Abstract
The objective of this study was to develop caffeine citrate orally disintegrating tablet (ODT) formulations utilizing hot-melt extrusion technology and evaluate the ability of the formulation composition to mask the unpleasant bitter taste of the drug using in vitro and in vivo methods. Ethylcellulose, along with a suitable plasticizer, was used as a polymeric carrier. Pore forming agents were incorporated into the extruded matrix to enhance drug release. A modified screw configuration was applied to improve the extrusion processability and to preserve the crystallinity of the API. The milled extrudates were subjected to dissolution testing in an artificial salivary fluid and investigations using e-tongue, to assess the extent of masking of bitter taste of the API. There was an insignificant amount of drug released from the formulation in the salivary medium while over 80% of drug released within 30 min in 0.1 N HCl. ODTs were also developed with the extrudate mixed with mannitol and crospovidone. The quality properties such as friability and disintegration time of the ODTs met the USP specifications. The lead extrudate formulations and the ODTs prepared using this formulation were subjected to human gustatory evaluation. The formulations were found to mask the unpleasant taste of caffeine citrate significantly.
Keywords: Hot-melt extrusion, Taste-masking, pediatric and geriatric, Modified screw design, Ethylcellulose (EC)
1. INTRODUCTION
Some active pharmaceutical ingredients (API’s) are generally associated with unpleasant taste. The formulations containing such APIs are poorly accepted by patients and the adherence to treatment is adversely affected. Bad taste is a primary barrier while administrating drugs to children. For example, more than 90% of pediatricians reported that the taste and palatability were the greatest hurdles to complete treatment (Mennella et al., 2013). Therefore, it is necessary to discover robust approaches to formulate the dosage forms to mask the unpleasant taste of the API to improve the ease of administration and palatability (Jacqz-Aigrain and Choonara, 2006).
Traditionally, liquid type oral dosage forms incorporated with excess amounts of sweeteners is one of the common approaches to mask the unpleasant taste of APIs, particularly in pediatric medications. However, this type of formulation is associated with various shortcomings such as physical and chemical stability, use of alcohol and dose variability (Niazi, 2004). A World Health Organization (WHO) working document recommends refraining from incorporating coloring and flavoring agents in pediatric dosage forms (Susan and Walters, 2011). Thus, solid oral dosage forms become one of the more attractive choices for pediatricians and care takers as the usage of these excipients is less required in this case. Moreover, solid oral dosage forms tend to exhibit improved storage and transportation qualities in addition to more accurate dose precision (Strickley et al., 2008). However, in the context of pediatric populations, conventional tablets may be difficult to swallow and, therefore, present choking hazards. ODTs are a solid oral dosage form, which disintegrates very rapidly, typically within 30 seconds, with or without the administration of additional water. These are particularly convenient for people suffering from dysphagia, stroke, thyroid disorders, Parkinson’s disease, multiple sclerosis and cerebral palsy (Badgujar and Mundada, 2011).
Some common techniques for the development of taste masked solid dosage formulations are complexation (Dinge and Nagarsenker, 2008; Mahesh et al., 2010), freeze-drying (Seager, 1998), microencapsulation (Al-Omran et al., 2002; Shah et al., 2008a), fluidized-bed coating (Behzadi et al., 2008; Hamashita et al., 2008) and supercritical fluids (Benoit et al., 2000; Hanna and York, 2006). Recently, HME has demonstrated its utility in the development of taste masked formulations (Gryczke et al., 2011; Maniruzzaman et al., 2012a) by using a physical barrier between bitter drugs and taste receptors. Hot-melt extrusion (HME) is a viable technology for processing pharmaceutical formulations as it is potentially a single step and continuous process, which helps to decrease the formulation steps. HME has become a well established pharmaceutical processing technique over the last two decades. It is primarily employed for solubility improvement, but has also shown considerable utility for various usages such as controlled release formulations and targeted drug delivery including taste masking systems (Maniruzzaman et al., 2012b; Maniruzzaman et al., 2013).
HME processing involves the physical mixing of a formulation at elevated temperatures, and pressure(s) while also being under high shear. Nakamichi et al demonstrated that the kneading paddles play an important role in changing the crystallinity and dissolution characteristics of a solid dispersion (Nakamichi et al., 2002). Formulations containing a poorly soluble API require more aggressive screw designs, which include multiple mixing zones along with other screw elements (Figure. 1a). This design tends to impart improved solubility, which translates to improved bioavailability for BCS II APIs (Deng et al., 2013; Mohammed et al., 2012). However, the specific screw design is dictated by the particular properties that are desired in the final product, which is determined on a case-by-case basis. The physical transformation of the drug from the crystalline to the amorphous state is responsible for the improvement in dissolution. However, sometimes it is favorable to retain the crystalline form to avoid excess release of a highly soluble drug in short time periods for controlled release formulations or improvement in the stability of BCS class II drug solid dispersions (Reitz et al., 2012).
Figure 1.
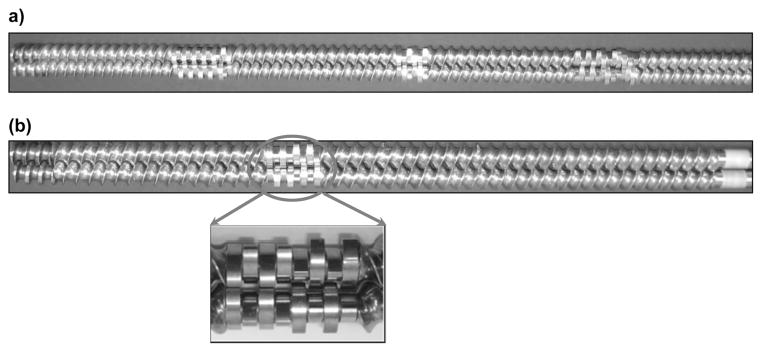
Twin screw extruder screws; (a) for high shear, (b) for low shear and its magnified image of the kneading zone.
Caffeine citrate is a bitter, white crystalline and highly water soluble API, which is classified as a BCS class I drug (Wu and Benet, 2005). Caffeine citrate is a central nervous stimulant and helps to restore alertness. Pharmaceutically, caffeine citrate is used in the treatment of apnea in newborns; also it is the component of many of the analgesic formulations, where it demonstrates a synergistic effect with those analgesics (Comer et al., 2001; Sawynok, 1995).
The main purpose of this study was to develop a robust formulation to mask the bitter taste of a model API, caffeine citrate, by a hot-melt extrusion process and evaluate the taste masked effects with in vitro and in vivo methods. In this work, the authors employed multiple techniques, including a human taste panel, dissolution and e-tongue analysis in a single study.
