Abstract
The clinical diagnosis and classification of neurodegenerative diseases based on clinical examination or available biomarkers are currently insufficiently accurate. Although histological examination is considered the gold standard for diagnosis, brain biopsies have been avoided because of the high risk-benefit ratio. However, brain biopsies have previously been performed with a craniotomy and excision of approximately 1 cc of cerebral cortex tissue, and it is possible that needle core brain biopsies would have a lower morbidity and mortality risk. Here, we compared the ability of simulated needle core biopsy versus simulated open biopsy to detect the frontal cortex histopathology associated with common neurodegenerative diseases in the elderly using 144 autopsy-proven cases. Simulated needle core biopsy, as compared to simulated open biopsy, gave close to 90% sensitivity and specificity for identifying graded densities of β-amyloid and neuritic plaques, neurofibrillary tangles, phosphorylated α-synuclein, and phosphorylated TDP-43 pathology. This study shows that the presence and densities of the most common molecular pathologies may be histopathologically assessed in simulated frontal cortex needle biopsies with accuracy very close to that obtained by open cortical biopsy. An accurate estimation of the morbidity and mortality risk associated with cortical needle core biopsy will require specifically designed clinical trials in appropriate subjects.
Keywords: Alzheimer disease, Amyloid plaque, Brain biopsy dementia with Lewy bodies, Frontotemporal dementia, Neurofibrillary tangle, Neuritic plaque, TDP-43
INTRODUCTION
Brain biopsy for dementia has generally been restricted to subjects with rapidly progressive disease or to exclude a potentially reversible cause such as vasculitis (1, 2). Because of the perception of a high risk-benefit ratio, brain biopsies when a primary neurodegenerative disorder is suspected have been contra-indicated for more than 30 years. In the absence of effective disease-modifying therapies, the benefit has been perceived to be low, whereas the risks, which include hemorrhage, infection, seizures and death, have been considered too high. One reason why the benefits of biopsy have been considered to be low is that the clinical diagnostic accuracy for neurodegenerative dementing diseases such as Alzheimer disease (AD) has been overestimated. A recent study of US National Institutes on Aging AD centers has shown that a standard neurological diagnostic workup for AD has a sensitivity and specificity of only approximately 70% when using the most common neuropathological criteria as the gold standard (3). For dementia with Lewy bodies (DLB), which is often regarded as the second most common cause of dementia, sensitivity is very poor, ranging from 12% to 32% (4). Moreover, clinical examination also has a very limited ability to determine the underlying molecular pathology in subjects with frontotemporal dementia (5, 6). If biopsy could improve clinical diagnostic accuracy, this might be of great benefit to patients because the chances of having successful clinical trials for promising new therapies might be much greater.
The failure, over the last 3 decades or more to find new effective therapeutic agents may be due, at least in part, to clinical diagnostic inaccuracy (7). Small effect size is the most common reason for phase II trial failures across medical fields (8). When new agents are designed to target specific disease-altered molecular pathways, and 30% or more of subjects in a clinical trial do not have those specific molecular alterations, the perceived effect size of the tested agent may be too low to warrant further trials. This problem is compounded by disease heterogeneity introduced by the frequent concurrence of different neurodegenerative conditions (9–11). Although there are many new clinical biomarkers in development, only those for amyloid are FDA-approved for the clinical setting (12–14). If brain biopsy could allow a much more accurate subject selection for clinical trials, patients may ultimately derive immeasurable benefits from the successful development and licensing of new, effective therapies. Additionally, brain biopsy might allow more rapid validation, as compared to autopsy, of less-invasive diagnostic clinical biomarkers.
Just as the benefits of brain biopsy may have been underestimated, the risks may have been overestimated because past experience has been primarily derived from the results of open brain biopsy, which requires craniotomy and approximately 1 cc of brain tissue. In the intervening years, stereotactic needle core brain biopsies have been extensively used to diagnose other brain diseases and the risks of complications and death appear to be much lower as compared with open biopsies. This seems to be particularly true at centers performing frequent needle core biopsies. At one such center, the mortality rate in 500 needle core biopsies was 0.2 % (15), as compared to an estimated 1% mortality for open biopsies (1). In a review encompassing multiple studies and more than 2500 stereotactic brain biopsies, Kongkham et al found that the morbidity rate ranged from 0.4% to 17.2%, with an average of 4.9% (16), whereas for open biopsies, Warren et al found that morbidity ranged from 2% to 14%, with an average of 11.5% (1). All of the studies of stereotactic biopsy were aimed at a mix of deep and superficial brain targets and it is likely that a needle biopsy of cerebral cortex would be less hazardous than one of the basal ganglia, thalamus or other diencephalic structures.
In support of this, in a study of stereotactic biopsy in 58 cases, Nishihara et al found that there were 3 subjects with procedure-related symptomatic hematoma; all 3 were in diencephalic structures (thalamus and basal ganglia) while of 43 biopsies from cerebral cortex or lobar white matter, there were 0 complications (17). A similar increased risk of biopsy of deep targets have been reported by Kongkham et al (16), Sawin et al (18), and McGirt et al (19). By contrast, Chen et al (20), and Paleologos et al (21) did not find an increased risk based on location. Also, many of the studies of safety of stereotactic biopsy have reported that biopsies of gliomas, lymphomas and other tumors, which would only very rarely be present in biopsies of subjects with presumed neurodegenerative dementia, account for a majority of the complications, particularly for hemorrhage (16–19, 22, 23).
