Abstract
Purpose
To quantify the predictive strength of anterior chamber area (ACA), anterior chamber volume (ACV), anterior chamber width (ACW), lens vault (LV), iris thickness (IT), and iris area (IArea) for two angle width parameters, trabecular-iris space area (TISA750) and angle opening distance (AOD750) at 750 μm from the scleral spur, in different ethnicities.
Design
Prospective, cross-sectional study.
Methods
Anterior segment optical coherence tomography images for 166 white, 90 African, 75 Hispanic, and 132 Chinese subjects were analyzed. First, ACA, ACV, ACW, LV, IT, and IArea were compared among ethnic groups. Second, associations of TISA750 and AOD750 with ACA, ACV, ACW, LV, IT and IArea were investigated within each ethnic group using multivariable linear regression models, standardized regression coefficients (β), and coefficients of determination (R2).
Results
Significant ethnic differences were observed in ACA, ACV, ACW, LV, IT, and IArea (all P<0.05). ACA, ACV, and LV were significant predictors of TISA750 and AOD750 in all ethnic groups (all P<0.001). ACW and IT were significant predictors of AOD750 in whites and Africans (all P<0.05). ACW and IT were significant predictors of TISA750 in whites (all P<0.05). IArea was a significant predictor of AOD750 in Chinese (P<0.05). ACA, ACV, and LV had the highest predictive strength for both TISA750 and AOD750 in all ethnic groups based on β and R2.
Conclusions
Despite ethnic differences in ACA, ACV, ACW, LV, IT, and IArea, the same three anterior segment parameters (ACA, ACV, and LV) were the strongest predictors of angle width (TISA750 and AOD750) in all four ethnic groups.
Keywords: Ethnicity, anterior chamber angle width, trabecular-iris space area, angle opening distance, anterior chamber area, anterior chamber volume, anterior chamber width, lens vault, iris thickness, iris area
Introduction
Primary angle closure glaucoma (PACG) is one of the leading causes of irreversible blindness worldwide.1 Although this condition is most commonly described among those of Asian descent, it can occur in all races. A review of the literature published between the years 1966 and 2006 indicated reproducible evidence of ethnic variations in the prevalence of PACG.2 Evaluation of the anterior chamber angle width is considered the key factor in diagnosis since this anatomic parameter primarily determines the risk for PACG development. One method of quantifying angle width is by using diagnostic imaging modalities such as anterior segment optical coherence tomography (AS-OCT). Analysis of AS-OCT images with customized software, the Zhongshan Angle Assessment Program (Zhongshan Ophthalmic Centre, Guangzhou, China), allows objective and reproducible quantification of anterior segment biometric parameters.3
Two parameters that closely reflect angle width are trabecular-iris space area and angle opening distance. Foo et al. showed in Chinese subjects that angle width is largely dependent on variations in anterior chamber area, anterior chamber volume, anterior chamber width, lens vault, iris thickness measured at 750 μm from the scleral spur, and iris area.4 Using a predictive mathematic model comprised of these six anterior segment biometric parameters, their study explained 81.4% of the variability in trabecular-iris space area at 750 μm from the scleral spur and 85.5% of the variability in angle opening distance at 750 μm from the scleral spur.
However, their findings in an ethnic Chinese population may not necessarily apply to other ethnic groups. Quantifying the predictive ability of anterior segment biometric parameters associated with anterior chamber angle width in various ethnic groups can provide insight into mechanisms of angle closure in different ethnicities. The purpose of the present study was to objectively compare the predictive strength of anterior chamber area, anterior chamber volume, anterior chamber width, lens vault, iris thickness measured at 750 μm from the scleral spur, and iris area for anterior chamber angle width among white, African, Hispanic, and Chinese populations.
Methods
Approval for this prospective, cross-sectional, single-center, multiethnic, clinic-based study was obtained from the University of California, San Francisco (UCSF) Committee on Human Research. The study was carried out in accordance with the tenets of the Declaration of Helsinki and written consent was obtained from all patients. The study population was composed of 4 different ethnic cohorts (white, African, Hispanic, and Chinese) consecutively recruited from the UCSF general ophthalmology and glaucoma clinics between March 1, 2008 and September 28, 2010. Ethnicities were self-designated by the patients.
Inclusion criteria for subject enrollment included: (1) adult patients (age >18 years); (2) patients who consented to undergo standardized ophthalmic examination and AS-OCT imaging; (3) absence of corneal abnormalities that may obscure the view of anterior segment structures; (4) absence of prior laser or incisional eye surgery; (5) absence of previous ocular trauma; and (6) self-reported white, African, Hispanic, and Chinese ancestry in both parents (the term “white” for the purposes of this study included only European-derived whites). Exclusion criteria for enrollment included the following: (1) aphakic or pseudophakic eyes; (2) inability to complete the standardized ophthalmic examination and AS-OCT imaging; and (3) poor AS-OCT imaging quality (evaluated on the basis of corneal reflection, continuity of anterior segment structures, motion artifacts, and indeterminate scleral spurs).
All enrolled subjects received intraocular pressure measurement by Goldmann applanation tonometry (model AT900, Haag-Streit AG, Koeniz, Switzerland), central corneal thickness measurement by ultrasound pachymetry (Model DGH-550 Pachette 2; DGH Technology Inc., Exton, PA, USA), manual refraction, and gonioscopy with a Zeiss-style 4-mirror lens (Model OPDSG; Ocular Instruments, Inc., Bellevue, WA, USA). A single trained ophthalmologist (Shan C. Lin) performed gonioscopy at x16 magnification with slit-lamp biomicroscopy in a darkroom setting. The study population was further categorized into narrow angle and open angle groups. The Shaffer gonioscopic classification was used to determine the anterior chamber angle grading in all four quadrants: an angle between the iris and the trabecular meshwork surface of 35° to 45° was classified as grade 4, between 20° and 35° was classified as grade 3, between 10° to 20° was classified as grade 2, and less than 10° was classified as grade 1. Grade 0 was assigned if angle structures were not observed.5 For this study, eyes with narrow angles were defined as those with Shaffer grades of 2 or less in 3 or more quadrants, whereas eyes with open angles were defined as those with Shaffer grades of 3 to 4 in three or more quadrants. This definition of narrow angle has previously been used by other studies.6–13 Both the narrow angle and open angle groups included nonglaucomatous and glaucomatous subjects.
