Abstract
Biomarkers for diffuse axonal injury could have utilities for the acute diagnosis and clinical care of concussion, including those related to sports. The calpain-derived αII-spectrin N-terminal fragment (SNTF) accumulates in axons after traumatic injury and increases in human blood after mild traumatic brain injury (mTBI) in relation to white matter abnormalities and persistent cognitive dysfunction. However, SNTF has never been evaluated as a biomarker for sports-related concussion. Here, we conducted longitudinal analysis of serum SNTF in professional ice hockey players, 28 of whom had a concussion, along with 45 players evaluated during the preseason, 17 of whom were also tested after a concussion-free training game. Compared with preseason levels, serum SNTF increased at 1 h after concussion and remained significantly elevated from 12 h to 6 days, before declining to preseason baseline. In contrast, serum SNTF levels were unchanged after training. In 8 players, postconcussion symptoms resolved within a few days, and in these cases serum SNTF levels were at baseline. On the other hand, for the 20 players withheld from play for 6 days or longer, serum SNTF levels rose from 1 h to 6 days postconcussion, and at 12–36 h differed significantly from the less-severe concussions (p=0.004). Serum SNTF exhibited diagnostic accuracy for concussion, especially so with delayed return to play (area under the curve=0.87). Multi-variate analyses of serum SNTF and tau improved the diagnostic accuracy, the relationship with the delay in return to play, and the temporal window beyond tau alone. These results provide evidence that blood SNTF, a biomarker for axonal injury after mTBI, may be useful for diagnosis and prognosis of sports-related concussion, as well as for guiding neurobiologically informed decisions on return to play.
Key words: : calpain, diffuse axonal injury, mild traumatic brain injury, sports concussion, tau
Introduction
Mild traumatic brain injury (mTBI) presenting with negative head computed tomography (CT) findings, often referred to as concussion, is the most common neurological injury and is an increasing concern for participants in contact sports. For athletes and other mTBI sufferers, postconcussion symptoms (PCS) commonly resolve within hours or days, but for a small proportion of cases, brain dysfunction and disability can persist, sometimes for a year or longer.1–4 The main structural correlate for the long-lasting brain dysfunction that can occur after mTBI is thought to be diffuse axonal injury.5–8 Unfortunately, there is currently no therapeutic or rehabilitative intervention clinically proven to ameliorate diffuse axonal injury (DAI) or improve long-term brain function.9–11 Moreover, there is no established method for identifying, at an early and potentially treatable stage, those individuals suffering mTBI with negative head CT findings that nevertheless are at risk of developing white matter (WM) damage and lasting brain dysfunction. For athletes, challenges remain to make neurobiologically informed decisions on suitability for return to play and vulnerability to repetitive injuries.12,13
A blood biomarker whose levels relate to mTBI-induced DAI and persistent brain dysfunction would have major diagnostic and prognostic utilities for the clinical research and treatment of mTBI. A number of proteins have been evaluated as candidate blood biomarkers for mTBI, including neuron-specific enolase, ubiquitin C-terminal hydrolase L1, and αII-spectrin C-terminal fragments, as well as the glia-enriched S100β and glial fibrillary acidic protein. Unfortunately, none of these markers has a prognostic relationship with patient outcomes for mTBI with negative head CT findings.14–21 One study, however, showed an increase in serum tau in concussed hockey players correlating with the duration of PCS.22 We identified calpain-derived 1176 residue N-terminal fragment of α-spectrin, SNTF, as a protein that accumulates preferentially in damaged axons23–25 and increases measurably in the blood after TBI, including CT-negative mTBI.26,27 SNTF is normally undetectable in axons, but is generated subsequent to stretch injury by intra-axonal calcium overload and spectrin proteolysis mediated by the calpain family of calcium-activated proteases.25,28,29 In a preliminary study of mTBI treated in the emergency room, blood SNTF levels correlated with WM abnormalities detectable with diffusion tensor imaging (DTI) as well as cognitive dysfunction that persisted for at least 3 months.27 Consequently, SNTF is a promising, biologically plausible blood biomarker for the DAI of functionally deleterious mTBI. It has not been studied before as a marker for sports-related concussion.