It is not currently clear whether or not a needle core biopsy taken from a single cortical site would reliably give useful diagnostic information. Two recent studies investigated the theoretical diagnostic accuracy that might be obtained through open brain biopsies of the cerebral cortex (24, 25). These studies, done on postmortem cerebral cortex from subjects with neuropathologically confirmed diagnoses, showed that with approximately 1 cc of cortical tissue, cortical biopsy would be a highly sensitive and specific diagnostic tool capable of detecting the major causes of elderly dementia, including AD, DLB and frontotemporal lobar degeneration with TDP-43 pathology (FTLD-TDP). Additionally, studies of frontal cortex needle cores taken during shunt placement for normal pressure hydrocephalus have shown that the β-amyloid (Aβ) and tau pathology in the cores significantly correlated with Mini Mental State Examination scores as well as CSF levels of Aβ 1–42 and phosphorylated tau (p-tau) protein (26). Building on these studies, we undertook a comparison of simulated cortical needle core biopsy with open cortical biopsy, to determine whether the much smaller sample of tissue obtained with the needle biopsy would still give a useful level of diagnostic accuracy. The results indicate that cortical needle core biopsy may have useful sensitivity and specificity. Therefore, further evaluation may be warranted to determine more clearly the risks of cortical needle core biopsy in cognitively impaired subjects presumed to have a primary neurodegenerative cause.
MATERIALS AND METHODS
Human Subjects
Brain necropsy and complete neuropathological examination were performed on deceased elderly subjects who had volunteered for the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND) and Brain and Body Donation Program ([BBDP]; www.brainandbodydonationprogram.org). All enrolled subjects or their legal representatives signed an Institutional Review Board-approved informed consent form allowing both clinical assessments during life and several options for brain and/or bodily organ donation after death. All subjects were clinically characterized with annual standardized test batteries that include general neurological, cognitive, and movement disorder components (27, 28). Subjects were chosen by searching the BBDP database for cases neuropathologically diagnosed as AD (n = 63); Parkinson disease ([PD] n = 19); PD/AD (n = 18); DLB (n = 35); and FTLD-TDP43 (n = 9) (Table 1). Some cases had additional complicating pathologies such as vascular dementia, hippocampal sclerosis, and incidental Lewy bodies (Table 2). Cases were chosen to represent a wide range of frontal cortex Aβ plaques, neuritic plaques, neurofibrillary tangles, p-tau, phosphorylated α-synuclein (p-synuclein), and phosphorylated TDP-43 (p-TDP-43) histopathological lesion densities (Table 3).
Table 1.
General Characteristics of the Study Subjects by Neuropathologic Diagnosis, Age, Gender and Postmortem Interval
| Diagnosis (N) | Age (SD) | Gender (% M) | PMI, hours, mean (SD) | Last MMSE (SD) |
|---|---|---|---|---|
| AD (63) | 85.4 (8.9) | 53.4 | 3.4 (1.2) | 14.4 (8.4) |
| PD (37) | 76.9 (9.4) | 67.6 | 4.0 (3.0) | 18.6 (8.8) |
| DLB (35) | 81.2 (7.7) | 57.1 | 3.4 (1.2) | 11.7 (9.3) |
| FTLD-TDP43 (9) | 65.7 (10.8) | 66.7 | 3.2 (1.0) | 20.5 (10.9) |
AD, Alzheimer disease; PD, Parkinson disease; DLB, Dementia with Lewy bodies; FTD-TDP43, frontotemporal lobar degeneration with TDP-43 pathology; N, number of subjects; M, male; PMI, postmortem interval; SD, standard deviation; MMSE, Mini Mental State Examination.
Table 2.
Additional Neuropathological Findings in Study Subjects
| Diagnosis (N) | Alzheimer Disease | Parkinson Disease | Dementia with Lewy Bodies | Vascular Dementia (%) | Incidental Lewy Bodies (%) | Hippocampal Sclerosis (%) |
|---|---|---|---|---|---|---|
| Alzheimer disease (63) | NA | 0 | 0 | 12 (19) | 22 (35) | 3 (5) |
| Parkinson Disease (37) | 18 (49) | NA | 0 | 0 | 0 | 0 |
| Dementia with Lewy Bodies (35) | 32 (91) | 0 | NA | 1 (3) | 0 | 0 |
| Frontotemporal lobar degeneration with TDP-43 pathology (9) | 0 | 0 | 0 | 0 | 2 (22) | 4 (44) |
N, number of subjects; NA, not applicable.
Table 3.
Distribution of Histopathological Lesion Densities Within Source Blocks by Number and Percentage of Blocks with Each Density
| Density Estimate | Total Plaques Thioflavin S N (%) | Neuritic Plaques Thioflavin S N (%) | Total Plaques IHC N (%) | NF Change IHC N (%) | NFTs Thioflavin N (%) | P-synuclein IHC N (%) | P-TDP43 IHC N (%) |
|---|---|---|---|---|---|---|---|
| 0 | 26 (18) | 23 (16) | 27 (19) | 29 (20) | 85 (59) | 63 (44) | 12(44) |
| >0 | 118 (82) | 121 (84) | 117 (81) | 110 (79) | 59 (41) | 81(56) | 15 (56)* |
| ≥1 | 114 (79) | 115 (80) | 116 (81) | 97 (70) | 48 (33) | 80 (56) | NA |
| ≥2 | 105 (73) | 75 (52) | 103 (72) | 64 (46) | 37 (26) | 58 (40) | NA |
| ≥3 | 70 (49) | 12 (8) | 85 (59) | 46(33) | 21 (15) | 32 (22) | NA |
| ≥4 | NA | NA | NA | NA | NA | 11 (8) | NA |
The density scales used ranged from 0–3 for plaques, neurofibrillary change and tangles; for p-synuclein, a 0–4 scale was used. Total n = 144, except for p-tau (n = 139) and TDP43 (n = 35). Histopathology for p-TDP-3 was based on presence (>0) or absence (0). P= phosphorylated; IHC = immunohistochemical method; NF = neurofibrillary changes; NFT = neurofibrillary tangles; N= number of subjects; NA = not applicable.