2. MATERIALS AND METHODS
2.1. MATERIAL
Caffeine Citrate (CC), Calcium Carbonate, Calcium Phosphate and Triethyl citrate (TEC) were purchased from Fisher Scientific (Pittsburgh PA, USA). Magnesium oxide USP light was ordered from PCCA (Houston, TX, USA). Aqualon™ Ethylcellulose N7 (EC) Polyplasdone™ (grades XL and XL-10) was supplied by Ashland Specialty Ingredients (Wilmington, DE, USA). Magnesium stearate was purchased from Spectrum Chemical Mfg. Corp (Gardena, CA, USA), Pearlitol® 50C-mannitol was supplied by Roquette America Inc (Keokuk, IA, USA).
2.2. Preparation of melt extrudate formulations
Caffeine citrate (20% w/w) was blended with EC, triethyl citrate (TEC) and with or without pore formers in an amount outlined in Table 1 using a V-shell blender (GlobePharma, Maxiblend, New Brunswick, NJ, USA) after passing through ASTM #30 mesh. The blends were melt-extruded using a co-rotating twin-screw extruder (11 mm Process 11, ThermoFisher Scientific, Pittsburgh, PA, USA) with a modified screw design at 50 rpm over a temperature range of 125–130°C. The extrudate was milled using a comminuting mill (Fitzpatrick, Model “L1A”, Elmhurst, IL, USA) and sieved through an ASTM # 40/35. The portion retained by an ASTM # 35 sieve was stored in foil lined polyethylene bags for further analysis and processing.
Table 1.
HME Formulations
| Formulations (%w/w) | CC1 (control) | CC2 | CC3 | CC4 | CC5 | CC6 | CC7 | CC8 | CC9 | CC10 | CC11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Caffeine Citrate | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| TEC | 5 | 5 | -- | -- | 10 | 10 | 5 | 5 | 5 | 5 | 5 |
| Stearic acid | -- | -- | 5 | 5 | -- | -- | -- | -- | -- | -- | -- |
| Mannitol | -- | 3 | -- | 3 | -- | 3 | 5 | 10 | -- | -- | -- |
| Calcium Carbonate | -- | -- | -- | -- | -- | -- | -- | -- | 15 | -- | -- |
| Magnesium Oxide | -- | -- | -- | -- | -- | -- | -- | -- | -- | 15 | -- |
| Calcium Phosphate | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 15 |
| Aqualon™ Ethyl Cellulose (ECN7) | 75 | 72 | 75 | 72 | 70 | 67 | 70 | 65 | 60 | 60 | 60 |
2.3. Thermogravimetric analysis (TGA)
Thermogravimetric analysis studies (Perkin Elmer Pyris 1, Shelton, CT, USA) were performed to estimate the thermal stability of the API and excipients during HME processing. The data was analyzed using Pyris software. The API excipients were heated from 30 – 200°C at 20°C/min.
2.4. Differential scanning calorimetry (DSC)
DSC studies were performed with a Perkin Elmer Diamond differential scanning calorimeter (DSC) equipped with Pyris software (Shelton, CT, USA). Samples were prepared by sealing 3–5 mg of pure API, physical mixtures and milled extrudates in hermetically sealed aluminum pans and heated from the temperature range of 30°C to 180°C at the heating rate of 20°C/min under an inert nitrogen atmosphere at a flow rate of 20 mL/min.
2.5. Fourier transforms infrared spectroscopy (FTIR)
FTIR spectra of the API, polymer, physical mixtures and milled extrudates were recorded using an Agilent Cary 660 FTIR spectrophotometer (Santa Clara, CA, USA) to investigate any possible interactions between the drug, polymer and other excipients.
2.6. Analytical method
A Waters High performance liquid chromatography (HPLC) system equipped with a Water 600 binary pump, Waters 2489 UV/detector, and Waters 717 plus autosampler (Waters Technologies Corporation, 34 Maple St., Milford, MA 0157) and a Phenomenex Luna 5um C18 (2) 250 × 4.6 mm column (Torrance, CA, USA) were used at a detection wavelength of 273 nm. The mobile phase consisted of Methanol and water at a ratio of 70:30 (v/v). The mobile phase flow rate was maintained at 1.0mL/min. and an injection volume of was 20 μL was used (O’connell and Zurzola, 1984). HPLC data was analyzed using Empower V. software (Milford, MA, USA).
2.7. In vitro dissolution studies
In vitro dissolution studies were performed by a modified dissolution method, as currently there are no regulatory (USP or FDA) guidelines available to evaluate taste masked formulations in salivary dissolution media. Briefly this study was conducted using 500 mL of salivary media with expeditious sampling points to mimic the oral environment and to minimize the handling and sampling error, which could arise in low volume salivary dissolution.
Dissolution studies were performed on the milled extrudates and ODTs using USP apparatus I (50 rpm) in 500mL of artificial salivary fluid adjusted to pH 6.8 for oral drug release (Azarmi et al., 2007) (Table 2). The samples were analyzed at 5 second intervals using a Rainbow Dynamic Dissolution Monitor® System powdered by Indigo™ software (Pion Inc., Billerica, MA, USA). Gastric drug release was assessed in 900 mL of 0.1 N HCl using USP apparatus I (50 rpm). The gastric release samples were analyzed by HPLC. In both instances the dissolution temperature was maintained at 37 ± 0.5°C using a Hanson SR8-Plus dissolution testing system.
Table 2.
Artificial saliva dissolution media (pH 6.8)
| Compound | Concentration (g/L) |
|---|---|
| CaCl2·2H2O | 0.228 |
| MgCl2·6H2O | 0.061 |
| NaCl | 1.017 |
|
| |
| K2CO3·1.5H2O | 0.603 |
| Na2HPO4·7H2O | 0.204 |
| NaH2PO4·H2O | 0.273 |
2.8. Evaluation using E-Tongue
An Astree e-tongue system equipped with an Alpha M.O.S sensor set # 2 (composed of 7 specific sensors, ZZ, AB, BA, BB, CA, DA, JE; Alpha MOS America, Hanover, MD, USA) was employed. The conditioning step was performed using 0.01 N HCl to hydrate the sensors and assess for noise or drift. After the instrument passed the conditioning step, the E-tongue was calibrated in 0.01 N HCl with reference to the manufacturer’s specified set of target values and margin of error (Siddiqui et al., 2013). After calibration, hydrochloric acid, sodium chloride and methyl sodium glutamate were utilized to check the discrimination of sour, salty and umami tests by the sensors.
Approximately 0.05 g of sample was dispersed in 50 mL of phosphate buffer solution (pH 6.8) and gently shaken for 30 seconds. The suspension was filtered with a 0.45 micron syringe filter (nylon membrane) into a 25 mL beaker and the acquisition time was set at 120 seconds (Maniruzzaman et al., 2012a; Siddiqui et al., 2013). Ten samples for each run were required per manufacturer’s guidelines.
