Abstract
Estimating effects of diabetes on cognitive change among older Mexican Americans is important, yet challenging because diabetes and cognitive decline both predict mortality, which can induce survival bias. Older Mexican Americans in the Sacramento Area Latino Study on Aging (n=1,634) completed Modified Mini-Mental State Exams (3MSE) and diabetes assessments up to seven times (1998-2007). We examined baseline and new onset diabetes and cognitive decline with joint longitudinal-survival models to account for death. At baseline, 32.4% of participants had diabetes and 15.8% developed diabetes during the study. Over the study, 22.8% of participants died. In joint longitudinal-survival models, those with baseline diabetes experienced faster cognitive decline (p=0.003) and higher mortality (HR=1.88, 95% CI 1.48-2.38) than those without diabetes. Cognitive decline and mortality were similar for those with new onset diabetes and those without diabetes. For a typical person, 3MSE scores declined by 2.3 points among those without diabetes and 4.3 points among those with baseline diabetes during the last 6 years of study. Ignoring the impact of death yielded a 17.0% smaller estimate of the effect of baseline diabetes on cognitive decline. Analyses that overlook the association between cognitive decline and mortality may underestimate the effect of diabetes on cognitive aging.
Introduction
Cognitive decline and dementia are major causes of disability and death for older adults1, and strategies to prevent or treat dementia remain elusive. A growing body of evidence suggests that older adults with type 2 diabetes are 50-100% more likely to develop dementia than those without diabetes2,3. Type 2 diabetes is a growing epidemic in the United States and globally4 Certain racial and ethnic groups, including Mexican Americans, experience a disproportionate burden of diabetes. However, there is very limited research on dementia and related outcomes among this potentially vulnerable minority population.
To understand whether diabetes contributes to dementia pathogenesis, it is important to examine the association between diabetes and rate of cognitive decline. Dementia onset is influenced by both level of cognitive function prior to onset of decline and rate of cognitive decline. Thus, the association between diabetes and dementia could be confounded by shared determinants of diabetes and level of cognitive function prior to onset of decline, such as early life social factors. Findings on diabetes and rate of cognitive decline have been inconsistent: many studies report an association between diabetes and decline in one or more domains, but across studies, there is not a consistent association between diabetes and cognitive decline5-8. A recognized methodological limitation that could contribute to inconsistent results of prior work is selective survival. Since both diabetes9 and accelerated cognitive decline10,11 are associated with higher mortality, ignoring attrition due to death may lead to underestimation of the effect of diabetes on cognitive decline. Although the potential bias from selective survival is well understood, few prior studies of diabetes and cognitive change have implemented statistical tools to account for this bias. To address gaps in the existing literature on the effects of diabetes on cognitive aging, this study will examine the effect of type 2 diabetes on rate of cognitive change while accounting for mortality over up to ten years among older Mexican Americans.
Methods
Study population
The Sacramento Area Latino Study on Aging (SALSA) is a population-based longitudinal study of older Mexican Americans living in the Sacramento Valley area of California who were 60-101 years old at baseline in 1998-1999. SALSA was designed to examine the effects of metabolic and cardiovascular risk factors on dementia and cognitive decline in this understudied ethnic group. A total of 1,789 participants were interviewed and underwent clinical examinations, including a cognitive assessment, in their homes every 12-15 months through 2007 for up to seven examinations. Participants were also contacted every six months by telephone to update contact information and health status. Study questionnaires were validated in Spanish and English, and interviews were conducted in the language that participants preferred. A detailed description of study procedures has been published previously12. SALSA was approved by the Institutional Review Boards of the University California San Francisco and Davis and the University of Michigan.
Measures
Type 2 diabetes
At every study visit, diabetes classification was based on fasting glucose level ≥126 mg/dL, anti-diabetic medication use, or self-report of a physician diagnosis of diabetes at the baseline examination. Fasting glucose was measured with the Cobas Mira Chemistry Analyzer (Roche Diagnostics Corporation, Indianapolis, IN) and medication use was ascertained by inspection of medications. The majority (75.5%) of participants with diabetes at baseline met at least two criteria and 24.5% met one (10.8% fasting glucose level, 2.1% medication use, and 11.7% self-report). Due to the advanced age of the cohort, most diabetes cases are likely to be type 2 diabetes. At each wave, individuals were designated as having one of the following: baseline diabetes, diabetes at the current wave that was diagnosed since baseline (diabetes diagnosed during study), or no diabetes.