Anterior Segment Optical Coherence Tomography
All qualified study subjects received imaging in the dark with AS-OCT (Visante OCT; Carl Zeiss Meditec, Inc., Dublin, CA, USA), a non-contact optical coherence tomographic system using 1310-nm wavelength light to capture high-resolution cross-sectional images of the anterior segment of the eye. AS-OCT imaging was performed under standardized dark conditions with illuminations below 1 lux as measured by EasyView Digital Light Meter (Model EA30; Extech Instruments, Inc., Waltham, MA, USA). Patients were allowed 5 minutes for dark adaptation before image acquisition. Each AS-OCT scan captured both the temporal and nasal quadrants (nasal-temporal 0–180°) in a single image while the patient looked straight ahead. An experienced operator, masked to the standardized ophthalmic examination findings, performed all the AS-OCT scans. Three to five images were acquired for the one randomly selected eye of each subject and the image with the best quality was selected for analysis using the Zhongshan Angle Assessment Program. Image quality was evaluated on the basis of a steady central fixation as judged by a clear corneal reflection, good visibility of the scleral spurs, the presence of continuity in anterior segment structures, and the absence of motion artifacts. The Zhongshan Angle Assessment Program contained algorithms that automatically defined the borders and curvatures of anterior segment structures after the scleral spurs were localized manually on the AS-OCT images. Measurements for both the nasal and temporal angles were simultaneously produced, but only the nasal angles were analyzed in this study. This procedure has previously been used in our other studies.11–14 The following parameters were derived from the Zhongshan Angle Assessment Program: trabecular-iris space area at 750 μm from the scleral spur, angle opening distance at 750 μm from the scleral spur, anterior chamber area, anterior chamber volume, anterior chamber width, lens vault, iris thickness measured at 750 μm from the scleral spur, iris area, and pupil diameter.
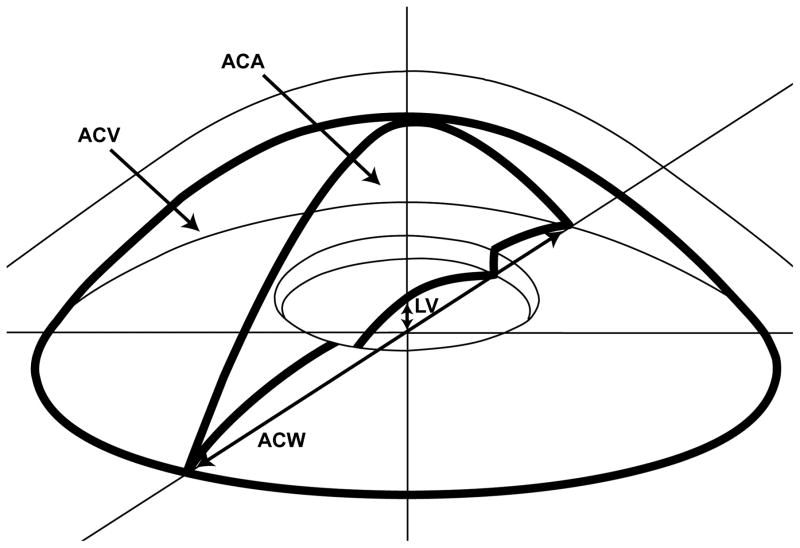
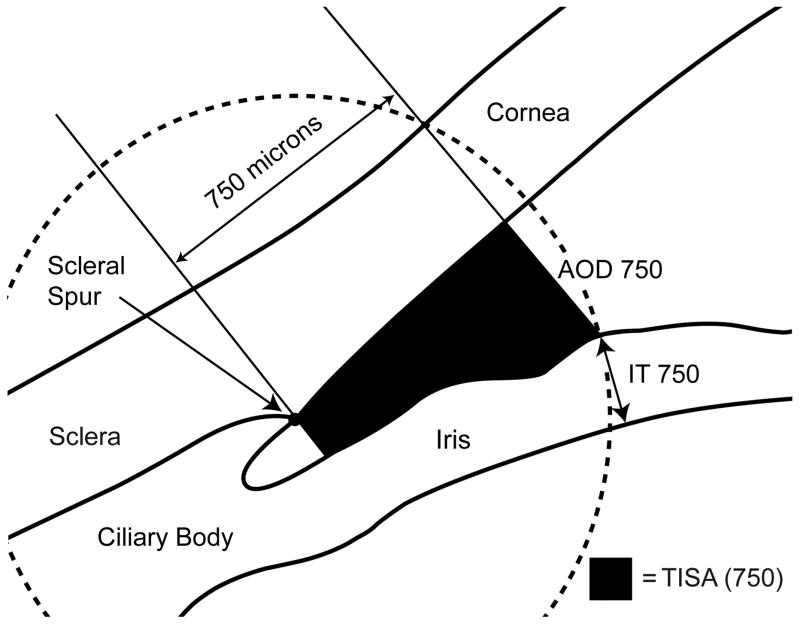
The above listed AS-OCT parameters were defined previously in other studies. Trabecular-iris space area at 750 μm from the scleral spur was defined as the trapezoidal area with the following boundaries: anteriorly, a perpendicular line between the inner corneoscleral wall and the iris surface at 750 μm anterior to the scleral spur; posteriorly, a line perpendicular to the inner corneoscleral wall extending from the scleral spur to the iris surface; superiorly, the inner corneoscleral wall; and inferiorly, the iris surface.4 Angle opening distance at 750 μm from the scleral spur was defined as the length of the line extending from the anterior iris to the corneal endothelium, perpendicular to the line drawn along the trabecular meshwork at 750 μm anterior to the scleral spur.15,16 Anterior chamber area was defined as the cross-sectional area of the anterior chamber bounded by the corneal endothelium anteriorly, and the anterior surface of iris and the anterior surface of lens (within the pupil) posteriorly.17 Anterior chamber volume was calculated by rotating the anterior chamber area 360 degrees around a vertical axis through the midpoint of the anterior chamber area.17 Anterior chamber width was defined as the horizontal scleral spur-to-spur distance.18 Lens vault was defined as the perpendicular distance between the anterior pole of the crystalline lens and the horizontal line joining the two scleral spurs.19 An illustration of anterior chamber area, anterior chamber volume, anterior chamber width, and lens vault is provided in Figure 1. For iris thickness, a circle centered at the scleral spur was drawn with a radius of 750 μm, and the point of intersection between the circle and the anterior surface of the iris was identified. The shortest distance from this point to the posterior surface of the iris was calculated as iris thickness measured at 750 μm from the scleral spur.20 An illustration of trabecular-iris space area at 750 μm from the scleral spur, angle opening distance at 750 μm from the scleral spur, and iris thickness measured at 750 μm from the scleral spur is provided in Figure 2. Iris area was calculated as the cumulative cross-sectional area of the full length of the iris.20 Pupil diameter was calculated by measuring the distance between the pupil edges on the cross-sectional images.21
Figure 1.
An illustration of anterior chamber area (ACA), anterior chamber volume (ACV), anterior chamber width (ACW), lens vault (LV). ACA was defined as the cross-sectional area of the anterior chamber bounded by the corneal endothelium anteriorly, and the anterior surface of iris and the anterior surface of lens (within the pupil) posteriorly. ACV was calculated by rotating the ACA 360 degrees around a vertical axis through the mid-point of the ACA. ACW was defined as the horizontal scleral spur-to-spur distance. LV was defined as the perpendicular distance between the anterior pole of the crystalline lens and the horizontal line joining the two scleral spurs.
Figure 2.