2.9. Preparation of Orally Disintegrating Tablets (ODTs)
The extrudates prepared as discussed in Section 2.2 were blended with mannitol, used as a diluent, and Polyplasdone™ XL or XL-10, used as a disintegrant (Table 3) utilizing a V-shell blender. Magnesium stearate was added when two minutes of blending remained. The final blend was evaluated for bulk & tap density as well as moisture content.
Table 3.
Composition of ODT formulations
| ODT Formulations (% w/w) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 |
|---|---|---|---|---|---|---|---|---|
| CC2 Extrudates | 50 | 50 | -- | -- | -- | -- | -- | -- |
| CC9 Extrudates | -- | -- | 50 | 50 | -- | -- | -- | -- |
| CC10 Extrudates | -- | -- | -- | -- | 50 | 50 | -- | -- |
| CC11 Extrudates | -- | -- | -- | -- | -- | -- | 50 | 50 |
| Mannitol | 44.5 | 44.5 | 44.5 | 44.5 | 44.5 | 44.5 | 44.5 | 44.5 |
| Polyplasdone XL | 5 | -- | 5 | -- | 5 | -- | 5 | -- |
| Polyplasdone XL-10 | -- | 5 | -- | 5 | -- | 5 | -- | 5 |
| Magnesium Stearate | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
The ODTs were prepared by direct compression on a Globe tablet press (MCTMI, Globe Pharma Inc. (New Brunswick, NJ, USA) using 10 mm standard concave tooling at a compression force of 5–10 kN.
2.10. Evaluation of Compressibility
Carr’s Index (Compressibility Index I) and Hausner’s ratio of ODT formulation blends with magnesium stearate were calculated by measuring the tapped bulk and poured bulk volume of powders after subjecting to 100 taps in a graduated cylinder.
2.11. Quality of ODTs
Tablet hardness, weight variation and average thickness were measured using a Smart Test 50 (Sotax Corporation, Westborough, MA, USA) and disintegration time was evaluated using Disi Test 20 (Sotax Corporation, Westborough, MA, USA) disintegration tester filled with simulated salivary fluid which was thermally equilibrated to 37±0.5°C prior to testing. Friability studies were performed using a dual scooping projection Vankel type Drum (Model 10801, Vankel Industries Inc. Edison, NJ, USA) for 4 minutes at 25 rpm.
2.12. Human taste evaluation
Taste masking evaluation was performed at the Institute for Drug Delivery and Biomedical Research, Bangalore India (Protocol number VIPS/2013/12). The subjects were recruited after obtaining informed consent. The study is also in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
2.12.1. Human subject selection criteria
Nine human subjects belonging to either sex were recruited. They were asked to abstain from coffee/tea and other beverages for 12 hours. The subjects were only allowed to drink water for the 12 hours. Moreover, they were asked not to eat chocolate or other candy for over 6 hours. Inclusion criteria was healthy human subjects of age 18–42 and exclusion criteria was subjects suffering from fever, smokers, mouth ulcers, dry mouth, cold, nose block and wounds.
2.12.2. Data collection
Before data collection the subjects were asked to wash their mouth with ambient temperature water. The surface temperature of the tongue was recorded using an IR thermometer and difference of ±5°C compared to body temperature was considered an exclusion criteria.
2.12.2.1. Bitterness perception
The subjects were asked to taste aqueous solutions of CC starting from a very dilute solution escalating to higher concentrations by placing 2 ml of solution for 30 seconds on the tongue/buccal cavity. The concentrations screened were 0, 0.5, 1, 5, 10 and 40 mg. The volunteers were asked to report the following perception each time as 1- I feel bitter taste, 2- I feel something but cannot identify the taste, and 3-I do not feel the taste.
The subjects who answer 2 or 3 were asked to taste higher concentration solution until they expressed perception 1. This was recorded as the threshold for an individual. The individuals who reported a score of 1 at least 1/5th the drug concentration of the actual dose were only allowed to test the product.
Above the individual’s perception threshold, a few higher concentration API solutions were made for tasting by the subjects and they were subsequently asked to provide a score for each of the solutions (Table.4). The highest concentration of solution contained caffeine citrate equivalent to the dose present in the tested products. The scoring pattern was followed according to modified hedonic scale where 0- no taste, 1-threshold, 2-slightly bitter, 3-moderately bitter, 4-bitter and 5-strongly bitter.
2.12.2.2 Formulation evaluation and data analysis
A washout interval of 12–24 hours was allowed after screening the standard solution. The individuals were asked to taste the products (physical mixture, hot-melt extruded formulation or ODT) randomly (blinded) and asked to score the product. The products were wetted with water (0.5 ml) and placed on the tongue/buccal cavity for a duration of 30 – 40 seconds and asked to score the bitterness on a scale of 0–5 for each product. Sufficient washout time was allowed between the products and allowed to drink copious amounts of water after tasting each product.
The scores given by all individuals was averaged and expressed as mean standard deviation. The mean scores between the physical mixture and formulation was compared using a student t-test at 95% confidence level and P<0.05 was considered statistically significant.
Results and Discussion
3.1. Preparation of hot-melt extrudates utilizing modified screw configuration
Ethyl cellulose is a suitable polymer for HME due to its thermoplastic property, however it can be a challenge to process due to a thermally narrow processing window. EC has a high glass transition temperature (129–133°C), while thermal processing above 150°C induces slight color changes due to oxidation and degradation (Ashland, 2002). These limitations, a narrow processing window and hydrophobicity obstruct, can be resolved using processing and formulation approaches through low shear screw configurations, compatible plasticizers and pore forming agents.
EC, proved difficult to extrude using the standard screw configuration (Figure 1a) as a result of the three mixing zones therein. Additionally caffeine citrate is a highly soluble drug and the employment of this configuration resulted in the complete conversion of crystalline caffeine citrate to the amorphous form, which will result in rapid drug release in the oral cavity and further hinder taste masking efficiency. Therefore, for this study a modified screw design (Figure 1b), which could apply sufficient mixing without complete conversion of caffeine citrate into the amorphous phase, was utilized. Using a combination of approaches such as incorporation of plasticizer and a modified screw configuration, helped to extrude within a thermal processing window with very low torque, successfully producing white extrudates without any color change and nor degradation of API or polymer.
3.2. Physiochemical properties of hot-melt extrudates
DSC, TGA and FTIR were performed to determine the thermal stability at the extrusion conditions and the post extrusion physical characterization of the extrudates, respectively. The thermal stability of the drug, polymer and excipients was determined using TGA. All of the materials utilized for these studies showed no appreciable level of weight loss at the extrusion temperatures used (Figure 2).
Figure 2.
