar]
- 8.Labow D, Lee S, Ginsberg RJ, et al. Adenovirus vector-mediated gene transfer to regional lymph nodes. Hum Gene Ther. 2000;11:759–69. doi: 10.1089/10430340050015653. [DOI] [PubMed] [Google Scholar]
- 9.Spigel DR, Greco FA, Meluch AA, et al. Phase I/II trial of preoperative oxaliplatin, docetaxel, and capecitabine with concurrent radiation therapy in localized carcinoma of the esophagus or gastroesophageal junction. J Clin Oncol. 2010;28:2213–9. doi: 10.1200/JCO.2009.24.8773. [DOI] [PubMed] [Google Scholar]
- 10.Greer SE, Goodney PP, Sutton JE, et al. Neoadjuvant chemoradiotherapy for esophageal carcinoma: a meta-analysis. Surgery. 2005;137:172–7. doi: 10.1016/j.surg.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 11.Starling N, Rao S, Cunningham D, et al. Thromboembolism in patients with advanced gastroesophageal cancer treated with anthracycline, platinum, and fluoropyrimidine combination chemotherapy: a report from the UK National Cancer Research Institute Upper Gastrointestinal Clinical Studies Group. J Clin Oncol. 2009;27:3786–93. doi: 10.1200/JCO.2008.19.4274. [DOI] [PubMed] [Google Scholar]
- 12.Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110:1723–9. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology. 1999;90:896–905. doi: 10.1097/00000542-199903000-00034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.