Needle Core Biopsy
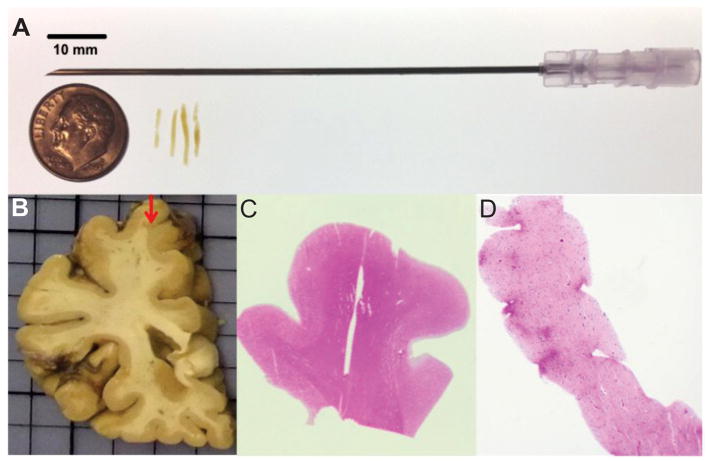
Four needle cores were obtained with an 18-gauge needle from fixed brain tissue of 144 diseased subjects chosen as described above (Fig. 1A). The mean length of the needle cores ± SD was 0.88 ± 0.24 cm. The cores were all collected from the crest of the superior frontal gyrus at the coronal level of the head of the caudate nucleus, at approximately the border of Brodmann areas 6 and 8 (Fig. 1B). A full-sized paraffin tissue block (approximately 2.0 x 2.0 cm), including the cortex immediately surrounding the four needle cores (“source block”), was also dissected. All the cores and the source blocks were embedded in paraffin and sectioned at 6 μm.
Figure 1.
Needle core biopsy of the frontal lobe cortex of autopsied subjects. (A) Four needle cores were collected with an 18-gauge needle from fixed tissue of deceased subjects with different neuropathologic diagnoses. (B) The cores were collected from the crest of the superior frontal gyrus at the level of the head of the caudate nucleus. (C, D) All the cores and the source blocks used for biopsy were embedded in paraffin and sectioned. All cores and source blocks were stained with hematoxylin and eosin to confirm the presence of gray and white matter. Core image in (D) was taken at a 20x magnification.
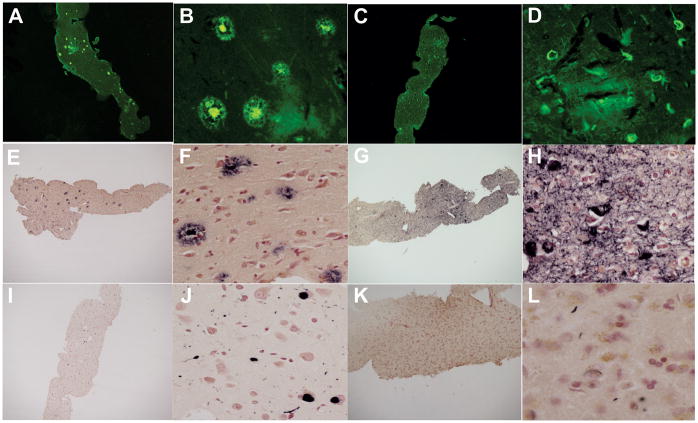
Sections from each of the cores and source blocks were stained with hematoxylin and eosin (n = 144) (Fig. 1C, D), Thioflavin S stain (n = 144) (29) (Fig. 2A, B), by immunohistochemistry using primary antibodies against p-tau (n = 139; clone AT8; Thermo Fisher Scientific, Waltham, MA), Aβ (n = 144; 6E10, Covance, Princeton, NJ), p-TDP-43 (n = 35), and p-synuclein (n = 144; Fig. 2E-L). The antibodies used for p-synuclein (raised against alpha-synuclein phosphorylated at serine 129) and p-TDP-43 (TDP-43 phosphorylated at serine 409/410) were privately developed and their characterization has been previously described (30–36). The signal development steps have been described in previous publications (10). These were identical in the procedures used for p-tau, Aβ, and pTDP-43 except for differing epitope exposure methods: 20 minutes in 80% formic acid for p-tau and Aβ; 20 minutes in boiling 0.1 M sodium citrate for p-TDP-43; and 20 minutes proteinase K pretreatment for p-synuclein. Primary antibody concentrations were 1:1,000 for p-tau and Aβ and 1:10,000 for p-synuclein and p-TDP-43.
Figure 2.
Diagnostic staining. (A-D) All core and source sections were stained with Thioflavin S, showing plaques (A, B) and tangles (C, D). (E-H) Immunohistochemistry was performed for β-amyloid (E, F), phosphorylated tau protein (G, H), phosphorylated-synuclein (I, J), and phosphorylted-TDP-43 (K, L). Images were taken at 20x (A, C, E, G, I, K) and 200x (B, D, F, H, J, L) magnification.