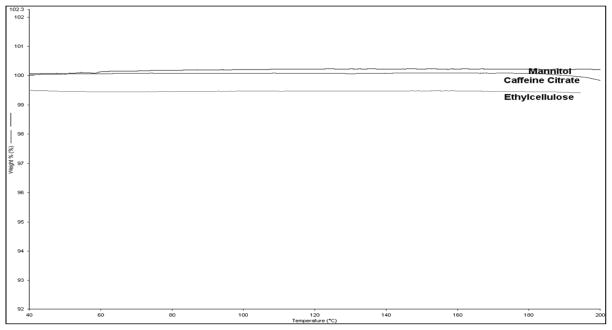
TGA Thermograms of Mannitol, caffeine citrate and Ethyl cellulose.
DSC studies were conducted to evaluate the physical state of the drug in the polymer matrix. Pure caffeine citrate (CC) exhibited a sharp endothermic peak onset at 168°C shown in Figure. 3 while EC exhibited an absence of a melting peak, which confirmed the complete amorphous nature of ethylcellulose (thermogram not shown). DSC thermograms of all of the extrudates illustrated a shifting of the endothermic peak associated with caffeine citrate, which may be due to the incorporation of TEC (as a plasticizer). Formulations with TEC and mannitol 3%, 5% and 10% showed a decrease in the melting temperature of CC. Additionally, with an increase in the concentration of mannitol, the peak became shifted and broadened due to the very close melting point of caffeine citrate and mannitol. Formulations CC9 and CC11 with calcium carbonate and calcium phosphate showed a shifting of the sharp endothermic peak; however, the formulation CC10 with magnesium oxide resulted in a very weak melting peak due to partial solubilization of caffeine citrate during extrusion. Also, this result confirmed TEC’s role as a suitable plasticizer for EC which helped to reduce the glass transition temperature (Tg) and melt viscosity due to an increase in the free volume of the ethyl cellulose chain (Crowley et al., 2004).
Figure 3.
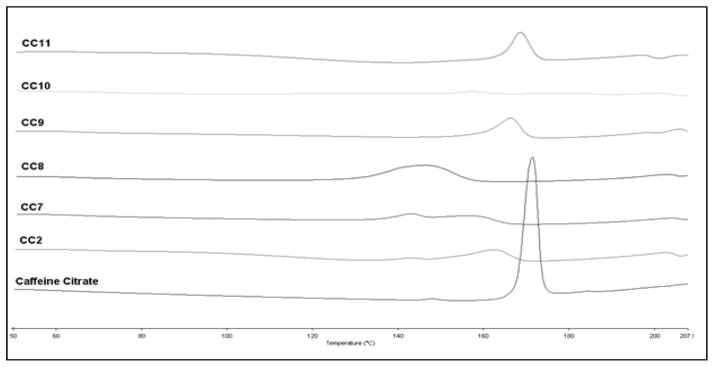
DSC Thermograms of extruded formulations and caffeine citrate; (CC2) caffeine citrate with EC/TEC and 3%mannitol, (CC7) 5%mannitol, (CC8) 10%mannitol, (CC9) 15% calcium carbonate, (CC10) 15% magnesium oxide, (CC11) 15%calcium phosphate.
Any significant quantity of a compound in which intermolecular interactions are present, hydrogen bonding for example, contains numerous interacting species, each of which would be involved in the interactions to varying extents. Therefore, what should be evident in the event of an intermolecular interaction would be a broadening of the spectral region in question, as the resulting spectrum is an average of these individual responses. Similarly, this broadening of the spectral region would result in a shift in the peaks center, which is represented by Figure 4. This is evident in neither the physical mixtures nor the extruded formulations. To the contrary, what is evident in the spectra is not a broadening of the spectral bands, nor a shift in the peaks center, but a very narrow and sharp response from the ethyl cellulose hydroxyl moiety near 2972cm−1, which is indicative of a lack of hydrogen bonding. This region is of particular interest as it is the only region with hydrogen bonding potential on the polymer chain that is not subject to steric hindrance. Likewise, the numerous potential hydrogen bond acceptors/donors available on caffeine citrate are neither broadened nor shifted, but exhibit a marked decrease in intensity resulting from the API’s dilution in the carrier. This indicates that the taste masking properties of the extruded formulations are due solely to physical entrapment of the API in the hydrophobic carrier.
Figure 4.
FTIR spectra of extrudates; (CC) caffeine citrate, (CC2) with 3%mannitol, (CC7) with 5%mannitol, (CC8) with 10%mannitol, (CC9) with 15%calcium carbonate, (CC10) with 15% magnesium oxide, (CC11) with 15%calcium phosphate.
Thermal stabilities of all of the excipients were established and the DSC investigations confirmed the presence of crystalline caffeine citrate in all of the extrudates. Hence, these data affirmed the effectiveness of a modified screw design to retain the physical state of the drug during the extrusion process. FTIR data proved the absence of interaction(s) of drug with carrier or other excipients during HME.
3.3. Dissolution studies and the role of pore forming agents
Ethyl cellulose (EC) is one of the most widely explored polymers for the formulation of microencapsulations, controlled release matrix systems, taste masking, as well as solvent extrusion granulations. EC has acceptable properties for taste masked formulations, however due to its considerable hydrophobicity, it cannot be employed for taste masked immediate release formulations. Therefore, it is necessary to incorporate release modulators or pore forming agents to assist in more rapid drug diffusion from the carrier (Mohammed et al., 2012). These agents could allow rapid entry of dissolution media into the matrix, which ultimately leads to rapid drug release. This type of sugar alcohols are highly water soluble compounds, and at higher loadings in a formulation may undesirably increase the release of the drug in oral cavity. Thus, the use of pH dependent pore formers is suitable for taste masked formulations as well as subsequent gastric release. With this in mind, calcium carbonate, calcium phosphate and magnesium oxide were selected as they are soluble in an acidic environment (Lai et al., 2013), which circumvents drug release in the mouth while allowing rapid gastric release.