The aim of the present study was to evaluate serum SNTF as a diagnostic and prognostic biomarker for concussion in a prospective cohort study involving professional ice hockey players in the Swedish Hockey League. This study design and player cohort was used recently to evaluate other candidate serum biomarkers for concussion in professional athletes.22 Here, in a parallel approach, we compared the serum levels of SNTF longitudinally postconcussion with their baseline levels before the start of the season, evaluated relationships between serum SNTF concentrations and the severity of PCS, and conducted multi-variate analyses of SNTF and the other markers in relation to the incidence and severity of concussion.
Methods
Study population
The prospective study of concussion among professional ice hockey players from the Swedish Hockey League has been described before.22 The study was approved by the Ethics Committee for Medical Research at the University of Gothenburg (Molndal, Sweden) and by the Swedish Ice Hockey Association. Written informed consent was obtained from all 288 study participants comprised of 24 players from each of the 12 teams. The physicians for each team documented signs and symptoms of concussion and performed physical examinations in the event of concussion during the first half of the 2012–2013 season. The diagnosis of concussion (n=28) was made according to the latest guidelines on sports-related concussion.30 Blood samples were obtained at 1 (n=25), 12 (n=22), 36 (n=20), and 144 h (n=18) after concussion as well as on the day of return to play (n=10). Physicians recorded the date of concussion and the date at which players completely recovered from their injuries and returned to unrestricted competition. In addition, before the start of the season, players from two teams were sampled for baseline serum biomarker levels (n=45), and players from one of these teams provided blood samples 1 and 12 h after a training game without concussion incident (n=17).
Serum biomarkers
Blood samples were collected and sera prepared by the methods described before.22 SNTF was quantified in the deidentified sera using an electrochemiluminescence-based sandwich immunoassay27 by an experimenter blinded to the data on PCS severity and serum levels of the other biomarkers. Briefly, standard 96-well plates with an underside electrode (Meso Scale Discovery, Gaithersburg, MD) were coated with purified and highly cross-species adsorbed goat anti-mouse immunoglobulin G (IgG; Southern Biotechnology, Birmingham, AL) at 50 ng per well in phosphate-buffered saline containing 0.03% Triton X-100, air dried, and stored overnight at 4°C. The next day, wells were blocked with 0.5% bovine serum albumin (BSA) in TTBS (Tris-buffered saline [pH 7.4] containing 0.05% Tween-20), then washed with TTBS. The capture antibody (Ab), a mouse monoclonal to the SH3 domain in the N-terminal half of spectrin αII-subunit31 (Covance, Kalamazoo, MI) was applied as ascites fluid at 25 ng per well in 0.2% BSA/TTBS for 1 h, then the wells were washed with TTBS. Next, human sera diluted to 40% or SNTF standards mixed in 0.2% BSA/TTBS were added in sextuplicate (25 μL per well) for 2 h, and then the wells were washed with TTBS. A standard curve was generated using a preparation of αII-spectrin isolated from mouse brain membranes by high salt extraction and ammonium sulfate precipitation, followed by digestion with purified erythrocyte calpain I to generate SNTF, as described before.23,27,32 The detecting Ab was a cleavage site-specific purified rabbit IgG raised against the calpain-generated neoepitope in the C-terminus of SNTF ending at αII-spectrin residue 1176. This Ab was prepared in our laboratory and characterized extensively by Western blotting, immunohistochemistry, protease digest, and solid-phase immunoassay for specific reactivity with SNTF, but not the spectrin holoprotein or other spectrin proteolytic fragments.23,26 Negative controls were evaluated in triplicate for every serum sample by replacing the SNTF-specific IgG with purified IgG prepared from preimmune rabbit serum and used at the same concentration (200 ng/mL). The reporter Ab was goat anti-rabbit IgG conjugated to ruthenium (Sulfotag; Meso Scale Discovery) and diluted to 1:500 in BSA/TTBS. After three washes in TTBS, read buffer T containing tripropylamine (Meso Scale Discovery) was added to each well and, using a Sector 6000 system current, was applied to the plates and the electrochemiluminescent product generated in each well was quantified. Specific SNTF signal was calculated as the difference in signal between triplicate wells containing either the SNTF IgG or preimmune IgG and normalized to brain SNTF standards. One unit of SNTF is defined as the signal generated by 1 nL of the protein standard per mL. The lower limit of detection (LLOD) was determined experimentally to be 14 units.