Cognitive function
Cognitive function was assessed every 12-15 months with the Modified Mini Mental State Exam (3MSE). The 3MSE is a test of global cognitive function that was designed to have fewer ceiling effects and better reliability and validity than the Mini-Mental State Examination13. Scores on the 3MSE range from 0-100, where higher test scores indicate better cognitive function. The distribution of 3MSE scores was left-skewered. As such, we examined the log-transformation of the errors on the 3MSE (log(101 –3MSE score)) to correspond more closely to a normal distribution. More errors indicate poorer cognitive function, and an increase in log(3MSE errors) over time indicates cognitive decline.
Death
Ascertaining mortality involved the following methods: online surveillance of death notices, review of the Social Security Death Index, the National Death Index, vital statistics data files from the state of California, and interviews with family members when participants could not be reached for annual study visits or interim six month phone calls. This analysis is restricted to deaths during active follow-up for the study (1998-2007).
Other variables
At baseline, age, sex, years of education, country of birth, and history of stroke, myocardial infarction, congestive heart failure, angina pectoris, atrial fibrillation, intermittent claudication, and deep vein thrombosis were collected from a structured baseline interview. Waist circumference, height, weight, blood pressure, and depressive symptoms were measured. Waist circumference was measured around the point of greatest indentation on the abdomen when the participant bent to one side, and categorized according to American Heart Association sex-specific cut-points for abdominal obesity (>40 inches for males, >35 inches for females)14. BMI (kg/m2) was calculated from direct measurements of height and weight. Hypertension was defined based on measured systolic blood pressure >140 mmHG or diastolic blood pressure >90 mmHG, self-report of a physician diagnosis, or anti-hypertensive medication use. Depressive symptoms were measured by the Center for Epidemiologic Studies Depression (CESD) Scale, a widely used scale (range 0–60), and elevated depressive symptoms was defined as CESD≥1615. Cardiovascular disease was defined as history of one or more of the following conditions: myocardial infarction, congestive heart failure, angina pectoris, atrial fibrillation, intermittent claudication, and deep vein thrombosis.
Statistical analysis
To examine the association between diabetes status and rate of change in cognitive function while accounting for the dependence between cognitive decline and death, we used a joint longitudinal-survival model16 to simultaneously model cognitive decline and risk of death. This modeling approach corrects for selective survival to the extent that the model recovers the association between diabetes and cognitive function that would have been obtained using a separate linear mixed effects model if mortality were independent of rate of cognitive change. The joint model was comprised of two sub-models that use a shared parameter for rate of cognitive change (random effect for slope): a sub-model for repeated measures of cognitive function (linear mixed effects model with random effects for intercept and slope17.) and a sub-model for time to death (piecewise exponential model18). We ran the joint models using PROC NLMIXED following the approach described by Guo and Carlin19. In both sub-models, diabetes was modeled as a time-dependent variable: at each wave, individuals were designated as having baseline diabetes, diabetes at the current wave that was diagnosed since baseline diabetes, or no diabetes. Both sub-models used time (in years) from enrollment as the time scale. For the piecewise exponential sub-model, we divided the time scale into five two-year intervals because this division corresponded reasonably well with the spacing of the cognitive assessments and the hazard function appeared relatively constant within each two-year interval.
In the linear mixed effects sub-model, we included indicators for the first and second testing occasions to account for practice effects, which were evident when we examined average 3MSE scores at each visit among participants who remained under study through the final assessment. Practice effects (also called retest or learning effects) refer to improvements in cognitive test performance attributable to increased familiarity with the cognitive testing procedures, and have been demonstrated in other studies of older adults with cognitive assessments administered a year or more apart20,21. Accounting for the practice effect improved the model fit over a simple linear or linear quadratic model form. We estimated the difference in average annual rate of change in cognitive function associated with diabetes with a multiplicative interaction term between diabetes status and time in years (baseline diabetes*time and diabetes diagnosed during study*time).
We fit a series of models to assess the joint effects of diabetes on cognitive decline and mortality. First, we fit a model with diabetes as the only predictor (Model 1). Next, we included age, sex, and years of education (Model 2). Finally, we additionally adjusted for baseline abdominal obesity, history of stroke, hypertension, history of cardiovascular disease, and elevated depressive symptoms (Model 3). For ease of interpretation, all continuous variables were centered at the mean baseline value for the study sample.