An illustration of trabecular-iris space area (TISA750), angle opening distance (AOD750), and iris thickness (IT750) measured at 750 μm from the scleral spur. TISA750 was defined as the trapezoidal area with the following boundaries: anteriorly, a perpendicular line between the inner corneoscleral wall and the iris surface at 750 μm anterior to the scleral spur; posteriorly, a line perpendicular to the inner corneoscleral wall extending from the scleral spur to the iris surface; superiorly, the inner corneoscleral wall; and inferiorly, the iris surface. AOD750 was defined as the length of the line extending from the anterior iris to the corneal endothelium, perpendicular to the line drawn along the trabecular meshwork at 750 μm anterior to the scleral spur. For iris thickness, a circle centered at the scleral spur was drawn with a radius of 750 μm, and the point of intersection between the circle and the anterior surface of the iris was identified. The shortest distance from this point to the posterior surface of the iris was calculated as IT750.
Statistical Analysis
For analysis, one eye was selected for each subject. The default protocol was to use images from the right eye for analysis. However, the left eye’s data was used when the right eye did not meet the inclusion criteria. For each ethnic group, mean and standard deviation were calculated for all variables. Comparisons were made between ethnic groups for subjects with only narrow angles, only open angles, and both narrow and open angles. A p-value <0.05 was considered statistically significant. Continuous data were analyzed using one-way ANOVA with Tukey’s post-hoc test for between-ethnic group comparisons. Continuous data were analyzed using independent Student t tests for within-ethnic group comparisons. Categorical data was analyzed with Pearson chi-square test. Multivariable linear regression models were used to investigate the association of trabecular-iris space area at 750 μm from the scleral spur and angle opening distance at 750 μm from the scleral spur with anterior chamber area, anterior chamber volume, anterior chamber width, lens vault, iris thickness measured at 750 μm from the scleral spur, iris area, while controlling for age, sex, pupil diameter, and spherical equivalent. Both standardized regression coefficients (β) and coefficients of determination (R2) were calculated from the multivariable linear regression models. To determine the effects of glaucoma on the association of trabecular-iris space area at 750 μm from the scleral spur and angle opening distance at 750 μm from the scleral spur with anterior chamber area, anterior chamber volume, anterior chamber width, lens vault, iris thickness measured at 750 μm from the scleral spur, and iris area, the same multivariable linear regression models were applied to only nonglaucomatous subjects. Spherical equivalent was derived from the refraction using the following formula: sphere plus half of the cylinder. All statistical analyses were performed with JMP statistical software (Ver. 10.0; SAS Institute, Cary, NC, USA). AS-OCT images were analyzed with Zhongshan Angle Assessment Program. Scleral spurs were localized by a single observer (BC) while masked to the patient’s ophthalmic examination results. To evaluate intra-observer reproducibility, 25 images were randomly selected and anterior segment parameters were re-measured by the same observer (BC) on two separate occasions. The intra-observer reproducibility was assessed with an intra-class correlation coefficient.
Results
This study enrolled a total of 474 white, African, Hispanic, and Chinese patients who met the inclusion criteria and were willing to participate. Among them, 11 patients were excluded due to poor AS-OCT imaging quality (evaluated on the basis of corneal reflection, continuity of anterior segment structures, motion artifacts, and indeterminate scleral spurs). The narrow angle subjects included 61 whites, 41 Chinese, 28 Africans, and 36 Hispanics. The open-angle subjects included 105 whites, 91 Chinese, 62 Africans, and 39 Hispanics. Among the 463 included patients, 398 subjects did not have a diagnosis of glaucoma and 65 subjects had a diagnosis of glaucoma. Table 1 summarizes the comparisons of anterior chamber area, anterior chamber volume, anterior chamber width, lens vault, iris thickness measured at 750 μm from the scleral spur, iris area, age, sex, pupil diameter, and spherical equivalent between ethnic groups.
Table 1.
Comparison of Demographics and Anterior Segment Biometric Parameters between Ethnic Groups
| Ethnicity | White | Chinese | African | Hispanic | P Value |
|---|---|---|---|---|---|
| No. Patients [Total] | 166 | 132 | 90 | 75 | 0.069Φ |
| Narrow | 61 | 41 | 28 | 36 | |
| Open | 105 | 91 | 62 | 39 | |
| Age [Total] (years) | 66.3 ± 13.0* | 64.6 ± 16.2* | 62.7 ± 13.0* | 67.1 ± 13.2* | 0.146Ω |
| Narrow (years) | 68.3 ± 12.6* | 70.4 ± 14.9* | 64.9 ± 9.8* | 66.7 ± 13.0* | 0.339Ω |
| Open (Years) | 65.1 ± 13.1* | 62.0 ± 16.1* | 61.7 ± 14.1* | 67.4 ± 13.6* | 0.108Ω |
| Sex [Total] (% female) | 53.61% | 57.58% | 64.44% | 65.33% | 0.218Φ |