All of the sections were graded blindly by 2 observers except the results from the p-synuclein and Thioflavin S staining, which were each graded by single observers. Total plaque, neuritic plaque, neurofibrillary change and neurofibrillary tangle densities were graded on a 0–3 scale using the templates published by the Consortium to Establish a Registry for Alzheimer’s Disease (37–40). Frequencies of occurrence of Lewy-type α-synucleinopathy were graded on a 0–4 scale by reference to the DLB Consortium III template (32, 41). For pTDP-43 pathology, lesion density was graded as simply present or absent; a smaller subset of cores and sources was used due to the availability of fewer subjects with pTDP-43 pathology (Table 1). All histopathological lesion types were graded in all of the cores and both the average and highest density estimate from each core histopathological lesion was compared to the histopathological density of the same lesion in the source block.
Statistical Methods
No adjustments were made for age, gender, or other subject characteristics. Analysis of variance was performed for comparing group means. Cohen’s Kappa analysis was used to calculate percentage agreement between observers. Spearman rank correlation was used to compare the histopathology density scores obtained from needle cores and source blocks. Sensitivity and specificity were calculated for the ability of varying dichotomous density classes of core histopathologies to predict the same density classes in source sections.
RESULTS
The subjects were all middle-aged to elderly. Group means differed significantly (p < 0.001), with the youngest group (FTLD-TDP43) had a mean age of 65.7 years whereas the oldest group (AD) mean was 85.4 years (Table 1). The male to female ratio varied from 53% to 67%; the mean postmortem interval was 3.3 hours. The Mini Mental State Examination score means ranged from 11.7 to 20.5 points (Table 1).
Cohen’s Kappa analysis for inter-observer variability showed that source histopathology density estimates varied between 26% and 0% depending on the histopathological lesion type. Inter-observer variability was greatest for amyloid plaques and least for p-tau neurofibrillary change. Needle core histopathology density estimates correlated significantly (p < 0.0001) with their respective source block density estimates for all the lesions of interest (Table 4). A total of 144 cases were used for all the staining except for p-tau (n = 139) and TDP-43 (n = 35). Five cases stained for p-tau were lost, whereas a smaller subset of cases was stained with pTDP-43 because of the small percentage of cases with FTLD diagnoses.
Table 4.
Spearman Rank Correlation Coefficients between Histopathology Density Estimates with Simulated Needle Core Biopsy and Open Biopsy
| Correlation Coefficient Highest Core | Correlation Coefficient Average Core | |
|---|---|---|
| Staining | Density | Density |
| Total plaques Thioflavin S |
0.80 | 0.82 |
| Neuritic plaques Thioflavin S |
0.83 | 0.83 |
| Total plaques IHC | 0.80 | 0.79 |
| Neurofibrillary change IHC |
0.89 | 0.89 |
| Neurofibrillary tangles Thioflavin S |
0.90 | 0.90 |
| Phosphorylated synuclein IHC |
0.96 | 0.94 |
| Phosphorylated TDP-43 IHC |
0.80 | 0.82 |
Correlations are shown for 2 situations: when the highest density of all cores is compared to the source blocks and when the average density of all cores is compared to the source blocks. All correlations were significant with p < 0.0001. IHC, immunohistochemistry.
Needle core sensitivity and specificity for varying densities and types of source block histopathology are shown in Table 5. Varying densities of amyloid plaques were detected with a sensitivity that ranged from 41% to 95%, depending on the stain and targeted density level. For most categories, sensitivity and specificity were between 90% and 100%. The only sensitivities and specificities lower than 80% were for Thioflavin-S core predictions of the highest source plaque density categories. The core sensitivity for detecting source p-tau neurofibrillary changes ranged from 83% to 93% whereas the specificity ranged from 83% to 99%. For the detection of neurofibrillary tangles with Thioflavin-S, sensitivities and specificities were all greater than 85% except when predicting the highest tangle density, where sensitivity was only 62%. Core Lewy-type α-synucleinopathy densities predicted source densities with uniformly high accuracy, with sensitivities ranging between 91% and 97% and specificities ranging between 95% and 97%. Cores detected the presence or absence of p-TDP43 source pathology with a sensitivity and specificity of 87% and 100%, respectively.
Table 5.
Sensitivity and Specificity of Needle Core Findings for Source Block Findings, Stratified for Several Histopathological Severity Levels
| Density Estimate | Density Estimate | Density Estimate | Density Estimate | Density Estimate | ||
|---|---|---|---|---|---|---|
| Staining | >0 | ≥1 | ≥2 | ≥3 | ≥4 | |
| Total plaques | Sensitivity | 93 | 92 | 90 | 87 | NA |
| Thioflavin S | Specificity | 100 | 100 | 95 | 69 | NA |
| Neuritic plaques | Sensitivity | 93 | 90 | 91 | 41 | NA |
| Thioflavin S | Specificity | 100 | 97 | 78 | 98 | NA |
| Total plaques | Sensitivity | 95 | 95 | 87 | 80 | NA |
| IHC | Specificity | 100 | 100 | 93 | 88 | NA |
| Neurofibrillary change IHC | Sensitivity | 88 | 83 | 86 | 89 | NA |
| Specificity | 88 | 95 | 99 | 99 | NA | |
| Neurofibrillary tangles | Sensitivity | 91 | 92 | 86 | 62 | NA |
| Thioflavin S | Specificity | 92 | 97 | 97 | 98 | NA |
| p-synuclein | Sensitivity | 91 | 93 | 93 | 97 | 90 |
| IHC | Specificity | 95 | 97 | 95 | 95 | 97 |
| p-TDP-43 | Sensitivity | 87 | NA | NA | NA | NA |
| IHC | Specificity | 100 | NA | NA | NA | NA |
IHC, immunohistochemistry; p-synuclein, phosphorylated synuclein; p-TDP-43, phosphorylated TDP-43; NA, not applicable.
