Abstract
Aortoesophageal fistula (AEF) is a rare and life-threatening cause of gastrointestinal haemorrhage which requires emergency surgical intervention. We report a case of aortoesophageal fistula due to previous endovascular stent grafting to the ascending thoracic aorta after previous traumatic injury. The patient presented with catastrophic haematemesis which was managed by repeated deployment of endovascular stent graft and aortic bypass surgery; however, the patient ultimately died due to massive haemoptysis.
Background
Aortoesophageal fistula is a rare cause of upper gastrointestinal bleeding which mandates an early and aggressive resuscitation once diagnosed, in addition to appropriate surgical intervention. This case report will highlight thoracic endovascular aortic repair (TEVAR)-related aortoesophageal fistula (AEF) and management plan and the complex hospital course which elaborates the outcome of such patients.
Case presentation
A 22-year-old male patient presented to the hospital with a history of black stool for 2 days. He had a motor vehicle accident 1 year prior to admission, complicated by proximal dissection of the aortic arch and underwent successful aortic grafting. The patient mentioned a history of the use of non-steroidal anti-inflammatory drugs (NSAID) for back pain and occasional marijuana inhalation.
The patient appeared pale, but not jaundiced. His temperature was 36.4°C; blood pressure 100/71 mm Hg; heart rate 93 beats/min without postural changes; respiratory rate was 18/min and oxygen saturation 99% on 24% oxygen. Systemic examination was normal except for mild back tenderness.
Investigations
WBC 12.5×109/l, haemoglobin 135 g/l, platelet count 277×109/l. coagulation and renal profiles were normal. The arterial blood gas was normal. Toxicology screening was negative. The patient underwent upper gastrointestinal (UGI) endoscopy, which showed an AEF in the mid-oesophagus and mild gastritis (figures 1 and 2).
Figure 1.

Aortoesophageal fistula (arrow).
Figure 2.

Non-steroidal anti-inflammatory drug-related gastropathy.
Treatment
After UGI endoscopy, the patient developed massive haematemesis requiring aggressive fluid resuscitation, in addition to 8 units of red cell packs and 8 units of fresh frozen plasma transfusion. A Sengstaken-Blakemore tube was inserted into the oesophagus to minimise the bleeding.
The patient was immediately taken to the operation room, and an aortic stent proximal to the left common carotid artery was inserted after identifying the site of leak through the AEF, in addition to oesophageal stenting by the interventional radiologist and vascular surgeon, and was then brought to the intensive care unit (figures 3 and 4).
Figure 3.

Three-dimensional CT angiogram showing the old endovascular stent graft covering the left subclavian artery and landing at the origin of the left common carotid artery after the traumatic dissection of the descending thoracic aorta.
Figure 4.

Three-dimensional CT angiogram showing the deployment of new stent graft at the left common carotid artery after the initial upper gastrointestinal bleeding episode.
After 3 h, the patient developed rebleeding. CT aortic angiogram showed the pseudoaneurysm on the inferior aspect of the aortic arch, with evidence of leak. The patient had a right to left carotid-to-carotid bypass, occlusion of the left proximal common carotid artery and extension of the thoracic endograft proximally to cover the left common carotid artery (figure 5).
Figure 5.
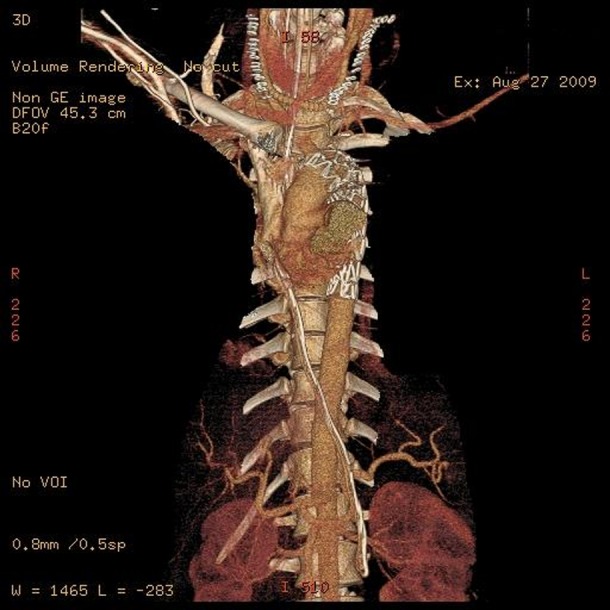
Three-dimensional CT angiogram shows the extension of the thoracic endograft to cover the left common carotid artery, right carotid to left carotid arteries bypass after the proximal occlusion of left common carotid artery.
Outcome and follow-up
He was extubated after 6 days of surgery, as he developed metabolic encephalopathy due to contrast nephropathy. His course was also complicated by aspiration pneumonia, due to the impaired oesophageal motility attributed to the placement of the oesophageal stent. Therefore, he was intubated again for airway protection and continued on enteral nutrition.
Blood stream infection with Staphylococcus aureus was reported on day 8 postsurgery, and all vascular catheters were changed. However, catheter tips cultures were negative, and the patient improved after modifying his antibiotics to intravenous vancomycin.
Follow-up CT aortic angiogram revealed patent endovascular graft and a pseudoaneurysm which appeared at the proximal landing zone of the extended stent near to the origin of the brachio-cephalic artery, mostly related to blood stream infection during the patient hospital stay (figure 6). The patient underwent endovascular/surgical intervention through replacement of a vascular graft from the aortic root to the brachiocephalic artery by the vascular and cardiac surgeons (figure 7). Aortic vessel biopsy was negative for vasculitic pathology or connective tissue disease.
Figure 6.