| Narrow (% female) | 63.93% | 70.73% | 78.57% | 83.33% | |
| Open (% female) | 47.62% | 51.65% | 58.06% | 48.72% | |
| SE [Total] (diopters) | −0.4 ± 2.9* | −2.0 ± 4.0* | −0.1 ± 2.1* | 0.0 ± 3.0* | <0.0001Ω |
| Narrow (diopters) | 1.0 ± 2.2* | 0.8 ± 3.3* | −0.2 ± 1.6* | 0.9 ± 1.9* | 0.208Ω |
| Open (diopters) | −1.3 ± 3.0* | −3.1 ± 3.7* | −0.1 ± 2.4* | −1.0 ± 3.6* | <0.0001Ω |
| PD [Total] (mm) | 3.9 ± 1.3* | 4.1 ± 1.3* | 3.9 ± 1.0* | 4.0 ± 0.9* | 0.421Ω |
| Narrow (mm) | 3.7 ± 1.4* | 3.5 ± 1.5* | 3.8 ± 1.0* | 4.2 ± 0.7* | 0.164Ω |
| Open (mm) | 4.0 ± 1.3* | 4.4 ± 1.1* | 4.0 ± 0.9* | 3.9 ± 1.1* | 0.064Ω |
| ACA [Total] (mm2) | 21.1 ± 4.1* | 20.4 ± 4.3* | 21.2 ± 4.1* | 19.3 ± 3.7* | 0.009Ω |
| Narrow (mm2) | 18.3 ± 2.9* | 17.1 ± 2.3* | 18.2 ± 2.4* | 17.0 ± 2.2* | 0.032Ω |
| Open (mm2) | 22.7 ± 3.9* | 21.9 ± 4.1* | 22.6 ± 4.0* | 21.4 ± 3.5* | 0.279Ω |
| ACV [Total] (mm3) | 147.2 ± 36.9* | 137.4 ± 35.6* | 146.0 ± 35.3* | 129.7 ± 32.0* | 0.001Ω |
| Narrow (mm3) | 123.0 ± 25.5* | 110.6 ± 19.2* | 120.2 ± 20.4* | 110.8 ± 18.9* | 0.010Ω |
| Open (mm3) | 161.3 ± 35.3* | 149.4 ± 34.8* | 157.6 ± 34.5* | 147.1 ± 31.9* | 0.044Ω |
| ACW [Total] (mm) | 12.0 ± 0.5* | 11.7 ± 0.4* | 11.9 ± 0.4* | 11.7 ± 0.5* | <0.0001Ω |
| Narrow (mm) | 12.0 ± 0.5* | 11.5 ± 0.4* | 11.8 ± 0.4* | 11.7 ± 0.4* | <0.0001Ω |
| Open (mm) | 12.1 ± 0.5* | 11.7 ± 0.4* | 12.0 ± 0.4* | 11.8 ± 0.5* | <0.0001Ω |
| LV [Total] (mm) | 0.54 ± 0.31* | 0.41 ± 0.35* | 0.36 ± 0.30* | 0.50 ± 0.31* | <0.0001Ω |
| Narrow (mm) | 0.76 ± 0.23* | 0.68 ± 0.22* | 0.63 ± 0.21* | 0.69 ± 0.21* | 0.043Ω |
| Open (mm) | 0.41 ± 0.28* | 0.29 ± 0.33* | 0.24 ± 0.26* | 0.33 ± 0.28* | 0.002Ω |
| IT750 [Total] (mm) | 0.43 ± 0.08* | 0.47 ± 0.08* | 0.46 ± 0.09* | 0.46 ± 0.08* | 0.008Ω |
| Narrow (mm) | 0.43 ± 0.07* | 0.48 ± 0.10* | 0.49 ± 0.09* | 0.47 ± 0.09* | 0.015Ω |
| Open (mm) | 0.43 ± 0.08* | 0.46 ± 0.07* | 0.44 ± 0.09* | 0.44 ± 0.06* | 0.017Ω |
| IAREA [Total] (mm2) | 1.53 ± 0.24* | 1.59 ± 0.23* | 1.66 ± 0.26* | 1.61 ± 0.29* | <0.0001Ω |
| Narrow (mm2) | 1.58 ± 0.25* | 1.64 ± 0.25* | 1.71 ± 0.18* | 1.61 ± 0.26* | 0.114Ω |
| Open (mm2) | 1.50 ± 0.23* | 1.57 ± 0.23* | 1.64 ± 0.28* | 1.61 ± 0.32* | 0.003Ω |
| CCT [Total] (μm) | 559 ± 33* | 552 ± 43* | 538 ± 36* | 555 ± 37* | <0.0001Ω |
| Narrow (μm) | 562 ± 33* | 555 ± 52* | 540 ± 36* | 556 ± 30* | 0.106Ω |
| Open (μm) | 557 ± 34* | 551 ± 38* | 537 ± 38* | 554 ± 36* | 0.008Ω |
| IOP [Total] (mm Hg) | 16.3 ± 3.7* | 16.1 ± 3.0* | 16.7 ± 3.7* | 17.2 ± 4.7* | 0.222Ω |
| Narrow (mm Hg) | 16.4 ± 3.7* | 16.5 ± 3.7* | 17.2 ± 3.1* | 17.5 ± 5.6* | 0.529Ω |
| Open (mm Hg) | 16.2 ± 3.8* | 16.0 ± 2.7* | 16.5 ± 4.0* | 17.3 ± 3.4* | 0.367Ω |
SE = spherical equivalent; PD = pupil diameter; ACA = anterior chamber area; ACV = anterior chamber volume; ACW = anterior chamber width; LV = lens vault; IT750 = iris thickness measured at 750 μm from the scleral spur; IAREA = iris area; CCT = central corneal thickness; IOP = intraocular pressure.
Data are expressed as mean value ± standard deviation.
P values by Pearson Chi Square.
P values by one-way ANOVA with Tukey’s post-hoc test.
In the narrow angle group, white, Chinese, African, and Hispanic subjects significantly differed in anterior chamber area, anterior chamber volume, anterior chamber width, lens vault, and iris thickness measured at 750 μm from the scleral spur (all P < 0.05). In the open angle group, white, Chinese, African, and Hispanic subjects significantly differed in anterior chamber volume, anterior chamber width, lens vault, iris thickness measured at 750 μm from the scleral spur, and iris area (all P < 0.05). Merging both narrow angle and open angle groups together showed significant difference in anterior chamber area, anterior chamber volume, anterior chamber width, lens vault, iris thickness measured at 750 μm from the scleral spur, and iris area among white, Chinese, African, and Hispanic subjects (all P < 0.05).
Table 2 lists the comparisons of anterior chamber area, anterior chamber volume, anterior chamber width, lens vault, iris thickness measured at 750 μm from the scleral spur, iris area, age, sex, pupil diameter, and spherical equivalent between narrow angle and open angle subjects within each ethnic group. For the white group, significant differences were observed in anterior chamber area, anterior chamber volume, and lens vault between narrow angle and open angle subjects (all P < 0.05). For the Chinese group, significant differences were observed in pupil diameter, anterior chamber area, anterior chamber volume, anterior chamber width, and lens vault between narrow angle and open angle subjects (all P < 0.05). For the African group, significant differences were observed in anterior chamber area, anterior chamber volume, anterior chamber width, lens vault, and iris thickness measured at 750 μm from the scleral spur between narrow angle and open angle subjects (all P < 0.05). For the Hispanic group, significant differences were observed in anterior chamber area, anterior chamber volume, lens vault, and iris thickness measured at 750 μm from the scleral spur between narrow angle and open angle subjects (all P < 0.05).
Table 2.
