Abstract
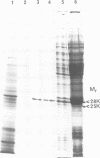
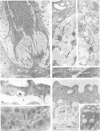
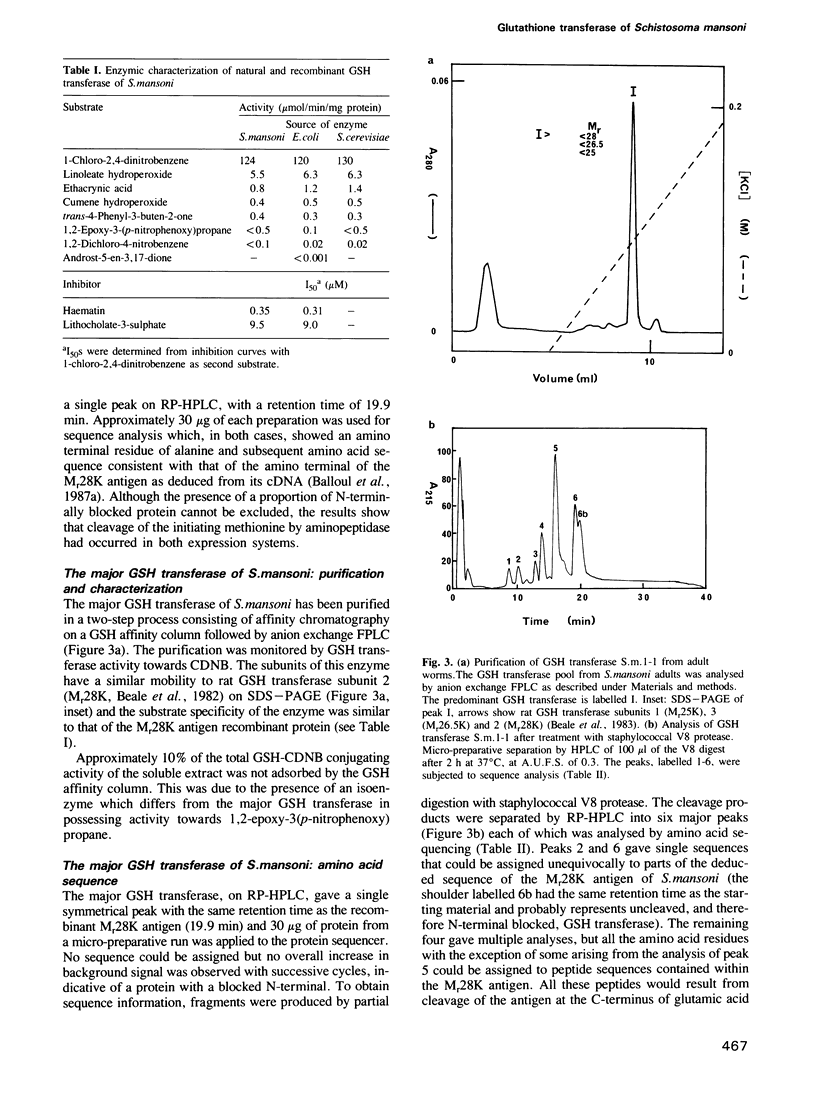
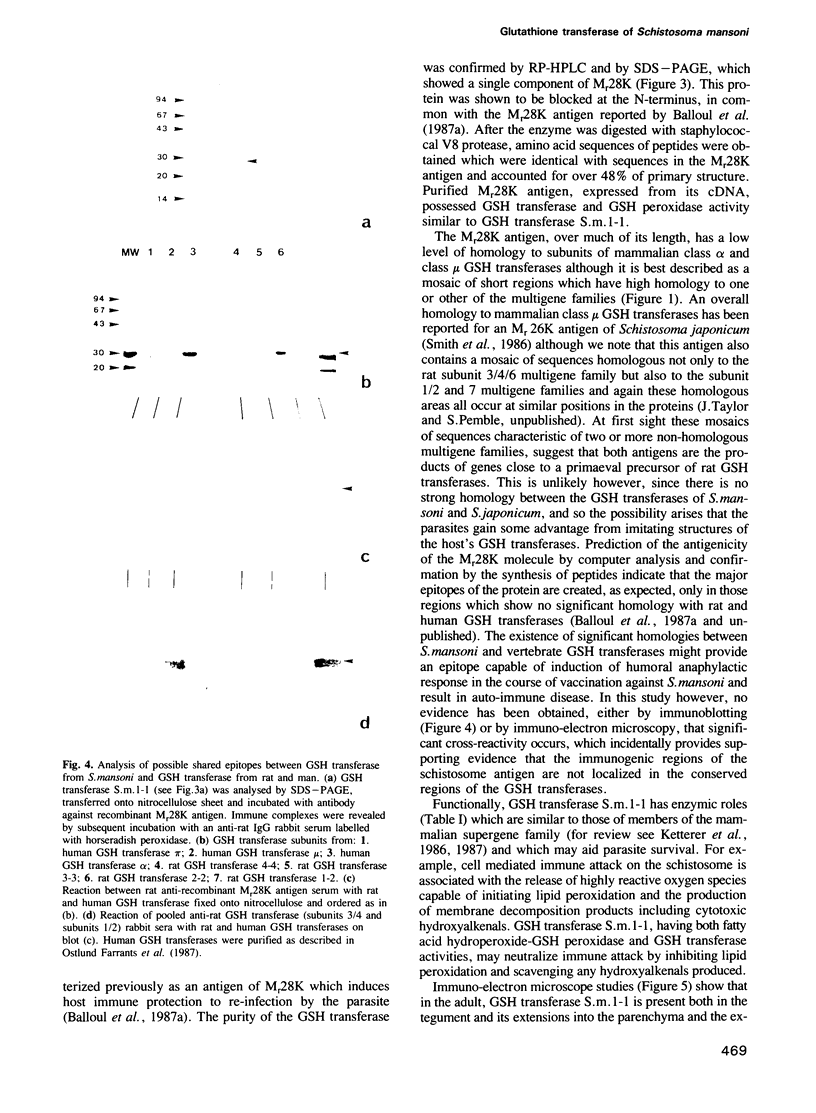
A protective Mr28K antigen of Schistosoma mansoni, expressed from its cDNA, has been purified in a single step and shown to possess glutathione (GSH) transferase activity as predicted from sequence homologies with two mammalian GSH transferase multigene families. It is notable for its high 1-chloro-2,4-dinitrobenzene GSH transferase and linoleic acid hydroperoxide GSH peroxidase activities. The major GSH transferase of S. mansoni has been purified and its subunit is identical to this Mr28K antigen by criteria of Mr, immunochemistry, substrate specificity and peptide sequence analysis. In the parasite, the antigen is present in the tegument, protonephridial cells and subtegumental parenchymal cells. No significant immunological cross-reactivity between the S.mansoni and mammalian (human and rat) GSH transferases was observed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovitz M., Listowsky I. Selective expression of a unique glutathione S-transferase Yb3 gene in rat brain. J Biol Chem. 1987 Jun 5;262(16):7770–7773. [PubMed] [Google Scholar]
- Balloul J. M., Pierce R. J., Grzych J. M., Capron A. In vitro synthesis of a 28 kilodalton antigen present on the surface of the schistosomulum of Schistosoma mansoni. Mol Biochem Parasitol. 1985 Oct;17(1):105–114. doi: 10.1016/0166-6851(85)90131-8. [DOI] [PubMed] [Google Scholar]
- Balloul J. M., Sondermeyer P., Dreyer D., Capron M., Grzych J. M., Pierce R. J., Carvallo D., Lecocq J. P., Capron A. Molecular cloning of a protective antigen of schistosomes. Nature. 1987 Mar 12;326(6109):149–153. doi: 10.1038/326149a0. [DOI] [PubMed] [Google Scholar]
- Beale D., Ketterer B., Carne T., Meyer D., Taylor J. B. Evidence that the Ya and Yc subunits of glutathione transferase B (ligandin) are the products of separate genes. Eur J Biochem. 1982 Sep 1;126(3):459–463. doi: 10.1111/j.1432-1033.1982.tb06802.x. [DOI] [PubMed] [Google Scholar]