To handle missing data, we used imputed data for variables missing for individuals prior to exit from the study (either due to death or censoring). A multiple-imputation approach was performed using the entire SALSA dataset to develop predictive models for missing data22. Five imputed datasets were created using Imputation and Variance Estimation Software23. The results from regression analyses from the five imputed datasets were summarized using the MI ANALYZE procedure.
All analyses were conducted in SAS version 9.3 (SAS Institute, Cary, North Carolina).
Results
Participants were followed from enrollment in 1998-1999 through 2007. A total of 1,789 participants enrolled in SALSA. Participants with dementia at baseline (n=87) and those lost to follow-up after only the baseline visit (n=68) were eliminated from analysis. The resulting sample size was 1,634 individuals.
The median follow-up time was 7.6 years (interquartile range: 4.8-8.2). A total of 530 (32.4%) had diabetes at baseline and an additional 258 (15.8%) developed diabetes during the study. Throughout the study, 372 (22.8%) participants died and 335 (20.5%) participants were lost to follow-up.
At baseline, participants with diabetes were more likely to have been born in the U.S., have higher BMIs, and have a higher prevalence of abdominal obesity, hypertension, history of stroke, and history of cardiovascular disease (Table 1). At baseline, 64.2% of participants with baseline diabetes were using an anti-diabetic medication. Among participants who developed diabetes during the study, the majority (75.7%) were not using an anti-diabetic medication at the visit of diagnosis.
Table 1.
Baseline characteristics of the sample by baseline diabetes status (n=1,634).
| Variable | No diabetes (n=846) | Baseline diabetes (n=530) | Diabetes diagnosed after baseline (n=258) | p-value |
|---|---|---|---|---|
|
| ||||
| mean (SD) or % | mean (SD) or % | mean (SD) or % | ||
|
| ||||
| Age (years) | 71.0 (7.1) | 69.9 (6.5) | 68.9 (6.0) | <0.001 |
|
| ||||
| Education (years) | 7.6 (5.3) | 7.3 (5.4) | 7.3(5.3) | 0.525 |
|
| ||||
| Female sex | 59.7 | 55.5 | 56.6 | 0.276 |
|
| ||||
| Born in U.S. | 47.6 | 56.4 | 45.0 | 0.001 |
|
| ||||
| Body mass index (kg/m2) | <0.001 | |||
| <25 | 25.1 | 12.3 | 14.7 | |
| 25-30 | 37.6 | 37.9 | 37.6 | |
| ≥30 | 37.4 | 49.8 | 47.7 | |
|
| ||||
| Abdominal obesity | 43.6 | 61.7 | 57.8 | <0.001 |
|
| ||||
| Hypertension | 61.9 | 81.7 | 70.9 | <0.001 |
|
| ||||
| Stroke | 6.4 | 13.0 | 5.4 | <0.001 |
|
| ||||
| Cardiovascular disease | 22.7 | 40.8 | 29.1 | <0.001 |
|
| ||||
| Elevated depressive symptoms | 23.3 | 27.4 | 23.6 | 0.214 |
Abdominal obesity defined as waist circumference >40 inches for males, >35 inches for females. Hypertension defined as systolic blood pressure >140 mm HG or diastolic blood pressure >90 mmHG, self-report of a physician diagnosis, or anti-hypertensive medication use. Cardiovascular disease includes self-report of myocardial infarction, congestive heart failure, angina pectoris, atrial fibrillation, intermittent claudication, or deep vein thrombosis. Elevated depressive symptoms defined as Center for Epidemiological Studies-Depression Scalescore ≥16. ANOVA was used for continuous variables, chi-square tests were used for categorical variables; p-values are two-sided.