Three-dimensional CT angiogram showing 3 cm size pseudoaneurysm (arrow) which formed at the proximal landing zone of the recently replaced stent near to the brachiocephalic artery.
Figure 7.

Three-dimensional CT angiogram showing the avascular graft from the aortic root to the brachiocephalic artery (arrow).
Five days later, he had sudden shortness of breath. CT chest showed resorping mediastinal haematoma with a small amount of air and left pulmonary artery pseduoaneurysm. Acute mediastinitis was excluded by serial chest images and negative mediastinal fluid cultures. Unfortunately, the patient developed sudden and massive haemoptysis, followed by respiratory arrest. The patient died after 50 min of aggressive resuscitation. The haemoptysis was probably due to the rupture of the left pulmonary artery pseudoaneurysm.
Discussion
Approximately 160 patients per 100 000 population are hospitalised annually for bleeding in the upper gastrointestinal tract in the USA.1 Duodenal and gastric ulcers, gastritis, oesophageal varices and Mallory-Weiss tears are the most common causes of gastrointestinal bleeding.1
AEF is a rare but often fatal cause of upper gastrointestinal bleeding.2 Chiari described the aortoesophageal syndrome in 1914 as consisting of a clinical triad of mid-thoracic chest pain, followed by sentinel arterial haemorrhage and fatal arterial exsanguination after a symptom free interval of hours to days.3
The aortoesophageal fistula is an abnormal communication between the oesophagus and the aorta that allows the high-pressure aortic blood to enter the oesophagus. Commonly, it is caused by thoracic aortic aneurysm, foreign body ingestion, oesophageal malignancy or postoperative complications.2
The diagnosis may be made on the basis of clinical findings alone (Chiari's triad). The bright red bleeding of the oxygenated arterial blood characteristically distinguishes the bleeding of aortoesophageal fistulas from that of oesophageal varices.4
Most diagnostic tests have significant individual limitations, and may not show any findings if the patient is not bleeding.
Endoscopy of the upper gastrointestinal tract should exclude alternative bleeding sources, and may show active pulsatile submucosal bleeding or a submucosal haematoma.5
Aortography may be useful during active haemorrhage to demonstrate the fistula, but results of aortography may be negative during the symptom-free interval, due to clot formation.5
Dynamic CT may be a more rapid alternative.6
For patients who are in a stable condition after the sentinel haemorrhage, a confirmatory test is often required.
Circulatory support with volume replacement and transfusion of blood products is essential in patients with active haemorrhage. A Sengstaken-Blakemore tube provides temporary control of the exsanguinating haemorrhage by direct pressure on the fistula.7
Patients in an unstable condition should undergo immediate surgery. Survival is now possible with rapid surgical intervention. Operative repair of an aortoesophageal fistula is necessary, as no survivors have been reported with nonsurgical management. Other measures, such as radiographic embolisation may be useful as a temporising measure; however, delayed exsanguination after embolisation can occur.8
TEVAR was introduced in 1999 as a nonsurgical modality for the management of acute and chronic aortic dissections.9
The retrospective study of Jonker et al stated that endovascular aortic repair of the AEF to prevent the immediate exsanguination as a bridge for future definitive oesophageal repair has improved survival. While awaiting operative intervention, long-term broad-spectrum antibiotics should be administered, as the oesophageal flora may invade the mediastinum or the aorta.10