Tau and S100β were quantified from the sera as published previously.22 One unit of tau and S100β are defined as the signal generated by their respective protein standards at 1 pg/mL.
Statistical analyses
For comparison of biomarker levels after concussion or training versus their preseason or pretraining levels, the two-tailed t-test was used. Longitudinal biomarker levels were compared by Mann-Whitney's U test between participants with return to play within less than 6 days and 6 days or greater. Linear regression analyses compared longitudinal postconcussion serum levels of SNTF with tau and S100β. For serum SNTF levels below the LLOD of 14 units, a value of 13 units was assigned for all statistical analyses. The area under the receiver operator characteristics curve (AUC) for SNTF levels postconcussion versus preseason or as a function of the delay in return to play was calculated using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA). Multi-variate analyses examined the combination of serum SNTF and tau after concussion in relation to preseason levels and with respect to the delay in return to play, using the statistical methods described above. Equal weighting was placed on SNTF and tau by representing each concentration as a fraction of the mean for that marker and summing the two fractional means for the combined measure.
Results
In this study of serum SNTF, all 28 hockey players suffering a concussion during the first half of the 2012–2013 season in the top professional league in Sweden were evaluated, along with 45 players analyzed during the preseason, 17 of whom were also tested before and after a training game without concussion incident. Mean ages were essentially the same for the groups of players tested during the preseason (27.6 years), before and after a training game (27.2 years), or subsequent to an in-season concussion (27.2 years). Among the concussion cases, 3 suffered a loss of consciousness and all experienced PCS, including headache, confusion, dizziness, or nausea. Based on grading according to the latest guidelines for sports concussion,30 8 of the players became symptom free within a few days of their injury, but in 20 players, symptoms persisted for 6 days or longer. Persistent symptoms that delayed return to play included dizziness, confusion, headache, cognitive impairment, nausea, insomnia, and irritability.
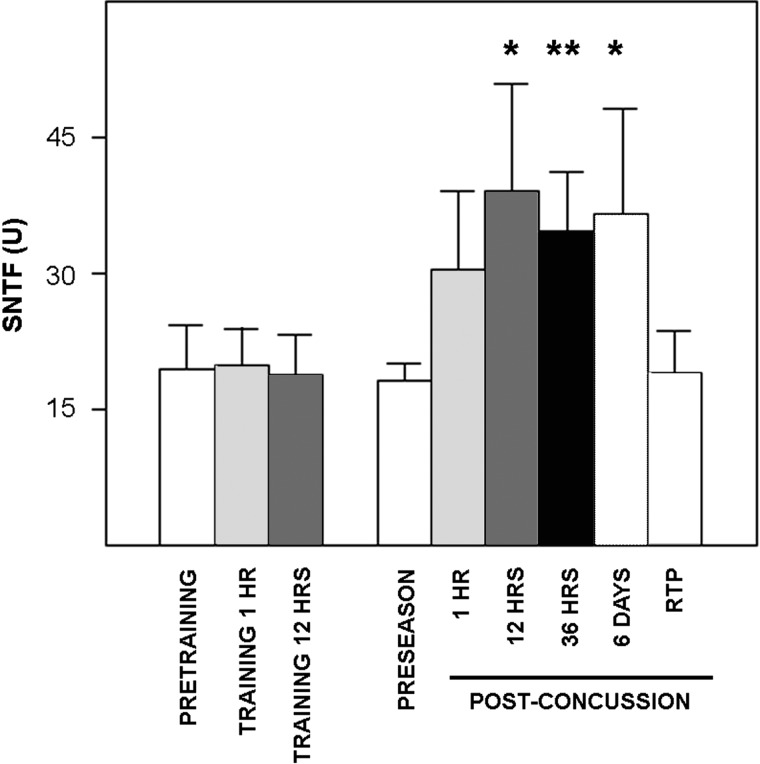
Serum SNTF levels were variable and generally low in samples taken during the preseason, with concentrations being below the LLOD in 58% of the players. Detection of serum SNTF above the lower limit in a subset of experienced professional athletes (levels were at least twice the LLOD in 16% of cases) contrasts with a pilot study of serum SNTF in nonprofessional athlete healthy controls, whose levels were below the LLOD in 100% of cases.27 Moreover, during the hockey season, serum SNTF concentrations increased in a rapid and prolonged fashion in players who sustained a concussion. Mean SNTF concentration was up to 2-fold higher at 1, 12, 36, and 144 h postconcussion, compared with preseason levels, and the increases at the latter three time points were statistically significant (Fig. 1). By the time players were symptom free and permitted to return to play, SNTF levels returned to near preseason baseline. To distinguish the effects of concussion on serum SNTF separate from any influence of physical exertion, the marker was measured serially in 17 players during the preseason and at two time points after a concussion-free training game. In contrast to the pronounced effects of concussion, serum SNTF levels were unchanged at 1 or 12 h after a training game, compared with their pregame levels.