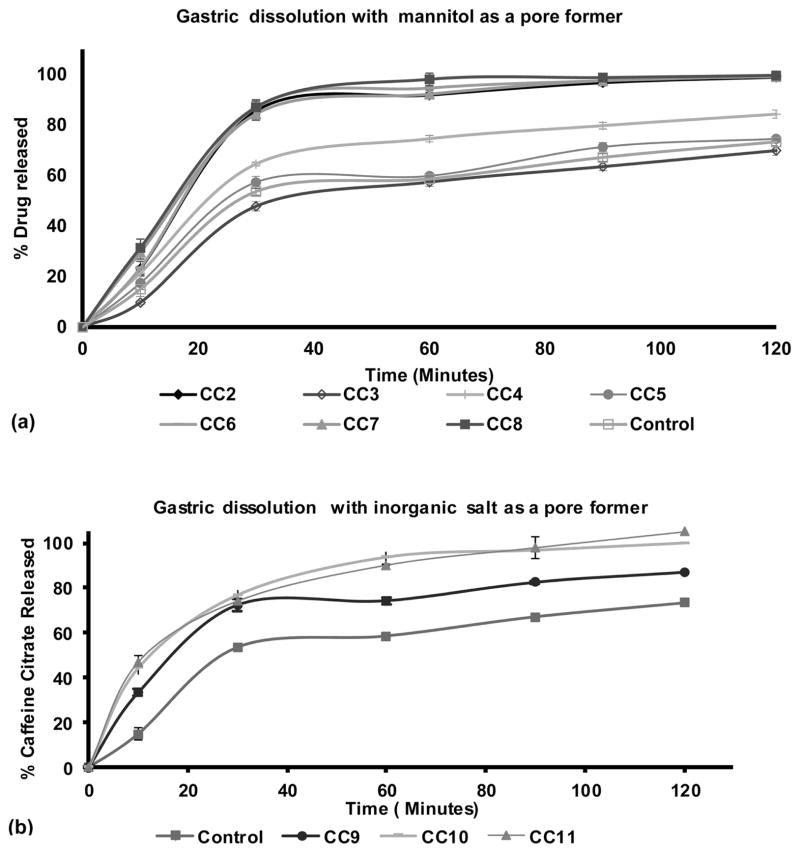
Dissolution profiles with pore formers showed an appreciable change in the rate and extent of release in 0.1 N HCl. Formulations without pore formers showed around 50% release in 30 minutes (Figure 5a). However, while some pore formers improve drug release in the gut, some may also improve drug release in the oral cavity, which is to the detriment of taste masking. Therefore, the selection of an appropriate pore former is critical to obtain a balance of oral cavity release and gastrointestinal release. For this study, we evaluated a water soluble sugar alcohol mannitol and pH dependent inorganic salts calcium phosphate, calcium carbonate and magnesium oxide. Formulations CC2, CC7 and CC8 with 3, 5 and 10% mannitol (Figure 5a) improved the release to more than 80% in 30 minutes and more than 92% in 60 minutes. On the other hand, formulations CC10 and CC11 with 15% of magnesium oxide (MgO) and calcium phosphate ((Ca)2PO4) exhibited 75% release in 30 minutes (Figure 5b) and above 90% in 60 minutes. CC9 with 15% calcium carbonate demonstrated 72% release in 30 minutes and approximately 75% in 60 minutes. The release mechanism of caffeine citrate in the ethyl cellulose matrix can be explained in that when a matrix is composed of a hydrophilic drug and hydrophobic polymer, the drug release occurs by dissolution of the active ingredient through capillaries composed of interconnecting drug particle clusters and the pore network. As drug release continues, the interconnecting cluster increases the pore network through which interior drug clusters can diffuse (Crowley et al., 2004). Despite this interconnecting cluster, the drug dissolution was not sufficient to obtain the targeted release profile without pore formers.
Figure 5.
Dissolution profiles of caffeine citrate in EC/TEC extrudates (n=3); (a) gastric dissolution profile with mannitol as pore former, control (CC1) caffeine citrate with EC/TEC, (CC2) with 3%mannitol, (CC7) with 5% mannitol, (CC8) with 10% mannitol; (b) gastric dissolution profile with inorganic salts, (CC9) caffeine citrate with EC/TEC and 15%calcium carbonate, (CC10) with 15% magnesium oxide, (CC11) with 15% calcium phosphate.
Formulation CC3 and CC4 with stearic acid as a plasticizer without and with 3% mannitol as a pore former, CC5 and CC6 with 10% TEC as a plasticizer without and with 5% mannitol did not demonstrate relevant results with the aim of this project so the results are not discussed in detail.
3.4. In vitro -Taste masking evaluations
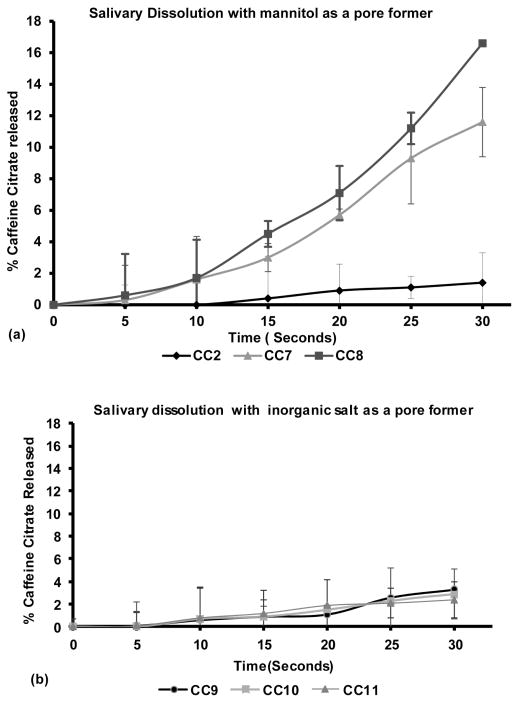
Dissolution testing using artificial salivary media (pH 6.8) was used as a primary screening method for taste masking ability of the formulation. The outcome as shown in Figure 6a and 6b from this concise study helped to understand the release behaviors which were extrapolated to oral release of formulations.
Figure 6.
Salivary dissolution profiles of caffeine citrate in EC/TEC extrudates (n=3); (a) dissolution profile with mannitol: (CC2) with 3% mannitol, (CC7) with 5% mannitol, (CC8) with 10% mannitol; (b) dissolution profile with inorganic salts, (CC9) with 15% calcium carbonate, (CC10) with 15% magnesium oxide, (CC11) with 15% calcium phosphate.
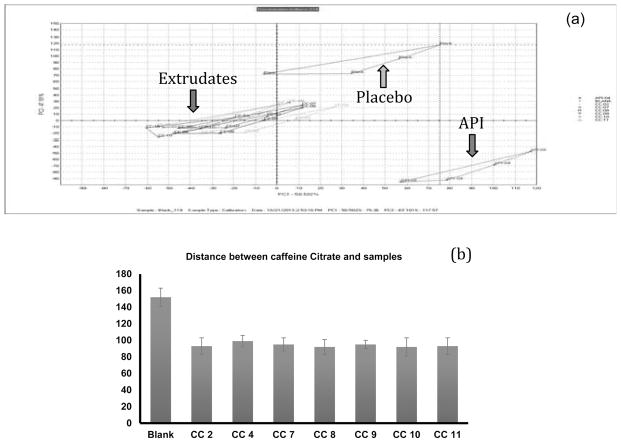
Successful formulations which showed low drug release in salivary fluid were evaluated using e-tongue where the outputs of these e-tongue studies were analyzed using a principal component analysis (PCA) where bitterness of the formulations was evaluated based on the distance between placebo and samples. The greater the proximity of samples to placebo, the more efficient the taste masking property was presumed to be. For this study, we evaluated ten samples from each beaker and used data from five samples (the first 3 and last 2 readings values were disregarded). This method helped to reduce an intra-sample variance, which is a common shortcoming of the electronic tongue that has been previously reported in the literature (Lorenz et al., 2009; Rahman et al., 2012; Siddiqui et al., 2013).