DISCUSSION
The clinical diagnosis of dementia in the elderly is challenging. When diagnosing AD, using the most commonly used combination of the clinical and neuropathological definitions of AD, sensitivity and specificity are both only approximately 70% (3). Of the many clinical biomarkers in development, only amyloid PET imaging has been approved by the US FDA, but amyloid imaging cannot assess the distribution of p-tau or neurofibrillary tangles, fully define the pathological stage of AD or identify diverse types of pathological heterogeneity (9, 10), which could cause differential subject responses in clinical trials of new potential therapies. Furthermore, while a negative amyloid PET scan would effectively exclude AD as a cause of dementia, there are no clinical methods available to identify the molecular basis of any of the non-AD dementias. Tau and synuclein imaging ligands are in development but have not yet been validated in autopsy studies.
Brain biopsies as a diagnostic tool for older individuals with a presumed neurodegenerative cause of dementia have been avoided because of the invasiveness and associated risks, which include seizures, bleeding and infection (1, 2). Needle core brain biopsies are likely to have much lower complication and mortality rates because of the much smaller volume of tissue removed. However, at present, there is still insufficient published relevant information on the safety of cortical needle biopsy in the setting of primary degenerative dementia in elderly subjects; this procedure would ultimately have to be assessed with specifically designed clinical trials.
Recently, there have been 2 published simulations of open cortical biopsy for the diagnosis of neurodegenerative diseases (24, 25). These proof-of-concept studies have demonstrated that biopsy of a single cortical site would likely have a relatively high sensitivity and specificity for diagnosing the underlying molecular pathology in common forms of neurodegenerative disease. The current study was conducted to determine whether the diagnostic accuracy obtained in these 2 studies might be replicated with a much smaller and safer tissue volume.
We found that needle cores generally predicted the histopathological lesion types and densities of the larger volume source block with sensitivities and specificities close to 90%. Hence, we propose that cortical needle core biopsies taken from a single cortical site may be a feasible and reliable means to detect the underlying molecular pathology in the majority of elderly subjects with dementia. To assess the ability of cortical needle biopsy to predict the causative disease entity, however, additional feasibility studies, as well as clinical trials of needle biopsy followed to autopsy, will be needed. We suggest that the use of cortical needle core biopsies would improve subject selection for clinical trials, and, similarly to autopsy but more expeditiously, could provide histopathological validation to research studies such as the one done by Elobeid et al in which AD pathology from frontal cortex needle cores taken during shunt placement for normal pressure hydrocephalus were correlated with Mini Mental State Examination scores as well as CSF levels of Aβ 1–42 and phosphorylated tau protein (26).
In order to optimize both safety and efficacy, we chose to sample frontal cortex for this study. Most of the open cortical biopsies performed to diagnose dementia have been done on the right (non-dominant) frontal lobe, anterior to primary motor cortex, in order to avoid motor and speech deficits (1, 42). Therefore, for safety reasons, this would also be the best choice for biopsy for neurodegenerative disease. In terms of having the best chance of predicting an underlying neurodegenerative disease, frontal cortex would also be a logical choice, as indicated by 2 studies of simulated brain biopsy for neurodegenerative disease. In the study of Venneti et al, simulated biopsies from the frontal cortex alone were compared to simulated biopsies from the frontal cortex in combination with 3 other regions (parietal cortex, superior temporal gyrus and lentiform nucleus) (25). Sensitivity in frontal cortex alone was relatively high for FTLD-TDP, AD and LBD (average of 82%); intermediate (71%, 66% and 66%) for multiple system atrophy, corticobasal degeneration and Pick disease, respectively, and poor (0%) for progressive supranuclear palsy. Average sensitivity with all conditions included was 64%, whereas specificity was 43%.
In the study by King et al, both frontal cortex (dorsolateral/anterior prefrontal (Brodmann area 9/10) and temporal cortex (Brodmann area 21) were evaluated in subjects with several different neurodegenerative conditions and there was no statistical advantage to either site in terms of agreement in predicting the neuropathological diagnosis obtained from examination of the entire brain (kappa = 0.84 and 0.86, respectively). The sensitivity for the frontal lobe was 81% while for temporal lobe it was 84%; specificity was 79% for the frontal lobe and 82% for the temporal lobe. Sensitivity, in frontal cortex alone, was 100% for AD, corticobasal degeneration, and multiple system atrophy whereas it was 60% for DLB and 67% for FTLD-TDP (43).
We obtained needle biopsies from the gyral crest of the superior frontal gyrus, approximately at the border of Brodmann areas 6 and 8. While Brodmann areas differ somewhat in extent on an individual basis, the selected cortical area was well away from primary sensory and motor areas. In retrospect, because area 6 is premotor cortex, with direct projections to spinal cord serving the muscles of the back and neck, and area 8 includes the frontal eye fields, where damage may result in deviation of the eyes towards the side of the injury, a diagnostic frontal lobe biopsy for neurodegenerative disease would better be taken from Brodmann areas 9 and 10, as in the simulation by King et al (43). We avoided gyral sulci, where leptomeningeal blood vessels are more concentrated, as discussed by Wong et al (42) and Czyz et al (44).