FIG. 1.
Sustained increase in serum SNTF concentrations in professional ice hockey players after concussion, but not concussion-free training. SNTF levels were measured in serum during the preseason (n=45) or serially after an in-game concussion (n=28), or before and after a training game (n=17). The mean serum SNTF levels (±standard error of the mean) were elevated at 1, 12, 36, and 144 h postconcussion, compared with the mean preseason baseline concentration, and the increases at the latter three time points were statistically significant (two-tailed t-test; *p<0.03; **p<0.002). At the time of return to play (RTP) after a period of rest, SNTF levels returned to their preseason baseline. In contrast to the pronounced effects of concussion, SNTF was unchanged 1 or 12 h after concussion-free training (p≥0.87). SNTF, calpain-derived αII-spectrin N-terminal fragment.
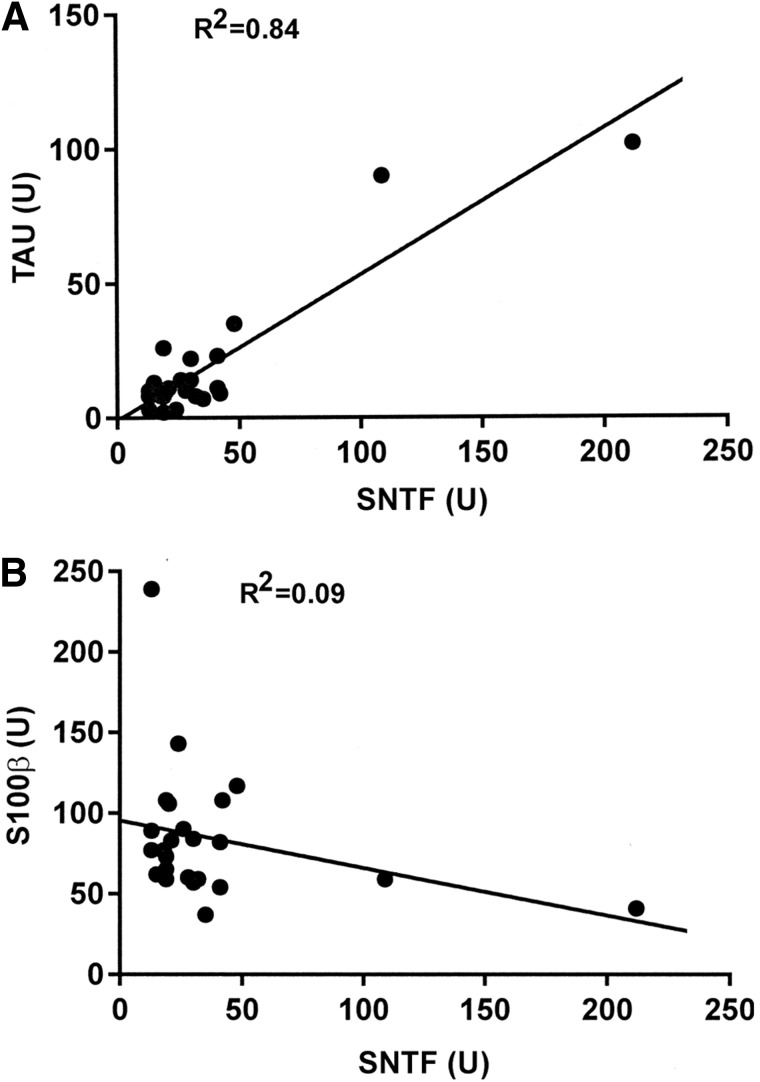
Serum concentrations of total tau are elevated in this cohort of concussed professional hockey players relative to preseason and pretraining game levels.22 Given that tau is an axon-enriched microtubule-associated protein,33 and SNTF accumulates preferentially in damaged axons after TBI,24,29,34 the serum elevations in these two cytoskeletal proteins may be mechanistically related to each other, as well as to TBI-induced DAI. To begin assessing this possibility, the serum levels of SNTF and tau were compared on a per-player basis. As shown in Figure 2A, the mean serum SNTF level at 12–36 h postconcussion was linearly related to serum tau level assessed 1 h postconcussion (R2=0.84; n=24). The relationship between serum SNTF and tau was less strong when either marker was evaluated at other times after concussion (data not shown). Serum concentrations of the astroglial-enriched S100β are also elevated at 1 h postconcussion, compared with its preseason baseline concentration, in this player cohort.22 However, in contrast to the correlation between serum concentrations of SNTF and tau, there was no relationship between serum levels of SNTF and S100β (Fig. 2B).
FIG. 2.
Serum levels of SNTF after concussion are related to serum tau, but not S100β. The mean serum SNTF concentration at 12 and 36 h postconcussion is linearly related to the serum tau concentration measured at 1 h postconcussion (A), but not to the serum level of S100β at 1 h (B). For measures of either SNTF or tau at all other time points, the correlation between the two markers is less strong (data not shown). Levels of each marker are represented in units (see Methods for more details). SNTF, calpain-derived αII-spectrin N-terminal fragment.
Diagnostic accuracy of serum SNTF
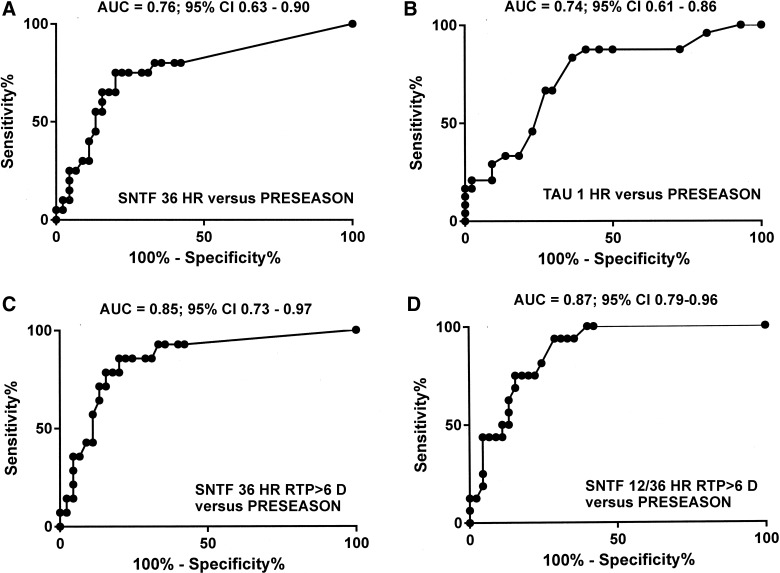
To assess the accuracy of serum SNTF for the diagnosis of sports-related concussion, the AUC was analyzed comparing SNTF levels tested at different times postconcussion with preseason SNTF concentrations. The AUC=0.76 for SNTF 36 h after concussion versus SNTF during the preseason (Fig. 3A), which compares favorably to the AUC for tau various times after concussion versus the preseason (highest AUC=0.74 at 1 h; Fig. 3B). SNTF at 36 h showed greater accuracy in diagnosing concussion in players experiencing persistent PCS that delayed return to play to 6 days or longer (AUC=0.85; Fig. 3C). Finally, the AUC=0.87 for the mean serum SNTF at 12–36 h in players with PCS lasting for at least 6 days, compared with preseason biomarker levels (Fig. 3D).