As it can be seen in Figure 6a and 6b, the amount of caffeine citrate released in CC2 with 3% mannitol in the EC/TEC matrix was around 2% in 30 seconds in artificial saliva whereas the formulation CC7 and CC8 with 5% and 10 % mannitol showed more than 13% in same amount of time. On the other hand, the release profile of formulations with inorganic salts, CC9, CC10 and C11, showed less than 3% release under the same conditions.
As illustrated in Figure 7a and 7b, the blank solution is at close distance with the extruded formulations. All of the formulations CC2, CC7 and CC8 with mannitol as a pore former had almost the same distance from the blank which confirmed the use of mannitol in the range of 3–10% without compromising the perceived taste. Formulations CC9, CC10 and CC11 with inorganic salts as a pore formers showed slightly better taste masking efficiency when compare to formulations with mannitol. More precisely, these inorganic pore formers only dissolve at acidic pH, which confirmed their importance in taste masking formulations. Formulations with magnesium oxide as a pore former exhibited a closer proximity to the blank compared to formulations with calcium carbonate and calcium phosphate.
Figure 7.
Taste masking evaluation using Astree e-tongue; (a) PCA chart for E-tongue results, (b) bar graph of distance between placebo and formulations.
Based on these findings from drug release profiles and e-tongue taste masking evaluations, the behaviors of low amounts of mannitol and organic salts as pore formers were very appropriate in a hydrophobic matrix such as ethylcellulose. These findings warranted studies of the taste masking effects in the mouth as well as evaluation of drug release in gastric fluid.
3.5. Preparation and Characterization for ODTs
The optimized extrudates which showed excellent taste masking were selected to develop an ODT according to FDA’s industrial guidelines (CDER, 2008). The caffeine citrate and EC extrudates with water soluble or insoluble pore formers were blended with super-disintegrates, Polyplasdone™ XL or XL-10, and mannitol as a diluent. ODT compositions in this study are provided in Table 3.
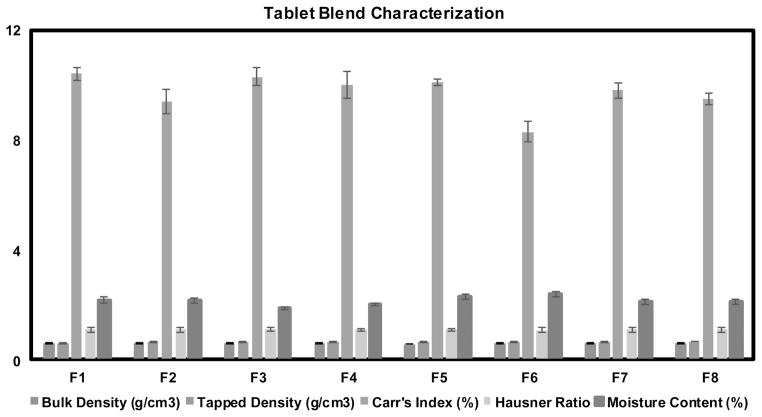
Before tablet compression, all tablet blends were evaluated for Hausner’s ratio and Carr’s index (Figure. 8), which were less than 1.12 and 11, respectively. Both these parameters are the most common indicators of the flowability of a powder blend. Hausner’s ratio of < 1.11 indicates good flowability whereas > 1.60 indicates poor flowability. Values between 1.12 and 1.18 is considered to be of acceptable flowability (Shah et al., 2008b). It is common to consider that the smaller the Carr’s index, the better the flowability. For example, values <10 indicate excellent, 11–15 good, 16 – 20 fair and > 26 poor flow (Shah et al., 2008b). In summary, all of the powder blends utilized for tableting demonstrated good flowability. For large scale tablet manufacturing processing, flowability is a critical parameter which determine the flow of powder from hopper to dies and it may affect content uniformity, weight and hardness of the tablet if the flowability is poor (Shah et al., 2008b). This results confirmed that the improvement in flowability and compressibility is mainly attributed to the use of EC in extrusion and mannitol as a diluent in the tablet blend.
Figure 8.
Schematic diagram of tablet blend characterizations, (F1) CC2 extrudates and polyplasdone XL, (F2) CC2 extrudates and polyplasdone XL-10, (F3) CC9 extrudates and polyplasdone XL, (F4) CC9 extrudates and polyplasdone XL-10, (F5) CC10 extrudates and polyplasdone XL, (F6) CC10 extrudates and polyplasdone XL-10, (F7) CC11 extrudates and polyplasdone XL, (F8) CC11 extrudates and polyplasdone XL-10.
Most of the studies revealed that a higher concentration of super-disintegrates affect disintegration time (DT) and hardness adversely. Thus, the optimized concentration is very important to obtain the DT below 30 seconds. In preliminary studies, formulations without super-disintegrates delayed disintegration time, which confirmed the importance of Polyplasdone™ XL and XL10 as a critical component for ODT disintegration (Results not shown). Moreover, with increasing the amount of super-disintegrates up to 5% showed an improvement in hardness, however with an increase in concentration above 10% exhibited lower hardness and increased disintegration time (Results not shown). These results are in agreement with a previously published study (Gryczke et al., 2011).
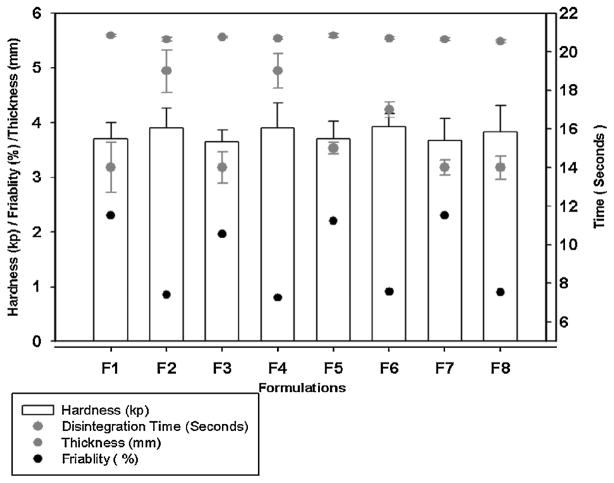
As per our study, the appropriate super-disintegrant concentration was found to be 5%, which showed the DT of 14 –19 seconds, which is less than the FDA’s guideline of 30 seconds (Figure. 9). The mechanism involved in the Polyplasdone™ XL and XL10 behavior for good DT was due to the rapid wicking of water into their porous particle size morphology. This generates rapid volume expansion by increasing the hydrostatic pressure that causes rapid tablet disintegration (Gryczke et al., 2011). All the tablets produced using HME had low hardness values (3.6– 3.9 kp); however, the friability was less than the acceptable range as it was measured to be below 1% in optimized formulations.