- Beale D., Meyer D. J., Taylor J. B., Ketterer B. Evidence that the Yb subunits of hepatic glutathione transferases represent two different but related families of polypeptides. Eur J Biochem. 1983 Dec 1;137(1-2):125–129. doi: 10.1111/j.1432-1033.1983.tb07805.x. [DOI] [PubMed] [Google Scholar]
- Ding G. J., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. Nucleotide sequence analysis of a Yb1 cDNA clone and prediction of the complete amino acid sequence of the Yb1 subunit. J Biol Chem. 1985 Oct 25;260(24):13268–13271. [PubMed] [Google Scholar]
- Drapeau G. R. The primary structure of staphylococcal protease. Can J Biochem. 1978 Jun;56(6):534–544. doi: 10.1139/o78-082. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B., Ketterer B., Mannervik B. Glutathione transferases: nomenclature. Biochem Pharmacol. 1984 Aug 15;33(16):2539–2540. doi: 10.1016/0006-2952(84)90621-x. [DOI] [PubMed] [Google Scholar]
- Kusel J. R., Mackenzie P. E. The measurement of the relative turnover rates of proteins of the surface membranes and other fractions of Schistosoma mansoni in culture. Parasitology. 1975 Oct;71(2):261–273. doi: 10.1017/s0031182000046709. [DOI] [PubMed] [Google Scholar]
- Lanar D. E., Pearce E. J., James S. L., Sher A. Identification of paramyosin as schistosome antigen recognized by intradermally vaccinated mice. Science. 1986 Oct 31;234(4776):593–596. doi: 10.1126/science.3094144. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. J., Beale D., Tan K. H., Coles B., Ketterer B. Glutathione transferases in primary rat hepatomas: the isolation of a form with GSH peroxidase activity. FEBS Lett. 1985 May 6;184(1):139–143. doi: 10.1016/0014-5793(85)80670-0. [DOI] [PubMed] [Google Scholar]
- Ostlund Farrants A. K., Meyer D. J., Coles B., Southan C., Aitken A., Johnson P. J., Ketterer B. The separation of glutathione transferase subunits by using reverse-phase high-pressure liquid chromatography. Biochem J. 1987 Jul 15;245(2):423–428. doi: 10.1042/bj2450423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemble S. E., Taylor J. B., Ketterer B. Tissue distribution of rat glutathione transferase subunit 7, a hepatoma marker. Biochem J. 1986 Dec 15;240(3):885–889. doi: 10.1042/bj2400885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. M., Zarlengo R. P., Tu C. P. The basic glutathione S-transferases from human livers are products of separate genes. Biochem Biophys Res Commun. 