Comparison of Demographics and Anterior Segment Biometric Parameters between Open and Narrow Angles within Ethnic Groups
| Ethnicity | Ethnicity | Narrow Angle | Open Angle | P Value |
|---|---|---|---|---|
| No. Patients | White | 61 | 105 | |
| Chinese | 41 | 91 | ||
| African | 28 | 62 | ||
| Hispanic | 36 | 39 | ||
| Age (years) | White | 68.3 ± 12.6* | 65.1 ± 13.1* | 0.128Ω |
| Chinese | 70.4 ± 14.9* | 62.0 ± 16.1* | 0.004Ω | |
| African | 64.9 ± 9.8* | 61.7 ± 14.1* | 0.216Ω | |
| Hispanic | 66.7 ± 13.0* | 67.4 ± 13.6* | 0.822Ω | |
| Sex (% female) | White | 63.93% | 47.62% | 0.042Φ |
| Chinese | 70.73% | 51.65% | 0.040Φ | |
| African | 78.57% | 58.06% | 0.059Φ | |
| Hispanic | 83.33% | 48.72% | 0.001Φ | |
| SE (diopters) | White | 1.0 ± 2.2* | −1.3 ± 3.0* | <0.0001Ω |
| Chinese | 0.8 ± 3.3* | −3.1 ± 3.7* | <0.0001Ω | |
| African | −0.2 ± 1.6* | −0.1 ± 2.4* | 0.951Ω | |
| Hispanic | 0.9 ± 1.9* | −1.0 ± 3.6* | 0.013Ω | |
| PD (mm) | White | 3.7 ± 1.4* | 4.0 ± 1.3* | 0.096Ω |
| Chinese | 3.5 ± 1.5* | 4.4 ± 1.1* | 0.002Ω | |
| African | 3.8 ± 1.0* | 4.0 ± 0.9* | 0.240Ω | |
| Hispanic | 4.2 ± 0.7* | 3.9 ± 1.1* | 0.201Ω | |
| ACA (mm2) | White | 18.3 ± 2.9* | 22.7 ± 3.9* | <0.0001Ω |
| Chinese | 17.1 ± 2.3* | 21.9 ± 4.1* | <0.0001Ω | |
| African | 18.2 ± 2.4* | 22.6 ± 4.0* | <0.0001Ω | |
| Hispanic | 17.0 ± 2.2* | 21.4 ± 3.5* | <0.0001Ω | |
| ACV (mm3) | White | 123.0 ± 25.5* | 161.3 ± 35.3* | <0.0001Ω |
| Chinese | 110.6 ± 19.2* | 149.4 ± 34.8* | <0.0001Ω | |
| African | 120.2 ± 20.4* | 157.6 ± 34.5* | <0.0001Ω | |
| Hispanic | 110.8 ± 18.9* | 147.1 ± 31.9* | <0.0001Ω | |
| ACW (mm) | White | 12.0 ± 0.5* | 12.1 ± 0.5* | 0.166Ω |
| Chinese | 11.5 ± 0.4* | 11.7 ± 0.4* | 0.011Ω | |
| African | 11.8 ± 0.4* | 12.0 ± 0.4* | 0.020Ω | |
| Hispanic | 11.7 ± 0.4* | 11.8 ± 0.5* | 0.657Ω | |
| LV (mm) | White | 0.76 ± 0.23* | 0.41 ± 0.28* | <0.0001Ω |
| Chinese | 0.68 ± 0.22* | 0.29 ± 0.33* | <0.0001Ω | |
| African | 0.63 ± 0.21* | 0.24 ± 0.26* | <0.0001Ω | |
| Hispanic | 0.69 ± 0.21* | 0.33 ± 0.28* | <0.0001Ω | |
| IT750 (mm) | White | 0.43 ± 0.07* | 0.43 ± 0.08* | 0.532Ω |
| Chinese | 0.48 ± 0.10* | 0.46 ± 0.07* | 0.372Ω | |
| African | 0.49 ± 0.09* | 0.44 ± 0.09* | 0.022Ω | |
| Hispanic | 0.47 ± 0.09* | 0.44 ± 0.06* | 0.075Ω | |
| IAREA (mm2) | White | 1.58 ± 0.25* | 1.50 ± 0.23* | 0.051Ω |
| Chinese | 1.64 ± 0.25* | 1.57 ± 0.23* | 0.137Ω | |
| African | 1.71 ± 0.18* | 1.64 ± 0.28* | 0.148Ω | |
| Hispanic | 1.61 ± 0.26* | 1.61 ± 0.32* | 0.999Ω | |
| CCT (μm) | White | 562 ± 33 | 557 ± 34 | 0.318Ω |
| Chinese | 555 ± 52 | 551 ± 38 | 0.678Ω | |
| African | 540 ± 36 | 537 ± 38 | 0.696Ω | |
| Hispanic | 556 ± 30 | 554 ± 36 | 0.760Ω | |
| IOP (mm Hg) | White | 16.4 ± 3.7 | 16.2 ± 3.8 | 0.753Ω |
| Chinese | 16.5 ± 3.7 | 16.0 ± 2.7 | 0.392Ω | |
| African | 17.2 ± 3.1 | 16.5 ± 4.0 | 0.421Ω | |
| Hispanic | 17.5 ± 5.6 | 17.3 ± 3.4 | 0.840Ω |
SE = spherical equivalent; PD = pupil diameter; ACA = anterior chamber area; ACV = anterior chamber volume; ACW = anterior chamber width; LV = lens vault; IT750 = iris thickness measured at 750 μm from the scleral spur; IAREA = iris area; CCT = central corneal thickness; IOP = intraocular pressure.
Data are expressed as mean value ± standard deviation.
P values by Pearson Chi Square.
P values by independent Student t tests.
Tables 3 and 4 provide the results of multivariable linear regression analyses that evaluated the association of trabecular-iris space area at 750 μm from the scleral spur and angle opening distance at 750 μm from the scleral spur with anterior chamber area, anterior chamber volume, anterior chamber width, lens vault, iris thickness measured at 750 μm from the scleral spur and iris area. The multivariable regression models adjusted for age, sex, pupil diameter, and spherical equivalent. Anterior chamber area, anterior chamber volume, and lens vault were significant predictors of trabecular-iris space area at 750 μm from the scleral spur and angle opening distance at 750 μm from the scleral spur in all ethnic groups (all P<0.001).
Table 3.
Multivariable linear regression models showing the association between six anterior segment biometric factors and angle opening distance at 750 μm from the scleral spur.
| Ethnicity | White | Chinese | African | Hispanic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | R2 | P | β | R2 | P | β | R2 | P | β | R2 | P | |
| ACA (mm2) | 0.771 | 0.675 | <0.001 | 0.885 | 0.671 | <0.001 | 0.903 | 0.747 | <0.001 | 0.757 | 0.708 | <0.001 |
| ACV (mm3) | 0.757 | 0.663 | < 0.001 | 0.864 | 0.676 | <0.001 | 0.887 | 0.755 | <0.001 | 0.752 | 0.687 | <0.001 |
| ACW (mm) | 0.219 | 0.252 | 0.004 | 0.075 | 0.322 | 0.533 | 0.283 | 0.282 | 0.031 | 0.093 | 0.266 | 0.569 |
| LV (mm) | −0.580 | 0.481 | < 0.001 | −0.732 | 0.556 | <0.001 | −0.816 | 0.702 | <0.001 | −0.672 | 0.639 | <0.001 |
| IT750 (mm) | −0.201 | 0.251 | 0.009 | −0.099 | 0.327 | 0.366 | −0.387 | 0.331 | 0.005 | 0.025 | 0.259 | 0.872 |
| IAREA (mm2) | −0.069 | 0.212 | 0.373 | −0.271 | 0.364 | 0.041 | −0.243 | 0.256 | 0.085 | 0.175 | 0.281 | 0.309 |
ACA = anterior chamber area; ACV = anterior chamber volume; ACW = anterior chamber width; LV = lens vault; IT750 = iris thickness measured at 750 μm from the scleral spur; IAREA = iris area; β = standardized regression coefficient; R2= coefficient of determination.
Multivariable linear regression models, adjusted for age, sex, pupil diameter, and spherical equivalent.
Table 4.