We took 4 needle cores (as is often done with stereotactic brain biopsies) to ensure the acquisition of sufficient tissue, but taking fewer cores may provide more safety. Stereotactic needle biopsies usually obtain between 1 and 4 needle cores. This varies with the surgeon or center as described in several reports. Kongkham et al state, “typically only a single biopsy is obtained” (16), whereas in Chen et al, “Two to 4 sequential biopsies were routinely taken along the chosen trajectory tract” (20), and in McGirt et al an average of 3 were taken (19). Dickerman et al state, “Most surgeons when performing Cosman-Roberts-Wells (CRW) brain biopsies will insert the cannulated brain needle and proceed to collect at least 4 separate specimens by spinning the needle within the cannula ‘in a clockwise fashion’ collecting at 12, 3, 6 and 9 o’clock, thus hopefully increasing the diagnostic yield” (45).
The risks of morbidity and mortality would likely increase with increasing numbers of needle cores obtained but this is not completely clear from review of the literature. Hence, Dickerman et al state, “Obviously, the more biopsies taken will increase the risk for hemorrhage” (45), while the review of Kongkham et al finds only “equivocal” evidence for this (16). McGirt et al found that more than 1 needle trajectory (total number of specimens not mentioned) increased the incidence of neurological deficits from 17% to 44% but only when the target was in the basal ganglia or thalamus, as there was no increased morbidity associated with multiple trajectories with biopsies limited to the cerebral cortex (19). Sawin et al reported higher morbidity with more biopsy attempts (18), while several other reports cited by Kongkham et al found no relationship between complications and the number of biopsy attempts (16).
It is possible that due to increased age, comorbid disease and frailty, the risks of stereotactic needle biopsy might be greater in those with presumed neurodegenerative dementia compared with the younger subject population that has previously undergone the procedure. Frailty and other age-related morbidity syndromes have been associated with increased mortality and perioperative complications (46–48). Approximately 10% of those over 65, and between 25% and 50% of those over age 85, have frailty (49), and there is evidence that those with AD are at increased risk of frailty (50). On the other hand, in a large review of more than 2500 cases of stereotactic brain biopsy, Kongkham et al found no evidence for an increased complication rate based on patient characteristics including age and hypertension, and concluded that risk associated with diabetes was conflicting in different reports (16). Elderly subjects with neurodegenerative dementia are not necessarily less robust than age-similar non-demented subjects (51), and there is some evidence suggesting that demented subjects may actually be healthier (52–56). In addition, assessment scales for frailty and cardiovascular disease could be used to screen out subjects at higher risk for complications (47, 51, 57).
It is important to emphasizes that open biopsy is still the preferred method to obtain a diagnosis of rapidly progressive dementia because of the need for a larger tissue volume with inclusion of substantial amounts of leptomeninges and white matter (2). Such patients include those with suspected vasculitis and other inflammatory conditions as well as those with suspected prion encephalopathy; however, these patients should be readily distinguished from those with a presumed primary neurodegenerative dementia on the basis on the basis of the characteristics and duration of the clinical syndrome. Particularly for Creutzfeldt-Jakob disease, which is the single most common cause of rapidly progressive dementia (1, 2, 58, 59), the disease duration alone would preclude such cases because less than 10% of Creutzfeldt-Jakob disease subjects survive beyond 1 year of clinical onset (59). For an additional level of safety for laboratory personnel, a PET blot for prion protein could be performed as a first procedure, as described by Wemheuer et al (60). We suggest that a needle core brain biopsy for presumed primary neurodegenerative dementia might be considered only for subjects with slowly progressive cognitive impairment that has been present for more than 2 years. Before this can be considered, however, there is a need for carefully designed Phase I clinical trials to assess fully the specific risks in the targeted subject population.
While restricting needle biopsy for neurodegenerative dementia to subjects with at least a 2-year clinical duration would make the procedure less useful for selecting subjects for clinical trials of early disease, this would seem to be at least initially necessary, not only to separate this group from those with rapidly-progressive dementia but also for ethical reasons. Until the risks of cortical needle biopsy in this subject population are rigorously defined, only those with a high probability of neurodegenerative dementia would be suitable for what is at present an experimental method. If the risks were determined to be low enough, the procedure might be extended to those closer to symptom onset. Clinical trials are still being performed, however, in those with well-established presumptive neurodegenerative dementia and the selection of subjects for such trials, as well as the interpretation of trial results, might be improved with knowledge of the underlying primary and concurrent molecular pathology.