1987 May 29;145(1):474–481. doi: 10.1016/0006-291x(87)91345-3. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Davern K. M., Board P. G., Tiu W. U., Garcia E. G., Mitchell G. F. Mr 26,000 antigen of Schistosoma japonicum recognized by resistant WEHI 129/J mice is a parasite glutathione S-transferase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8703–8707. doi: 10.1073/pnas.83.22.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telakowski-Hopkins C. A., Rodkey J. A., Bennett C. D., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. Construction of a cDNA clone complementary to a Yc mRNA and prediction of the complete amino acid sequence of a Yc subunit. J Biol Chem. 1985 May 10;260(9):5820–5825. [PubMed] [Google Scholar]
- Tessier L. H., Sondermeyer P., Faure T., Dreyer D., Benavente A., Villeval D., Courtney M., Lecocq J. P. The influence of mRNA primary and secondary structure on human IFN-gamma gene expression in E. coli. Nucleic Acids Res. 1984 Oct 25;12(20):7663–7675. doi: 10.1093/nar/12.20.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpier G., Capron M., Capron A. Structural changes of the tegumental membrane complex in relation to developmental stages of Schistosoma mansoni (Platyhelminthes: Trematoda). J Ultrastruct Res. 1977 Dec;61(3):309–324. doi: 10.1016/s0022-5320(77)80056-7. [DOI] [PubMed] [Google Scholar]
- Torpier G., Ouaissi M. A., Capron A. Freeze-fracture study of immune-induced Schistosoma mansoni membrane alterations. I. Complement-dependent damage in the presence of antisera to host antigenic determinants. J Ultrastruct Res. 1979 Jun;67(3):276–287. doi: 10.1016/s0022-5320(79)80028-3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Jagt D. L., Hunsaker L. A., Garcia K. B., Royer R. E. Isolation and characterization of the multiple glutathione S-transferases from human liver. Evidence for unique heme-binding sites. J Biol Chem. 1985 Sep 25;260(21):11603–11610. [PubMed] [Google Scholar]