Multivariable linear regression models showing the association between six anterior segment biometric factors and trabecular-iris space area at 750 μm from the scleral spur.
| Ethnicity | White | Chinese | African | Hispanic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | R2 | P | β | R2 | P | β | R2 | P | β | R2 | P | |
| ACA (mm2) | 0.767 | 0.628 | <0.001 | 0.790 | 0.569 | <0.001 | 0.918 | 0.714 | <0.001 | 0.718 | 0.652 | <0.001 |
| ACV (mm3) | 0.768 | 0.635 | <0.001 | 0.791 | 0.587 | <0.001 | 0.889 | 0.708 | <0.001 | 0.706 | 0.625 | <0.001 |
| ACW (mm) | 0.266 | 0.230 | 0.001 | 0.096 | 0.294 | 0.436 | 0.236 | 0.209 | 0.083 | 0.040 | 0.250 | 0.806 |
| LV (mm) | −0.517 | 0.388 | <0.001 | −0.622 | 0.459 | <0.001 | −0.835 | 0.676 | <0.001 | −0.692 | 0.651 | <0.001 |
| IT750 (mm) | −0.225 | 0.229 | 0.004 | −0.020 | 0.287 | 0.856 | −0.270 | 0.218 | 0.061 | 0.057 | 0.251 | 0.716 |
| IAREA (mm2) | −0.063 | 0.169 | 0.430 | −0.177 | 0.307 | 0.197 | −0.203 | 0.191 | 0.165 | 0.129 | 0.260 | 0.460 |
ACA = anterior chamber area; ACV = anterior chamber volume; ACW = anterior chamber width; LV = lens vault; IT750 = iris thickness measured at 750 μm from the scleral spur; IAREA = iris area; β = standardized regression coefficient; R2= coefficient of determination.
Multivariable linear regression models, adjusted for age, sex, pupil diameter, and spherical equivalent.
Among the six anterior segment biometric parameters, anterior chamber area, anterior chamber volume, and lens vault were the most significant predictors of variation in trabecular-iris space area at 750 μm from the scleral spur across all four ethnic groups. Anterior chamber area (β=0.767, R2=0.628, P<0.001), anterior chamber volume (β=0.768, R2=0.635, P<0.001), anterior chamber width (β=0.266, R2=0.230, P=0.001), lens vault (β=−0.517, R2=0.388, P<0.001), and iris thickness measured at 750 μm from the scleral spur (β=−0.225, R2=0.229, P=0.004) were significant predictor of trabecular-iris space area at 750 μm from the scleral spur in whites. Anterior chamber area (β=0.790, R2=0.569, P<0.001), anterior chamber volume (β=0.791, R2=0.587, P<0.001), and lens vault (β=−0.622, R2=0.459, P<0.001) were significant predictor of trabecular-iris space area at 750 μm from the scleral spur in Chinese. Anterior chamber area (β=0.918, R2=0.714, P<0.001), anterior chamber volume (β=0.889, R2=0.708, P<0.001), and lens vault (β=−0.835, R2=0.676, P<0.001) were significant predictor of trabecular-iris space area at 750 μm from the scleral spur in Africans. Anterior chamber area (β=0.718, R2=0.652, P<0.001), anterior chamber volume (β=0.706, R2=0.625, P<0.001), and lens vault (β=−0.692, R2=0.651, P<0.001) were significant predictor of trabecular-iris space area at 750 μm from the scleral spur in Hispanics.
Among the six anterior segment biometric parameters, anterior chamber area, anterior chamber volume, and lens vault were the most significant predictors of variation in angle opening distance at 750 μm from the scleral spur across all four ethnic groups. Anterior chamber area (β=0.771, R2=0.675, P<0.001), anterior chamber volume (β=0.757, R2=0.663, P<0.001), anterior chamber width (β=0.219, R2=0.252, P=0.004), lens vault (β=−0.580, R2=0.481, P<0.001), and iris thickness measured at 750 μm from the scleral spur (β=−0.201, R2=0.251, P<0.009) were significant predictor of AOD750 in whites. Anterior chamber area (β=0.885, R2=0.671, P<0.001), anterior chamber volume (β=0.864, R2=0.676, P<0.001), and lens vault (β=−0.732, R2=0.556, P<0.001) were significant predictor of AOD750 in Chinese. Anterior chamber area (β=0.903, R2=0.747, P<0.001), anterior chamber volume (β=0.887, R2=0.755, P<0.001), anterior chamber width (β=0.283, R2=0.282, P=0.031), lens vault (β=−0.816, R2=0.702, P<0.001), and iris thickness measured at 750 μm from the scleral spur (β=−0.387, R2=0.311, P=0.005) were significant predictor of AOD750 in Africans. Anterior chamber area (β=0.757, R2=0.708, P<0.001), anterior chamber volume (β=0.752, R2=0.687, P<0.001), and lens vault (β=−0.672, R2=0.639, P<0.001) were significant predictor of AOD750 in Hispanics.
Application of the multivariable linear regression models to only nonglaucomatous subjects showed similar results. Out the six anterior segment parameters, anterior chamber area, anterior chamber volume, and lens vault were the most predictive of trabecular-iris space area at 750 μm from the scleral spur and angle opening distance at 750 μm from the scleral spur across all four ethnic groups in terms of both standardized regression coefficients (β) and coefficients of determination (R2).
Table 5 displays the intraobserver reproducibility of anterior segment biometric parameters measurements in a randomly selected subset of 25 eyes. All parameters demonstrated fair to excellent reproducibility with intraclass correlations.
Table 5.
Reproducibility of Anterior Segment Biometric Parameter Measurements in a Random Subset of Eyes
| Parameters | ICC | Mean 1 | Mean 2 | Difference | 95% CI |
|---|---|---|---|---|---|
| PD (mm) | 0.894 | 4.29 | 4.37 | −0.08 | (−0.19, 0.03) |
| ACA (mm2) | 0.998 | 20.67 | 20.71 | −0.04 | (−0.11, 0.03) |
| ACV (mm3) | 0.995 | 140.64 | 141.10 | −0.46 | (−1.35, 0.42) |
| ACW (mm) | 0.934 | 11.80 | 11.82 | −0.02 | (−0.07, 0.03) |
| LV (mm) | 0.957 | 432.30 | 464.23 | −31.93 | (−58.47, −5.38) |
| IT750 (mm) | 0.796 | 0.48 | 0.47 | 0.01 | (−0.01, 0.02) |
| IAREA (mm2) | 0.957 | 1.61 | 1.60 | 0.01 | (−0.01, 0.04) |
| AOD750 (mm) | 0.932 | 0.40 | 0.39 | 0.01 | (−0.01, 0.04) |
| TISA750 (mm2) | 0.924 | 0.19 | 0.19 | 0.01 | (0.00, 0.02) |
ICC = intraclass correlation coefficient; CI = confidence interval; PD = pupil diameter; ACA = anterior chamber area; ACV = anterior chamber volume; ACW = anterior chamber width; LV = lens vault; IT750 = iris thickness measured at 750 μm from the scleral spur; IAREA = iris area; AOD750 = angle opening distance at 750 μm from the scleral spur; TISA = trabecular-iris space area at 750 μm from the scleral spur.