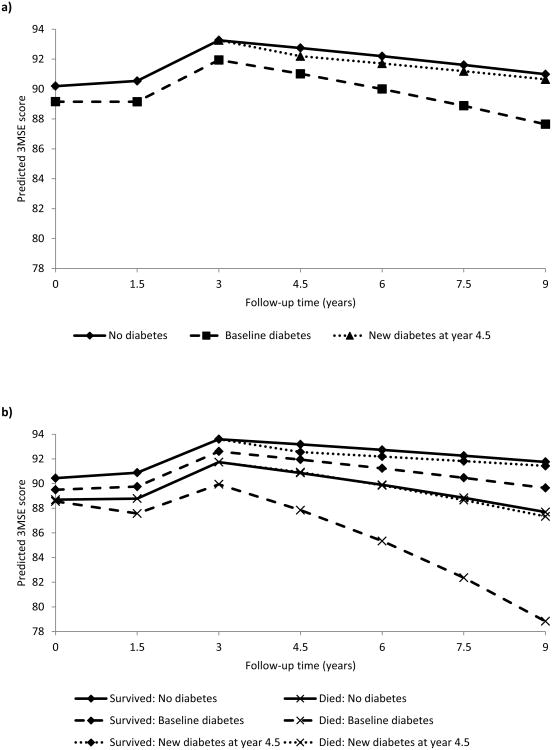
Individuals with baseline diabetes experienced substantially faster cognitive decline compared to those without diabetes, and baseline diabetes and rate of cognitive change were both associated with an increased risk of death (Table 2). New onset diabetes was not associated with rate of cognitive change or death. Adjustment for potential confounders had little impact on estimates of cognitive change. For example, for a typical person, 3MSE scores declined by 2.3 points among those without diabetes and 4.3 points among those with baseline diabetes during the last 6 years of follow up, and a standard deviation increase in rate of cognitive change was associated with 73% higher rate of death. Figure 1a illustrates predicted 3MSE score trajectories from Model 3 estimates for an individual with baseline diabetes, an individual who developed diabetes at year 4.5 in the study, and an individual who remained free of diabetes throughout the study period. An individual identified as having diabetes at the year 4.5 of the study remains in the no diabetes group until the cognitive assessment at year 3 and is part of the new onset diabetes group from year 4.5 forward. Overall, 3MSE scores increased through the third assessment, which we attribute to practice effects, and declined thereafter. Results from a separate linear mixed effects model for cognitive decline estimated a 17.0% smaller effect of baseline diabetes on rate of cognitive change (coefficient for baseline diabetes*time (in years): b=0.018, 95% CI 0.004-0.032) compared to the joint model for cognitive decline and death (Supplementary Table).
Table 2.
Regression coefficients (b) to describe the association between diabetes and rate of change in cognitive function (log(errors on Modified Mini Mental State Exam)) and hazard ratios (HR) to describe the associations of diabetes and rate of cognitive change with risk of death from joint longitudinal-survival models.
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Longitudinal sub-model | |||||||||
| Parameter | b | 95% CI | p-value | b | 95% CI | p-value | b | 95% CI | p-value |
| Intercept | 1.919 | (1.857, 1.982) | <0.001 | 1.939 | (1.874, 2.003) | <0.001 | 1.918 | (1.836, 2.000) | <0.001 |
| Wave 1 indicator | 0.471 | (0.412, 0.53) | <0.001 | 0.463 | (0.404, 0.522) | <0.001 | 0.462 | (0.403, 0.521) | <0.001 |
| Wave 2 indicator | 0.369 | (0.312, 0.426) | <0.001 | 0.366 | (0.309, 0.423) | <0.001 | 0.365 | (0.309, 0.422) | <0.001 |
| Baseline diabetes | 0.112 | (0.036, 0.188) | 0.004 | 0.103 | (0.042, 0.164) | <0.001 | 0.092 | (0.030, 0.154) | 0.003 |
| Diabetes diagnosed during study | 0.087 | (-0.083, 0.257) | 0.317 | 0.098 | (-0.070, 0.266) | 0.253 | 0.096 | (-0.074, 0.265) | 0.269 |
| Time (years) | 0.058 | (0.044, 0.072) | <0.001 | 0.045 | (0.030, 0.060) | <0.001 | 0.043 | (0.024, 0.062) | <0.001 |
| Baseline diabetes*Time | 0.024 | (0.009, 0.038) | 0.001 | 0.024 | (0.010, 0.038) | <0.001 | 0.022 | (0.007, 0.036) | 0.003 |
| Diabetes diagnosed during study* Time | -0.010 | (-0.038, 0.017) | 0.463 | -0.008 | (-0.036, 0.020) | 0.584 | -0.007 | (-0.035, 0.021) | 0.629 |
| Survival sub-model | |||||||||
| Parameter | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Baseline diabetes | 2.19 | (1.69, 2.84) | <0.001 | 2.19 | (1.72, 2.79) | <0.001 | 1.88 | (1.48, 2.38) | <0.001 |
| Diabetes diagnosed during study | 0.64 | (0.33, 1.26) | 0.193 | 0.71 | (0.38, 1.32) | 0.277 | 0.70 | (0.36, 1.35) | 0.278 |
| log(3MSE errors) slope (1 SD* unit) | 2.79 | (2.12, 3.68) | <0.001 | 1.80 | (1.45, 2.24) | <0.001 | 1.73 | (1.39, 2.16) | <0.001 |
SD=standard deviation. Model 1: unadjusted; Model 2: adjusted for age, sex, and years of education; Model 3: Model 2 + abdominal obesity, stroke, hypertension, cardiovascular disease, and elevated depressive symptoms.