FIG. 3.
Diagnostic accuracy of serum SNTF and tau assessed by receiver operator characteristics AUC analyses. (A) Serum SNTF levels at 36 h postconcussion versus preseason levels. (B) Serum tau levels at 1 h postconcussion versus preseason levels. (C) Serum SNTF levels at 36 h for concussions with return to play ≥6 days versus preseason levels. (D) Mean serum SNTF levels at 12–36 h for concussions with return to play ≥6 days versus preseason levels. SNTF, calpain-derived αII-spectrin N-terminal fragment; AUC, area under the curve; CI, confidence interval.
Serum SNTF
A fast, objectively quantified blood biomarker test could be valuable for the clinical research and management of sports-related concussion. As described above, the diagnostic accuracy of serum SNTF from 12 to 36 h after concussion was especially high for the subset of cases experiencing PCS persisting at least 6 days. To investigate further the relationship between longitudinal measures of serum SNTF and the persistence of PCS, we compared biomarker levels between concussion cases with return to play in less than 6 days with those requiring a delay in return to play of 6 days or longer (Table 1). At times ranging from 1 h to 6 days postconcussion, serum SNTF was essentially unchanged from preseason baseline levels for the subset of players with rapidly resolving PCS. In sharp contrast, for cases with persistent PCS lasting 6 days or longer, serum SNTF levels were higher by up to 2.5-fold from 1 h to 6 days postinjury, compared with concentrations either at preseason baseline or in players with less-severe PCS. The difference in serum SNTF concentration after concussion as a function of PCS severity was significant at the 36-h time point (p=0.014) and from the mean at 12 and 36 h (p=0.004). Serum tau levels also were higher in the subset of concussions requiring at least 6 days for return to play, compared with cases with shorter-lasting PCS, with the difference at 12 h being significant (p=0.039). On the other hand, whereas the serum level of S100β at 1 h postconcussion was above its preseason baseline concentration,22 there was no difference in S100β levels between concussions associated with relatively rapid (<6 days) and delayed (≥6 days) return to play. To determine whether the combined measure of serum SNTF and tau was associated more strongly with PCS severity than either marker alone, an equal-weight multi-variate analysis was performed. The multi-variate measure of two markers across all combinations of time points correlated less strongly with the dichotomized delay in return to play than serum SNTF concentrations alone. The addition of SNTF improved the correlation with PCS severity achieved with serum tau alone and broadened its temporal window (Table 1).
Table 1.
Longitudinal Serum SNTF, Tau, and S100β Concentrations in Relation to PCS Severity
| Time postconcussion | <6 days RTP | >6 days RTP | p value |
|---|---|---|---|
| SNTF, 1 h | 20.4±3.1 (n=8) | 35.5±12.9 (n=16) | >0.5 |
| 12 h | 19.0±2.4 (n=8) | 50.4±19.0 (n=14) | 0.087 |
| 36 h | 19.3±3.0 (n=6) | 41.0±8.4 (n=14) | 0.014 |
| 12/36 h mean | 18.6±2.1 (n=8) | 45.8±11.6 (n=17) | 0.004 |
| 6 days | 17.0±4.0 (n=5) | 44.6±15.4 (n=13) | 0.15 |
| Tau, 1 h | 9.0±2.1 (n=8) | 23.1±7.0 (n=17) | 0.070 |
| 12 h | 4.8±1.8 (n=8) | 17.9±7.4 (n=15) | 0.039 |
| 36 h | 5.7±1.6 (n=7) | 28.0±12.5 (n=14) | 0.11 |
| 6 days | 8.0±1.7 (n=5) | 35.6±14.5 (n=12) | 0.34 |
| S100β, 1 h | 106±21 (n=8) | 72.7±5.6 (n=17) | 0.27 |
| SNTF12/36+Tau12 | 69±8 (n=8) | 224±65 (n=17) | 0.011 |
The serum concentrations of the three markers (mean units±standard error of the mean) are presented in relation to the severity of PCS, dichotomized on the basis of a delay in RTP of <6 days or ≥6 days. SNTF and tau serum concentrations were higher in the players with more persistent PCS, whereas S100β serum levels were not. The increase in serum SNTF was statistically significant by Mann-Whitney's U test at 36 h and for the 12- to 36-h mean, whereas the elevation in serum tau was statistically significant at 12 h postconcussion. The multi-variate measure of serum SNTF (mean at 12–36 h) and tau (at 12 h) was also significantly different based on the persistence of PCS, but at no combination of time points were multi-variate analyses of SNTF and tau related more strongly to PCS severity than the mean SNTF levels at 12–36 h alone. The bolded p values are statistically significant.