Discussion
Foo et al. previously developed a mathematic model for predicting anterior chamber angle width using six anterior segment biometric parameters.4 By entering anterior chamber area, anterior chamber volume, anterior chamber width, lens vault, iris thickness measured at 750 μm from the scleral spur, and iris area into multivariable mathematic functions, their predictive mathematic model produced probability estimations that explained 81.4% of the variability in trabecular-iris space area at 750 μm from the scleral spur and 85.5% of the variability in angle opening distance at 750 μm from the scleral spur. However, their study consisted of only Chinese subjects. Concern exists as to the generalizability of their findings to other ethnic populations such as whites, Africans, and Hispanics. The present study sought to demonstrate the external validity of the anterior chamber angle width predictive model by comparing the predictive strength of the six anterior segment biometric parameters for two anterior chamber angle width parameters across multiple ethnic populations. Our results showed significant differences in anterior segment morphology among the four ethnic groups in the comparisons of subjects with only narrow angles, only open angles, and both narrow and open angles combined. Despite ethnic differences in anterior chamber area, anterior chamber volume, anterior chamber width, lens vault, iris thickness measured at 750 μm from the scleral spur, and iris area, three of the anterior segment biometric parameters showed significant association with both anterior chamber angle width parameters across in four ethnic groups. To our knowledge, this is the first study to demonstrate that anterior chamber area, anterior chamber volume, and lens vault were stronger predictors than anterior chamber width, iris thickness measured at 750 μm from the scleral spur, and iris area for trabecular-iris space area at 750 μm from the scleral spur and angle opening distance at 750 μm from the scleral spur in white, African, Hispanic, and Chinese subjects.
The association between angle-closure related pathology and measurements of anterior chamber area, anterior chamber volume, anterior chamber width, lens vault, iris thickness measured at 750 μm from the scleral spur and iris area has previously been reported. Wu et al. found smaller anterior chamber area and anterior chamber volume to be independently associated with narrow angles.17 Anterior chamber area and anterior chamber volume are the area and volume bounded by the cornea, the iris, and the anterior surface of the lens; therefore the characteristics of anterior chamber area and anterior chamber volume reflect the composite features of these tissues. Nongpiur et al. observed a smaller anterior chamber width in eyes with narrow angles and reasoned that because the circumference of a circle is estimated through diameter times pi (a value of 3.14), a unit change in the width (diameter) of the anterior chamber of the eye (approximated as a circle) would result in a circumferential change of 3.14 units.18 Under such conditions, a 1-mm decrease in the anterior chamber width from an average of 12-mm would result in a 3.14-mm decrease in the circumference of the anterior chamber drainage structures, which is equivalent to an 8.3% reduction. Additionally, Nongpiur et al. recognized greater lens vault as an anatomical parameter that is independently associated with angle closure.19 Lens vault quantifies the degree to which a lens is located anterior to the plane of the angle and increased bulk of the lens anterior to the plane of the scleral spur may push the peripheral iris against the trabecular meshwork and lead to angle crowding. Finally, greater iris thickness measured at 750 μm from the scleral spur and iris area have been shown to promote narrow angles because a thicker peripheral iris is likely to promote angle closure as the peripheral iris would be in closer proximity to the angle.20–22
The present study’s finding of ethnic differences in these six anterior segment biometric parameters may explain the variation in prevalence rate of PACG among the four ethnic groups. In all three categories of comparisons (only narrow angles, only open angles, and both narrow and open angles), our analyses indicated that whites possessed the highest mean values for anterior chamber area, anterior chamber volume, anterior chamber width, and lens vault, but simultaneously had the lowest mean values for iris thickness measured at 750 μm from the scleral spur and iris area. Chinese and Hispanic subjects shared the highest mean values for iris thickness measured at 750 μm from the scleral spur and the lowest mean values for anterior chamber area, anterior chamber volume, and anterior chamber width. African subjects exhibited the highest mean value for iris area and the lowest mean value for lens vault. Considering that smaller anterior chamber area, anterior chamber volume, and anterior chamber width, and greater lens vault, iris thickness measured at 750 μm from the scleral spur and iris area contribute to the development of narrow angles and the anterior chamber angle width is considered the key anatomic parameter that determines risk for PACG, the measurements in our study suggest that Chinese and Hispanics are more susceptible to PACG in comparison to whites and Africans. This outcome is in line with results from a recent study, which investigated the prevalence rate of PACG across different ethnicities and identified that PACG is more common in Asians and Hispanics. Stein et al. examined over 2 million eye care recipients and found the following prevalence rates of PACG: 1.35% in whites, 1.65% in Africans, 2.04% in Hispanics, and 3.01% in Asians.23
Whites displayed relatively greater lens vault, despite maintaining low prevalence rates of PACG. PACG is a multifactorial disease and the present study demonstrated that its key risk factor, the anterior chamber angle width, is determined by a combination of six anterior segment biometric parameters. Although anterior chamber area, anterior chamber volume, anterior chamber width, lens vault, iris thickness measured at 750 μm from the scleral spur and iris area contribute collectively to establish the angle configuration, certain anterior segment biometric parameters have more influence on the anterior chamber angle width than others. Among the six anterior segment biometric parameters, anterior chamber area, anterior chamber volume, and lens vault were the strongest determinants of both trabecular-iris space area at 750 μm from the scleral spur and angle opening distance at 750 μm from the scleral spur. High measurements in two angle-widening parameters may offset the effects of high measurements in one angle-narrowing parameter, leading to an overall wide anterior chamber angle in whites.
This study suffers from a common limitation inherent in most studies exploring ethnic differences in disease characteristics, namely the ethnic classification of study subjects. Ethnic groups in our study are not stratified according to specific anthropologically based criteria. Instead, the classification used in our study is based on the subjects’ self-reported ancestry of both parents. Additionally, the present study recruited subjects from a university-based general ophthalmology and glaucoma clinics, thus our study population may have suffered from selection bias despite consecutive subject enrollment. The study subjects are not population-based, and therefore, should not be expected to represent the general population. This study employed convenience sampling, which further limits generalizability. Our convenience sample may also be enriched in narrow angle subjects due to referral patterns. Another limitation lies in the reliance of the Zhongshan Angle Assessment Program on manual scleral spur identification as a measurement reference point for image processing. Manual scleral spur localization is subjective in nature, but our study design controlled for this by utilizing a single ophthalmologist, masked to the patient’s ethnicity and clinical examination results, to read all the AS-OCT images. We also selected a random subset of 25 AS-OCT images for reproducibility testing by the same ophthalmologist at a separate session. The random subset of 25 AS-OCT images selected for reproducibility testing yielded fair to excellent intraobserver correlation.
In summary, the results of our study add to the evidence that ethnic variation in anterior segment biometric parameters exists. Among the six anterior segment biometric parameters, anterior chamber area, anterior chamber volume, and lens vault were stronger than anterior chamber width, iris thickness measured at 750 μm from the scleral spur, and iris area in predicting variance of anterior chamber angle width (both TISA750 and AOD750) in all four ethnic groups. So while PACG is a multifactorial disease caused by a combination of variation in certain anterior segment structural features that are associated with anterior chamber angle width, some factors consistently predict anterior chamber angle width better than other factors across different ethnicities. Our results suggest that differences in anterior chamber area, anterior chamber volume, and lens vault may be one of the main causes of differences in the prevalence of PACG across ethnicities. Further population-based investigation with a larger sample size is warranted to confirm our findings.