Figure 1.
Predicted 3MSE scores from a) the joint longitudinal-survival model to describe the association between diabetes and rate of change in cognitive function and b) from the mixed linear effects model to describe the association between diabetes and rate of change in cognitive function by survival status. Predictions are for an individual who remains free of diabetes throughout the study (solid line), an individual with baseline diabetes (dashed line), and an individual with new diabetes at year 4.5 of the study (dotted line). All predictions are for a male individual 70 years of age with 7 years of education, waist circumference <40 inches, no history of stroke or cardiovascular disease, without hypertension or elevated depressive symptoms.
We further examined 3MSE score trajectories among participants who died during the study and those who survived with mixed linear effects models adding two-way interactions between death and time and death and diabetes status and three-way interactions among death, time, and diabetes status. Figure 1b illustrates the trajectories of change in 3MSE scores by diabetes status among participants who survived and those who died during the study period. Although the interaction terms with death were non-significant (death*time p=0.020, death*time*baseline diabetes p=0.11, death*time*new onset diabetes p=0.90), the observed trend was that rate of cognitive decline was faster among participants who died compared to those who survived, particularly for those with baseline diabetes.
Discussion
In this cohort of older Mexican Americans, those with prevalent diabetes at baseline experienced faster rates of cognitive decline than those without diabetes, but new onset diabetes was not associated with rate of cognitive decline. Baseline diabetes and faster rate of cognitive decline were both associated with an increased risk of death. Conventional models yielded smaller estimates of the effect of baseline diabetes on rate of cognitive decline compared to joint longitudinal-survival models, which account for the dependence between cognitive decline and mortality.
Our finding that separate longitudinal models estimated smaller effects of baseline diabetes on rate of cognitive decline compared to joint longitudinal-survival models suggests that conventional estimation techniques underestimate the effect of diabetes on rate of cognitive decline because diabetes and cognitive decline both predict mortality. This suggests that the effect of diabetes on rate of cognitive decline may have been underestimated in prior studies. Jointly modeling rate of cognitive decline and mortality in observational studies may be useful for planning future intervention studies for prevention of dementia. While the potential bias from selective survival has been widely recognized by researchers studying cognitive decline, few studies have attempted to address this issue. Future studies are needed to further quantify the potential degree of bias from selective survival in research on the effects of diabetes, as well as other exposures, on cognitive decline.
We previously found that diabetes is associated with a two-fold increased incidence of cognitive impairment and dementia in this same cohort24. Several previous studies examining the association between baseline diabetes and cognitive decline in non-Hispanic white and African American populations have found that diabetes is associated with greater cognitive decline in one or more cognitive domains among older adults5,8,25-28, although a few studies have reported no association7,29. Our finding that new onset diabetes was not associated with rate of cognitive decline is consistent with other recent studies showing that longer diabetes duration is associated with cognitive decline5,6,8,28,30. However, several of these studies found that the rate of cognitive decline among individuals who developed diabetes throughout the study fell between that of individuals who remained free of diabetes and those with baseline diabetes in one or more cognitive domain5,8,30. It is possible that certain cognitive domains are more sensitive to the effects of the early stages of diabetes, but the present study only measured global cognitive function. Characteristics of the study populations, length of study, and specific neuropsychological tests used may also contribute to differences in results across studies. The association between diabetes and cognitive decline over two years has previously been examined in SALSA31; no differences in change in cognitive function by diabetes status were observed over this short follow-up. To our knowledge, the Hispanic Established Populations for the Epidemiological Study of the Elderly is the only other study that has examined diabetes and change in cognitive function among older Mexican Americans32. The authors found that self-reported diabetes was associated with a higher risk of severe cognitive impairment but not moderate impairment over five years.