TEVAR has two major and devasting complications in the literature which are stroke secondary atherothombotic phenomena and paraplegia, which is secondary to prolonged ischaemic time to the neuronal cells in 1.5–19% of cases. Other rare complications are retrograde dissection of the ascending aorta, stent graft collapse, migration, torsion, thrombosis and AEF.9
Secondary AEF was reported to occur after 1–16 months of TEVAR. Eggebrecht et al reported an incidence rate of 1.9% among 268 patients in their case series.11
Our patient had AEF secondary to mechanical intimal tear to the aorta and invasion to the oesophageal wall was most likely. Other mechanisms of AEF formation in the literature are pressure necrosis of the oesophageal wall due to endovascular stent expansion force and decrease in blood supply to the oesophagus, leading to necrosis of the wall and infection.11
With the rapid diagnosis and surgical treatment of AEF, long-term survival is possible as the risk of re-bleeding, and mediastinal infection decreases.10
Learning points.
Aortoesophageal fistula (AEF) is a rare cause of upper gastrointestinal bleeding with high death rate.
The diagnosis of AEF is usually based on the clinical presentation with associated risk factors. Confirmation is required, with invasive aortograghy showing active bleeding.
Immediate resuscitation should be initiated, in addition to broad spectrum antibiotics, to prevent mediastinal infection.
Aggressive surgical approach including endovascular aortic stent grafting and oesophageal repair are the major interventions to increase the survival rate.
Rebleeding and infection are the main causes of death in patients with AEF.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Lewis JD, Bilker WB, Brensinger C, et al. Hospitalization and mortality rates from peptic ulcer disease and GI bleeding in the 1990s: relationship to sales of nonsteroidal anti-inflammatory drugs and acid suppression medications. Am J Gastroenterol 2002;97:2540–9. [DOI] [PubMed] [Google Scholar]
- 2.Fernando HC, Benfield JR. Surgical management and treatment of esophageal fistula. In: Meguid MM, Campos AC.eds. Surgical management of gastrointestinal fistulas—the surgical clinics of North America. Philadelphia: Saunders, 1996;76:1131–2. [DOI] [PubMed] [Google Scholar]
- 3.Carter R, Mulder GA, Snyder EN, Jr, et al. Aortoesophageal fistula. Am J Surg 1978;136:26–30. [DOI] [PubMed] [Google Scholar]
- 4.Albors J, Bahamonde JÁ, Sanchis JM, et al. Aortoesophageal fistula after thoracic stent grafting. Asian Cardiovasc Thorac Ann 2011;19:352–6. [DOI] [PubMed] [Google Scholar]
- 5.Khawaja FI, Varindani MK. Aortoesophageal fistula: review of clinical radiographic, and endoscopic features. J Clin Gastroenterol 1987;9:342–4. [DOI] [PubMed] [Google Scholar]
- 6.Molina PL, Strobl PW, Burstain JM. Aortoesophageal fistula secondary to mycotic aneurym of the descending thoracic aorta: CT demonstration. J Comput Assist Tomogr 1995;19:309–11. [DOI] [PubMed] [Google Scholar]
- 7.Magnussen I, Notander A, Rieger A, et al. Massive hematemesis due to an aortoesophageal fistula (case report). Acta Chir Scand 1987;153:317–19. [PubMed] [Google Scholar]
- 8.Reedy FM. Embolization of aortoesophageal fistula: a new therapeutic approach. J Vasc Surg 1988;8:349–50. [DOI] [PubMed] [Google Scholar]
- 9.Swee W, Dake MD. Endovascular management of thoracic dissections. Circulation 2008;117:1460–73. [DOI] [PubMed] [Google Scholar]
- 10.Jonker FH, Schlösser FJ, Moll FL, et al. Outcomes of thoracic endovascular aortic repair for aortobronchial and aortoesophageal fistulas. J Endovasc Ther 2009;16:428–40. [DOI] [PubMed] [Google Scholar]
- 11.Eggebrecht H, Mehta RH, Dechene A, et al. Aortoesophageal fistula after thoracic aortic stent-graft placement: a rare but catastrophic complication of a novel emerging technique. JACC Cardiovasc Interv 2009;2:570–6. [DOI] [PubMed] [Google Scholar]