Acknowledgments
Funding/Support: This study was supported by NIH-NEI EY002162 – Core Grant for Vision Research, Research to Prevent Blindness, and That Man May See, Inc.
Other Acknowledgments: None.
Biography

Roland Y. Lee received his undergraduate degree with High Distinction in General Scholarship from the University of California, Berkeley, where he was also inducted into Phi Beta Kappa. He is currently pursuing his medical education at the Wake Forest University School of Medicine with a merit-based, full-tuition scholarship. His research interests include causes of glaucoma, glaucoma predisposition and development in different ethnic groups, glaucoma surgical procedures, and ocular imaging.
Footnotes
Financial Disclosures:
Roland Y. Lee: none
Brian H. Chon: consultant for Transcend Medical.
Shuai-Chun Lin: none
Mingguang He: none
Shan C. Lin: consultant for Allergan.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Foster PJ. The epidemiology of primary angle closure and associated glaucomatous optic neuropathy. Semin Ophthalmol. 2002;17(2):50–8. doi: 10.1076/soph.17.2.50.14718. [DOI] [PubMed] [Google Scholar]
- 2.Yip JL, Foster PJ. Ethnic differences in primary angle-closure glaucoma. Curr Opin Ophthalmol. 2006;17(2):175–80. doi: 10.1097/01.icu.0000193078.47616.aa. [DOI] [PubMed] [Google Scholar]
- 3.Radhakrishnan S, See J, Smith SD, et al. Reproducibility of anterior chamber angle measurements obtained with anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48(8):3683–8. doi: 10.1167/iovs.06-1120. [DOI] [PubMed] [Google Scholar]
- 4.Foo LL, Nongpiur ME, Allen JC, et al. Determinants of angle width in Chinese Singaporeans. Ophthalmology. 2012;119(2):278–82. doi: 10.1016/j.ophtha.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 5.Shaffer RN. Primary glaucomas. Gonioscopy, ophthalmoscopy and perimetry. Trans Am Acad Ophthalmol Otolaryngol. 1960;64:112–27. [PubMed] [Google Scholar]
- 6.Lee RY, Huang G, Cui QN, He M, Porco TC, Lin SC. Association of lens vault with narrow angles among different ethnic groups. Curr Eye Res. 2012;37(6):486–91. doi: 10.3109/02713683.2012.669006. [DOI] [PubMed] [Google Scholar]
- 7.Huang G, Gonzalez E, Peng PH, et al. Anterior chamber depth, iridocorneal angle width, and intraocular pressure changes after phacoemulsification: narrow vs open iridocorneal angles. Arch Ophthalmol. 2011;129(10):1283–90. doi: 10.1001/archophthalmol.2011.272. [DOI] [PubMed] [Google Scholar]
- 8.Seider MI, Pekmezci M, Han Y, et al. High prevalence of narrow angles among Chinese-American glaucoma and glaucoma suspect patients. J Glaucoma. 2009;18(8):578–81. doi: 10.1097/IJG.0b013e3181996f19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung CY, Liu S, Weinreb RN, et al. Dynamic analysis of iris configuration with anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51(8):4040–6. doi: 10.1167/iovs.09-3941. [DOI] [PubMed] [Google Scholar]
- 10.Leung CK, Palmiero PM, Weinreb RN, et al. Comparisons of anterior segment biometry between Chinese and Caucasians using anterior segment optical coherence tomography. Br J Ophthalmol. 2010;94(9):1184–9. doi: 10.1136/bjo.2009.167296. [DOI] [PubMed] [Google Scholar]
- 11.Lee RY, Kasuga T, Cui QN, Huang G, He M, Lin SC. Association between baseline angle width and induced angle opening following prophylactic laser peripheral iridotomy. Invest Ophthalmol Vis Sci. 2013;54(5):3763–70. doi: 10.1167/iovs.13-11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee RY, Kasuga T, Cui QN, Huang G, He M, Lin SC. Comparison of anterior segment morphology following prophylactic laser peripheral iridotomy in Caucasian and Chinese eyes. Clin Experiment Ophthalmol. 2014;42(5):417–26. doi: 10.1111/ceo.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee RY, Kasuga T, Cui QN, et al. Association between Baseline Iris Thickness and Prophylactic Laser Peripheral Iridotomy Outcomes in Primary Angle-Closure Suspects. Ophthalmology. 2014;121(6):1194–202. doi: 10.1016/j.ophtha.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee RY, Kasuga T, Cui QN, Huang G, Wang SY, Lin SC. Ethnic differences in intraocular pressure reduction and changes in anterior segment biometric parameters following cataract surgery by phacoemulsification. Clin Experiment Ophthalmol. 2013;41(5):442–9. doi: 10.1111/ceo.12032. [DOI] [PubMed] [Google Scholar]
- 15.Pavlin CJ, Harasiewicz K, Foster FS. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am J Ophthalmol. 1992;113(4):381–9. doi: 10.1016/s0002-9394(14)76159-8. [DOI] [PubMed] [Google Scholar]
- 16.Narayanaswamy A, Sakata LM, He MG, et al. Diagnostic performance of anterior chamber angle measurements for detecting eyes with narrow angles: an anterior segment OCT study. Arch Ophthalmol. 2010;128(10):1321–7. doi: 10.1001/archophthalmol.2010.231. [DOI] [PubMed] [Google Scholar]
- 17.Wu RY, Nongpiur ME, He MG, et al. Association of narrow angles with anterior chamber area and volume measured with anterior-segment optical coherence tomography. Arch Ophthalmol. 2011;129(5):569–74. doi: 10.1001/archophthalmol.2011.68. [DOI] [PubMed] [Google Scholar]
- 18.Nongpiur ME, Sakata LM, Friedman DS, et al. Novel association of smaller anterior chamber width with angle closure in Singaporeans. Ophthalmology. 2010;117(10):1967–73. doi: 10.1016/j.ophtha.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Nongpiur ME, He M, Amerasinghe N, et al. Lens vault, thickness, and position in Chinese subjects with angle closure. Ophthalmology. 2011;118(3):474–9. doi: 10.1016/j.ophtha.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Wang BS, Narayanaswamy A, Amerasinghe N, et al. Increased iris thickness and association with primary angle closure glaucoma. Br J Ophthalmol. 2011;95(1):46–50. doi: 10.1136/bjo.2009.178129. [DOI] [PubMed] [Google Scholar]
- 21.Wang B, Sakata LM, Friedman DS, et al. Quantitative iris parameters and association with narrow angles. Ophthalmology. 2010;117(1):11–7. doi: 10.1016/j.ophtha.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Lee RY, Huang G, Porco TC, Chen YC, He M, Lin SC. Differences in iris thickness among African Americans, Caucasian Americans, Hispanic Americans, Chinese Americans, and Filipino-Americans. J Glaucoma. 2013;22(9):673–8. doi: 10.1097/IJG.0b013e318264ba68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein JD, Kim DS, Niziol LM, et al. Differences in rates of glaucoma among Asian Americans and other racial groups, and among various Asian ethnic groups. Ophthalmology. 2011;118(6):1031–7. doi: 10.1016/j.ophtha.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]