While our study addresses some of the biases of prior studies, it also has several limitations. Although mortality was the primary source of attrition in this cohort, some non-mortality attrition was present, and participants with diabetes and those beginning to experience cognitive decline may have been more likely to withdraw from the study, which could lead to underestimation of the effect of diabetes on cognitive decline. The 3MSE is a test of global cognitive function, so inferences about the effects of diabetes on specific cognitive domains cannot be drawn from this study.
The results of this study are nonetheless generalizable to community-dwelling older Mexican Americans, as well as other populations with similar risk factor profiles and mortality rates. SALSA is a longitudinal population-based study, and participants were representative of community-dwelling older Mexican Americans living in the Sacramento Area in 1998-199912. Study interviews and the 3MSE were validated in both English and Spanish.
In this population-based study of older Mexican Americans, we found that individuals with baseline diabetes experienced faster rates of cognitive decline than those without diabetes, but diabetes diagnosed after baseline was not associated with rate of cognitive decline during the study. Furthermore, our results suggest that conventional analysis approaches, which do not account for the dependence between cognitive decline and mortality, may underestimate the effect of diabetes on rate of cognitive decline. Our results are germane to potential public health interventions, as they suggest that prevention and management of diabetes could be successful strategies to preserve cognitive function and prevent dementia in this population.
Supplementary Material
Acknowledgments
Conflicts of Interest and Source of Funding: This work was supported by the National Institute on Aging (grants AG12975, AG033751, and K24-AG031155), the National Institute of Diabetes, Digestive, and Kidney Diseases (grant DK60753), and a grant from the American Health Assistance Foundation.
References
- 1.Thies W, Bleiler L, Alzheimer's A. 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 2013 Mar;9(2):208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Internal medicine journal. 2012 May;42(5):484–491. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 3.Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. The lancet Diabetes & endocrinology. 2014 Mar;2(3):246–255. doi: 10.1016/S2213-8587(13)70088-3. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nature reviews Endocrinology. 2012 Apr;8(4):228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 5.Yaffe K, Falvey C, Hamilton N, et al. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neurol. 2012 Sep;69(9):1170–1175. doi: 10.1001/archneurol.2012.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuligenga RH, Dugravot A, Tabák AG, et al. Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post-hoc analysis of the Whitehall II cohort study. Lancet Diabetes Endocrinol. 2014;2(3):228–235. doi: 10.1016/S2213-8587(13)70192-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011 Aug 2;77(5):461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spauwen PJ, Kohler S, Verhey FR, Stehouwer CD, van Boxtel MP. Effects of Type 2 Diabetes on 12-Year Cognitive Change: Results from the Maastricht Aging Study. Diabetes Care. 2013 Jun;36(6):1554–1561. doi: 10.2337/dc12-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011 Mar 3;364(9):829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muniz-Terrera G, van den Hout A, Piccinin AM, Matthews FE, Hofer SM. Investigating Terminal Decline: Results From a UK Population-Based Study of Aging. Psychology and aging. 2012 Dec 31; doi: 10.1037/a0031000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodge HH, Wang CN, Chang CC, Ganguli M. Terminal decline and practice effects in older adults without dementia: the MoVIES project. Neurology. 2011 Aug 23;77(8):722–730. doi: 10.1212/WNL.0b013e31822b0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003 Feb;51(2):169–177. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- 13.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987 Aug;48(8):314–318. [PubMed] [Google Scholar]