Acknowledgments
This study was supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center, P01 AG-017586, and P01 AG-032953), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
References
- 1.Warren JD, Schott JM, Fox NC, et al. Brain biopsy in dementia. Brain. 2005;128:2016–25. doi: 10.1093/brain/awh543. [DOI] [PubMed] [Google Scholar]
- 2.Schott JM, Reiniger L, Thom M, et al. Brain biopsy in dementia: clinical indications and diagnostic approach. Acta Neuropathol. 2010;120:327–41. doi: 10.1007/s00401-010-0721-y. [DOI] [PubMed] [Google Scholar]
- 3.Beach TG, Monsell SE, Phillips LE, et al. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J Neuropathol Exp Neurol. 2012;71:266–73. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson PT, Jicha GA, Kryscio RJ, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol. 2010;257:359–66. doi: 10.1007/s00415-009-5324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balasa M, Gelpi E, Martin I, et al. Diagnostic accuracy of behavioral variant frontotemporal dementia consortium criteria (FTDC) in a clinicopathological cohort. Neuropathol Appl Neurobiol. 2014 Nov 10; doi: 10.1111/nan.12194. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Knopman DS, Boeve BF, Parisi JE, et al. Antemortem diagnosis of frontotemporal lobar degeneration. Ann Neurol. 2005;57:480–8. doi: 10.1002/ana.20425. [DOI] [PubMed] [Google Scholar]
- 7.Beach TG. Alzheimer's disease and the "Valley Of Death": not enough guidance from human brain tissue? J Alzheimers Dis. 2013;33 (Suppl 1):S219–S233. doi: 10.3233/JAD-2012-129020. [DOI] [PubMed] [Google Scholar]
- 8.Arrowsmith J. Trial watch: Phase II failures: 2008–2010. Nat Rev Drug Discov. 2011;10:328–9. doi: 10.1038/nrd3439. [DOI] [PubMed] [Google Scholar]
- 9.Schneider JA, Arvanitakis Z, Bang W, et al. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 10.Dugger BN, Clark CM, Serrano G, et al. Neuropathologic heterogeneity does not impair florbetapir-positron emission tomography postmortem correlates. J Neuropathol Exp Neurol. 2014;73:72–80. doi: 10.1097/NEN.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovacs GG, Alafuzoff I, Al-Sarraj S, et al. Mixed brain pathologies in dementia: the BrainNet Europe consortium experience. Dement Geriatr Cogn Disord. 2008;26:343–50. doi: 10.1159/000161560. [DOI] [PubMed] [Google Scholar]
- 12.Clark CM, Pontecorvo MJ, Beach TG, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–78. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 13.Curtis C, Gamez JE, Singh U, et al. Phase 3 trial of flutemetamol labeled with radioactive fluorine 18 imaging and neuritic plaque density. JAMA Neurol. 2015;72:287–94. doi: 10.1001/jamaneurol.2014.4144. [DOI] [PubMed] [Google Scholar]
- 14.Sabri O, Sabbagh MN, Seibyl J, et al. Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer disease: Phase 3 study. Alzheimers Dement. 2015 Mar 28; doi: 10.1016/j.jalz.2015.02.004. pii: S1552–5260(15)00060-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Field M, Witham TF, Flickinger JC, et al. Comprehensive assessment of hemorrhage risks and outcomes after stereotactic brain biopsy. J Neurosurg. 2001;94:545–51. doi: 10.3171/jns.2001.94.4.0545. [DOI] [PubMed] [Google Scholar]
- 16.Kongkham PN, Knifed E, Tamber MS, et al. Complications in 622 cases of frame-based stereotactic biopsy, a decreasing procedure. Can J Neurol Sci. 2008;35:79–84. doi: 10.1017/s0317167100007605. [DOI] [PubMed] [Google Scholar]
- 17.Nishihara M, Sasayama T, Kudo H, et al. Morbidity of stereotactic biopsy for intracranial lesions. Kobe J Med Sci. 2011;56:E148–E153. [PubMed] [Google Scholar]
- 18.Sawin PD, Hitchon PW, Follett KA, et al. Computed imaging-assisted stereotactic brain biopsy: a risk analysis of 225 consecutive cases. Surg Neurol. 1998;49:640–9. doi: 10.1016/s0090-3019(97)00435-7. [DOI] [PubMed] [Google Scholar]
- 19.McGirt MJ, Woodworth GF, Coon AL, et al. Independent predictors of morbidity after image-guided stereotactic brain biopsy: a risk assessment of 270 cases. J Neurosurg. 2005;102:897–901. doi: 10.3171/jns.2005.102.5.0897. [DOI] [PubMed] [Google Scholar]
- 20.Chen CC, Hsu PW, Erich Wu TW, et al. Stereotactic brain biopsy: Single center retrospective analysis of complications. Clin Neurol Neurosurg. 2009;111:835–9. doi: 10.1016/j.clineuro.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Paleologos TS, Dorward NL, Wadley JP, et al. Clinical validation of true frameless stereotactic biopsy: analysis of the first 125 consecutive cases. Neurosurgery. 2001;49:830–5. doi: 10.1097/00006123-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Bernstein M, Parrent AG. Complications of CT-guided stereotactic biopsy of intra-axial brain lesions. J Neurosurg. 1994;81:165–8. doi: 10.3171/jns.1994.81.2.0165. [DOI] [PubMed] [Google Scholar]
- 23.Kreth FW, Muacevic A, Medele R, et al. The risk of haemorrhage after image guided stereotactic biopsy of intra-axial brain tumours--a prospective study. Acta Neurochir (Wien) 2001;143:539–45. doi: 10.1007/s007010170058. [DOI] [PubMed] [Google Scholar]
- 24.King A, Maekawa S, Bodi I, et al. Simulated surgical-type cerebral biopsies from post-mortem brains allows accurate neuropathological diagnoses in the majority of neurodegenerative disease groups. Acta Neuropathol Commun. 2013;1:53. doi: 10.1186/2051-5960-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venneti S, Robinson JL, Roy S, et al. Simulated brain biopsy for diagnosing neurodegeneration using autopsy-confirmed cases. Acta Neuropathol. 2011;122:737–45. doi: 10.1007/s00401-011-0880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elobeid A, Laurell K, Cesarini KG, et al. Correlations between mini-mental state examination score, cerebrospinal fluid biomarkers, and pathology observed in brain biopsies of patients with normal-pressure hydrocephalus. J Neuropathol Exp Neurol. 2015;74:470–9. doi: 10.1097/NEN.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 27.Beach TG, Sue LI, Walker DG, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–45. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beach TG, Adler CH, Sue LI, et al. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology. 2015 Jan 26; doi: 10.1111/neup.12189. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallet PG, Guntern R, Hof PR, et al. A comparative study of histological and immunohistochemical methods for neurofibrillary tangles and senile plaques in Alzheimer's disease. Acta Neuropathol. 1992;83:170–8. doi: 10.1007/BF00308476. [DOI] [PubMed] [Google Scholar]