Figure 9.
Schematic diagram of ODT characterizations; (F1) CC2 extrudates and polyplasdone XL, (F2) CC2 extrudates and polyplasdone XL-10, (F3) CC9 extrudates and polyplasdone XL, (F4) CC9 extrudates and polyplasdone XL-10, (F5) CC10 extrudates and polyplasdone XL, (F6) CC10 extrudates and polyplasdone XL-10, (F7) CC11 extrudates and polyplasdone XL, (F8) CC11 extrudates and polyplasdone XL-10.
All the ODT formulations showed very similar gastric dissolution release profiles as that of extrudates (results not shown). During compression of tablets, extrudates maintained their integrality and did not change dissolution behavior. The overall outcome was the robust, taste masked and very rapidly disintegrating ODT formulations with acceptable friability.
3.6. Evaluation of taste of the products in human volunteers
Taste evaluation was carried out with 9 healthy volunteers. Initially bitterness perception of each volunteer was assessed using different concentrations of API in 2 ml aqueous solution to understand sensitivity of each human subject to the API. Three subjects had bitterness thresholds at 0.5 mg, four subjects had a threshold at 1.0 mg and the remaining two subject’s threshold were 5.0 mg. As stated in Table 4, a dose of 5.0 mg or above indicated very high bitterness and only three volunteers could tolerate 40 mg solution of CC. Thus, this initial evaluation confirmed that the volunteers are appropriate to test the melt extruded products containing caffeine citrate.
Table 4.
Human taste perception response
| Amount of API/2ml | Volunteers | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 0 mg | |||||||||
| 0.5 mg | 1 | 1 | 1 | ||||||
| 1.0 mg | 1 | 1 | 1 | 4 | 3 | 1 | 2 | ||
| 5.0 mg | 2 | 1 | 4 | 1 | 3 | 5 | 4 | 4 | 4 |
| 10.0 mg | 4 | 2 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 40.0 mg | 5 | 4 | 5 | ||||||
Based on the taste evaluation using artificial salivary dissolution and e-tongue, formulations CC2 with 3% mannitol and CC11 with 15% calcium phosphate as a pore former were selected to reaffirm the outcome using a human taste panel. Results shown in Figure 10, the physical mixture of CC2 exhibited the unacceptable and intolerable bitterness in all of the human subjects (an average score of 4.6 on a scale of 1–5), it confirmed that the amount of mannitol and EC did not aid to minimize the bitterness produced due to caffeine citrate. When the milled extrudates were administrated, the average response dropped considerably (an average score of 2 on a scale of 1–5) and statistical analysis of taste responses confirmed a significant difference in bitter taste (p<0.0006) between the CC2 physical mixture and CC2 extrudates. Moreover, the results of formulation CC11 was comparable to that of CC2, where the average score for physical mixture was ~5; however the milled extrudates was only ~2.6 which, indicated the effectiveness of the extruded formulations compared to its physical mixture. The taste masking effectiveness of CC11 vs. its corresponding the physical mixture proved to be statistically significant (p<0.003). Remarkably, this result from human subjects has corresponded to e-tongue results as well as artificial saliva dissolution data. This outcome has demonstrated that the use of in vitro studies such as e-tongue and artificial saliva dissolution could be applicable to ascertain the taste masked efficiency of the formulations.
Figure 10.
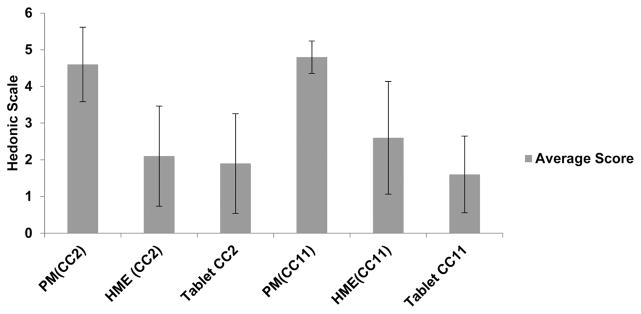
Human taste panel evaluations; PM (CC2): pre extrusion blend caffeine citrate with EC/TEC and 3%mannitol, HME (CC2): hot melt extrudate of CC2, Tablet CC2: ODT of CC2; PM(CC11): pre extrusion blend caffeine citrate with EC/TEC and 15% calcium phosphate, HME(CC11): hot melt extrudate of CC11, Tablet CC11: ODT of CC11.
In addition, HME technology proved the taste masking efficiency of formulations with an EC matrix and selected pore formers. This taste test for bitterness also indicated that the amount and type of pore former were critical factors for the development of taste masked formulations using hydrophobic polymeric matrices. Comparison of physical mixtures with extrudates affirmed the significance of the HME process and also proved the dilution of caffeine citrate is not adequate for producing taste masked formulations. These data asserted the theory of entrapment of CC in within the EC matrix incorporated via HME and its role to hinder the release in the oral cavity. The pH dependent and independent pore former type and concentration supported their importance without affecting the taste, as most of the pore formers facilitate release in the oral cavity.
Conclusion
This study demonstrated the effectiveness of an ethyl cellulose matrix with a pore former for the development of taste-masked formulations. To facilitate the processability of the high Tg of the ethylcellulose matrix, and to preserve the crystallinity of the model API, the screw configuration was modified to reduce the shear imparted during processing coupled with the incorporation of a plasticizer to decrease the Tg. Strikingly, the results from salivary dissolution and e-tongue data were in good correlation with the human taste panel. The development of taste-masked formulations required an appropriate processing technique, such as hot melt extrusion that could entrap the drug forming a barrier to achieve a palatable taste as well as demonstrating controlled release. This formulation could be used as a platform for unpleasant tasting, highly soluble drugs. In summary, the final ODTs were robust, taste masked dosage forms, adhering to acceptable ODT guidelines.