- 14.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006 Feb 14;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 15.Radloff LS. The CES-D Scale : A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 16.Diggle PJ, Sousa I, Chetwynd AG. Joint modelling of repeated measurements and time-to-event outcomes: the fourth Armitage lecture. Stat Med. 2008 Jul 20;27(16):2981–2998. doi: 10.1002/sim.3131. [DOI] [PubMed] [Google Scholar]
- 17.McCulloch CE, Searle SR, Neuhaus JM. Generalized, Linear and Mixed Models. Second. Hoboken, NJ: John Wiley & Sons; 2008. [Google Scholar]
- 18.Allison PD. Survival Analysis Using SAS®: A Practical Guide. Cary, N.C.: SAS Institute Inc.; 1995. [Google Scholar]
- 19.Guo X, Carlin BP. Separate and Joint Modeling of Longitudinal and Event Time Data Using Standard Computer Packages. The American Statistician. 2004 Feb;58(1):16–24. 2004. [Google Scholar]
- 20.Rabbitt P, Diggle P, Holland F, McInnes L. Practice and drop-out effects during a 17-year longitudinal study of cognitive aging. The journals of gerontology Series B, Psychological sciences and social sciences. 2004 Mar;59(2):84–97. doi: 10.1093/geronb/59.2.p84. [DOI] [PubMed] [Google Scholar]
- 21.Balasubramanian AB, Kawas CH, Peltz CB, Brookmeyer R, Corrada MM. Alzheimer disease pathology and longitudinal cognitive performance in the oldest-old with no dementia. Neurology. 2012 Aug 28;79(9):915–921. doi: 10.1212/WNL.0b013e318266fc77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raghunathan TE. What do we do with missing data? Some options for analysis of incomplete data. Annu Rev Public Health. 2004;25:99–117. doi: 10.1146/annurev.publhealth.25.102802.124410. [DOI] [PubMed] [Google Scholar]
- 23.IVEware: Imputation and variance estimation software survey methodology program. Ann Arbor, MI: Survey Research Center, Institute for Social Research, University of Michigan; 2009. computer program. [Google Scholar]
- 24.Mayeda ER, Haan MN, Kanaya AK, Yaffe K, Neuhaus J. Type 2 diabetes and 10 year risk of dementia and cognitive impairment among older Mexican Americans. Diabetes Care. 2013 doi: 10.2337/dc12-2158. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comijs HC, Kriegsman DM, Dik MG, Deeg DJ, Jonker C, Stalman WA. Somatic chronic diseases and 6-year change in cognitive functioning among older persons. Arch Gerontol Geriatr. 2009 Mar-Apr;48(2):191–196. doi: 10.1016/j.archger.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Hassing LB, Grant MD, Hofer SM, et al. Type 2 diabetes mellitus contributes to cognitive decline in old age: a longitudinal population-based study. J Int Neuropsychol Soc. 2004 Jul;10(4):599–607. doi: 10.1017/S1355617704104165. [DOI] [PubMed] [Google Scholar]
- 27.Kanaya AM, Barrett-Connor E, Gildengorin G, Yaffe K. Change in cognitive function by glucose tolerance status in older adults: a 4-year prospective study of the Rancho Bernardo study cohort. Arch Intern Med. 2004 Jun 28;164(12):1327–1333. doi: 10.1001/archinte.164.12.1327. [DOI] [PubMed] [Google Scholar]
- 28.Mayeda ER, Haan MN, Neuhaus J, et al. Type 2 Diabetes and Cognitive Decline Over 14 Years in Middle-Aged African Americans and Whites: The ARIC Brain MRI Study. Neuroepidemiology. 2014 Nov 13;43(3-4):220–227. doi: 10.1159/000366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Berg E, de Craen AJ, Biessels GJ, Gussekloo J, Westendorp RG. The impact of diabetes mellitus on cognitive decline in the oldest of the old: a prospective population-based study. Diabetologia. 2006 Sep;49(9):2015–2023. doi: 10.1007/s00125-006-0333-1. [DOI] [PubMed] [Google Scholar]
- 30.Nooyens AC, Baan CA, Spijkerman AM, Verschuren WM. Type 2 diabetes and cognitive decline in middle-aged men and women: the Doetinchem Cohort Study. Diabetes Care. 2010 Sep;33(9):1964–1969. doi: 10.2337/dc09-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu JH, Haan MN, Liang J, Ghosh D, Gonzalez HM, Herman WH. Impact of diabetes on cognitive function among older Latinos: a population-based cohort study. J Clin Epidemiol. 2003 Jul;56(7):686–693. doi: 10.1016/s0895-4356(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen HT, Black SA, Ray LA, Espino DV, Markides KS. Predictors of decline in MMSE scores among older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2002 Mar;57(3):M181–185. doi: 10.1093/gerona/57.3.m181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.