- 30.Hasegawa M, Arai T, Nonaka T, et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol. 2008;64:60–70. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beach TG, White CL, Hamilton RL, et al. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol. 2008;116:277–88. doi: 10.1007/s00401-008-0409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beach TG, Adler CH, Lue L, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–34. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujiwara H, Hasegawa M, Dohmae N, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–4. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 35.Obi K, Akiyama H, Kondo H, et al. Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. Exp Neurol. 2008;210:409–20. doi: 10.1016/j.expneurol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Walker DG, Lue LF, Adler CH, et al. Changes in properties of serine 129 phosphorylated alpha-synuclein with progression of Lewy-type histopathology in human brains. Exp Neurol. 2013;240:190–204. doi: 10.1016/j.expneurol.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 38.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 40.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–24. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 42.Wong SH, Jenkinson MD, Faragher B, et al. Brain biopsy in the management of neurology patients. Eur Neurol. 2010;64:42–5. doi: 10.1159/000315032. [DOI] [PubMed] [Google Scholar]
- 43.King A, Maekawa S, Bodi I, et al. Simulated surgical-type cerebral biopsies from post-mortem brains allows accurate neuropathological diagnoses in the majority of neurodegenerative disease groups. Acta Neuropathol Commun. 2013;1:53. doi: 10.1186/2051-5960-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Czyz M, Tabakow P, Weiser A, et al. The safety and effectiveness of low field intraoperative MRI guidance in frameless stereotactic biopsies of brain tumours-design and interim analysis of a prospective randomized trial. Neurosurg Rev. 2014;37:127–37. doi: 10.1007/s10143-013-0486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickerman RD, Mittler MA, Morgan JT. Stereotactic brain biopsies and operative complications: technique to further decrease risks. Acta Neurochir (Wien) 2005;147:911–2. doi: 10.1007/s00701-005-0566-7. [DOI] [PubMed] [Google Scholar]
- 46.Wang SY, Shamliyan TA, Talley KM, et al. Not just specific diseases: systematic review of the association of geriatric syndromes with hospitalization or nursing home admission. Arch Gerontol Geriatr. 2013;57:16–26. doi: 10.1016/j.archger.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Cohen RR, Lagoo-Deenadayalan SA, Heflin MT, et al. Exploring predictors of complication in older surgical patients: a deficit accumulation index and the Braden Scale. J Am Geriatr Soc. 2012;60:1609–15. doi: 10.1111/j.1532-5415.2012.04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26:1091–101. doi: 10.1093/annonc/mdu540. [DOI] [PubMed] [Google Scholar]
- 49.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–7. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 50.Buchman AS, Schneider JA, Leurgans S, et al. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology. 2008;71:499–504. doi: 10.1212/01.wnl.0000324864.81179.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koch G, Belli L, Giudice TL, et al. Frailty among Alzheimer's disease patients. CNS Neurol Disord Drug Targets. 2013;12:507–11. doi: 10.2174/1871527311312040010. [DOI] [PubMed] [Google Scholar]
- 52.Bergmann C, Sano M. Cardiac risk factors and potential treatments in Alzheimer's disease. Neurol Res. 2006;28:595–604. doi: 10.1179/016164106X130498. [DOI] [PubMed] [Google Scholar]
- 53.Beach TG, Maarouf CL, Brooks RG, et al. Reduced clinical and postmortem measures of cardiac pathology in subjects with advanced Alzheimer's Disease. BMC Geriatr. 2011;11:3. doi: 10.1186/1471-2318-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolf-Klein GP, Siverstone FA, Brod MS, et al. Are Alzheimer patients healthier? J Am Geriatr Soc. 1988;36:219–24. doi: 10.1111/j.1532-5415.1988.tb01804.x. [DOI] [PubMed] [Google Scholar]
- 55.Mielke MM, Zandi PP, Sjogren M, et al. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology. 2005;64:1689–95. doi: 10.1212/01.WNL.0000161870.78572.A5. [DOI] [PubMed] [Google Scholar]
- 56.Rosano C, Newman AB. Cardiovascular disease and risk of Alzheimer's disease. Neurol Res. 2006;28:612–20. doi: 10.1179/016164106X130407. [DOI] [PubMed] [Google Scholar]
- 57.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bucelli RC, Ances BM. Diagnosis and evaluation of a patient with rapidly progressive dementia. Mol Med. 2013;110:422–8. [PMC free article] [PubMed] [Google Scholar]
- 59.Geschwind MD, Shu H, Haman A, et al. Rapidly progressive dementia. Ann Neurol. 2008;64:97–108. doi: 10.1002/ana.21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wemheuer WM, Wrede A, Gawinecka J, et al. Filtration of protein aggregates increases the accuracy for diagnosing prion diseases in brain biopsies. J Neuropathol Exp Neurol. 2013;72:758–67. doi: 10.1097/NEN.0b013e31829d2799. [DOI] [PubMed] [Google Scholar]