Acknowledgments
The authors thank the Pii Center for Pharmaceutical Technology and Grant Number P20GM104932 from the National Institute of General Medical Sciences (NIGMS), a component of NIH for contributions to this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Omran MF, Al-Suwayeh SA, El-Helw AM, Saleh SI. Taste masking of diclofenac sodium using microencapsulation. Journal of microencapsulation. 2002;19:45–52. doi: 10.1080/02652040110055612. [DOI] [PubMed] [Google Scholar]
- Ashland. Aqualon Ethylcellulose. Ashland; 2002. [Google Scholar]
- Azarmi S, Roa W, Lobenberg R. Current perspectives in dissolution testing of conventional and novel dosage forms. Int J Pharm. 2007;328:12–21. doi: 10.1016/j.ijpharm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Badgujar BP, Mundada AS. The technologies used for developing orally disintegrating tablets: a review. Acta Pharm. 2011;61:117–139. doi: 10.2478/v10007-011-0020-8. [DOI] [PubMed] [Google Scholar]
- Behzadi SS, Toegel S, Viernstein H. Innovations in coating technology. Recent patents on drug delivery & formulation. 2008;2:209–230. doi: 10.2174/187221108786241633. [DOI] [PubMed] [Google Scholar]
- Benoit J-P, Rolland H, Thies C, Velde VV. Method of coating particles and coated spherical particles. Google Patents; 2000. [Google Scholar]
- CDER. Guidance for Industry Orally Disintegrating Tablets. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER); 2008. [Google Scholar]
- Comer AM, Perry CM, Figgitt DP. Caffeine Citrate. Paediatric drugs. 2001;3:61–79. doi: 10.2165/00128072-200103010-00005. [DOI] [PubMed] [Google Scholar]
- Crowley MM, Schroeder B, Fredersdorf A, Obara S, Talarico M, Kucera S, McGinity JW. Physicochemical properties and mechanism of drug release from ethyl cellulose matrix tablets prepared by direct compression and hot-melt extrusion. International journal of pharmaceutics. 2004;269:509–522. doi: 10.1016/j.ijpharm.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Dinge A, Nagarsenker M. Formulation and evaluation of fast dissolving films for delivery of triclosan to the oral cavity. AAPS PharmSciTech. 2008;9:349–356. doi: 10.1208/s12249-008-9047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczke A, Schminke S, Maniruzzaman M, Beck J, Douroumis D. Development and evaluation of orally disintegrating tablets (ODTs) containing Ibuprofen granules prepared by hot melt extrusion. Colloids and Surfaces B: Biointerfaces. 2011;86:275–284. doi: 10.1016/j.colsurfb.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Hamashita T, Matsuzaki M, Ono T, Ono M, Tsunenari Y, Aketo T, Watano S. Granulation of core particles suitable for film coating by agitation fluidized bed II. A proposal of a rapid dissolution test for evaluation of bitter taste of ibuprofen. Chemical & pharmaceutical bulletin. 2008;56:883–887. doi: 10.1248/cpb.56.883. [DOI] [PubMed] [Google Scholar]
- Hanna MH, York P. Particle formation methods and their products. Google Patents; 2006. [Google Scholar]
- Jacqz-Aigrain E, Choonara I. Paediatric clinical pharmacology. Taylor & Francis; 2006. [Google Scholar]
- Lai JW, Venkatesh GM, Qian KK. Taste-masked pharmaceutical compositions with gastrosoluble poreformers. Google Patents; 2013. [Google Scholar]
- Lorenz JK, Reo JP, Hendl O, Worthington JH, Petrossian VD. Evaluation of a taste sensor instrument (electronic tongue) for use in formulation development. Int J Pharm. 2009;367:65–72. doi: 10.1016/j.ijpharm.2008.09.042. [DOI] [PubMed] [Google Scholar]
- Mahesh A, Shastri N, Sadanandam M. Development of taste masked fast disintegrating films of levocetirizine dihydrochloride for oral use. Current drug delivery. 2010;7:21–27. doi: 10.2174/156720110790396454. [DOI] [PubMed] [Google Scholar]
- Maniruzzaman M, Boateng JS, Bonnefille M, Aranyos A, Mitchell JC, Douroumis D. Taste masking of paracetamol by hot-melt extrusion: an in vitro and in vivo evaluation. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e V. 2012a;80:433–442. doi: 10.1016/j.ejpb.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Maniruzzaman M, Boateng JS, Snowden MJ, Douroumis D. A review of hot-melt extrusion: process technology to pharmaceutical products. ISRN pharmaceutics. 2012b;2012 doi: 10.5402/2012/436763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniruzzaman M, Rana M, Boateng J, Mitchell J, Douroumis D. Dissolution enhancement of poorly water-soluble APIs processed by hot-melt extrusion using hydrophilic polymers. Drug development and Industrial Pharmacy. 2013;39:218–227. doi: 10.3109/03639045.2012.670642. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Spector AC, Reed DR, Coldwell SE. The bad taste of medicines: overview of basic research on bitter taste. Clinical therapeutics. 2013;35:1225–1246. doi: 10.1016/j.clinthera.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed NN, Majumdar S, Singh A, Deng W, Murthy NS, Pinto E, Tewari D, Durig T, Repka MA. Klucel EF and ELF polymers for immediate-release oral dosage forms prepared by melt extrusion technology. AAPS PharmSciTech. 2012;13:1158–1169. doi: 10.1208/s12249-012-9834-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niazi SK. Handbook of Pharmaceutical Manufacturing Formulations: Liquid Products. 3 of 6. CRC press; 2004. [Google Scholar]
- O’connell S, Zurzola F. Rapid quantitative liquid chromatographic determination of caffeine levels in plasma after oral dosing. Journal of pharmaceutical sciences. 1984;73:1009–1011. doi: 10.1002/jps.2600730742. [DOI] [PubMed] [Google Scholar]
- Rahman Z, Zidan AS, Berendt RT, Khan MA. Tannate complexes of antihistaminic drug: sustained release and taste masking approaches. Int J Pharm. 2012;422:91–100. doi: 10.1016/j.ijpharm.2011.10.033. [DOI] [PubMed] [Google Scholar]
- Sawynok J. Pharmacological rationale for the clinical use of caffeine. Drugs. 1995;49:37–50. doi: 10.2165/00003495-199549010-00004. [DOI] [PubMed] [Google Scholar]
- Seager H. Drug-delivery products and the Zydis fast-dissolving dosage form. The Journal of pharmacy and pharmacology. 1998;50:375–382. doi: 10.1111/j.2042-7158.1998.tb06876.x. [DOI] [PubMed] [Google Scholar]
- Shah PP, Mashru RC, Rane YM, Thakkar A. Design and optimization of mefloquine hydrochloride microparticles for bitter taste masking. AAPS PharmSciTech. 2008a;9:377–389. doi: 10.1208/s12249-008-9052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RB, Tawakkul MA, Khan MA. Comparative evaluation of flow for pharmaceutical powders and granules. AAPS PharmSciTech. 2008b;9:250–258. doi: 10.1208/s12249-008-9046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A, Shah RB, Khan MA. Oseltamivir phosphate-amberlite (TM) IRP 64 ionic complex for taste masking: preparation and chemometric evaluation. Journal of pharmaceutical sciences. 2013;102:1800–1812. doi: 10.1002/jps.23518. [DOI] [PubMed] [Google Scholar]
- Strickley RG, Iwata Q, Wu S, Dahl TC. Pediatric drugs--a review of commercially available oral formulations. Journal of pharmaceutical sciences. 2008;97:1731–1774. doi: 10.1002/jps.21101. [DOI] [PubMed] [Google Scholar]
- Susan, Walters HGK. Development of paediatric medicines: points to consider in pharmaceutical development. 2011. [DOI] [PubMed] [Google Scholar]
- Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharmaceutical research. 2005;22:11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]