SNTF, calpain-derived αII-spectrin N-terminal fragment; PCS, postconcussion symptom; RTP, return to play.
Discussion
SNTF reportedly increases in serum in a subset of cases after CT-negative mTBI,27 but until now had not been evaluated as a blood biomarker for sports-related concussion. Here, we demonstrate that SNTF is elevated in serum of professional ice hockey players who suffered a concussion in comparison with its preseason level. Longitudinal analysis finds that the serum concentration of SNTF increases as early as 1 h postconcussion and remains elevated significantly above preseason baseline for up to 6 days thereafter, before returning to baseline at the time of return to play (Fig. 1). The rise in serum SNTF levels is not simply a result of the physical exertion of hockey, given that the marker is unchanged in players evaluated serially before and after a concussion-free training game. Perhaps, most important, serum SNTF relates to the severity of the PCS, as assessed by the latest guidelines for sports concussions. For players whose PCS resolved within a few days, serum SNTF levels are essentially unchanged from their preseason baseline. On the other hand, for concussed players with persisting PCS requiring they be withheld from play for 6 days or longer, serum SNTF concentrations are significantly elevated from 12 to 144 h postinjury compared with preseason baseline and also from 12 to 36 h compared with concussed players whose symptoms resolved within a few days (Table 1; p=0.004). Serum SNTF has accuracy for diagnosing concussions, especially the subset with persisting PCS (Fig. 3; AUC=0.87). These results provide evidence that serum SNTF analyzed subacutely after injury may have utilities for the diagnosis and prognosis of sports-related concussion and might facilitate objective, neurobiologically informed decisions on fitness for return to play.
SNTF is a biologically plausible blood biomarker for sports-related concussion. Although the mechanisms underlying the serum rise in SNTF concentrations after mTBI are not completely understood, several findings suggest that it may be a mechanism-based marker for DAI. SNTF is absent from healthy neurons, but accumulates as a stable N-terminal 1176 residue fragment of the nonerythroid spectrin α-subunit35 in degenerating neurons after activation of calcium-dependent calpain proteases.23,36 Stretch-induced injury of axons in vitro triggers intra-axonal calcium overload, calpain activation, proteolysis of spectrin and other calpain substrates, and the accumulation of SNTF in the damaged axons.28,29,37 SNTF also builds up in axons after TBI in vivo,24,25 including in a head rotational acceleration large-animal model of sports-related concussion as well as in human TBI.34 In a recent human study of serum SNTF in CT-negative mTBI treated in the emergency room, the marker increased preferentially in the subsets of cases exhibiting WM abnormalities detectable with DTI and persistent postconcussion cognitive deficits.27 Together with the present findings linking serum SNTF to the incidence and severity of sports-related concussions, the collective data support the hypothesis that SNTF is a mechanism-based blood biomarker for the DAI underlying brain functional impairment after mTBI.5–7 The correlation on a per-player basis between the postconcussion serum level of SNTF with the axon-enriched tau, but not the glial-enriched S100β (Fig. 2), further supports this hypothesis.
In this study, serum level of SNTF is generally low in players during preseason sampling, but is above the LLOD in 42% of cases. The finding of detectible serum SNTF in a subset of professional ice hockey players during the off-season contrasts with a small pilot study of healthy controls not participating in contact sports, in which serum SNTF levels were below the LLOD in 100% of cases.27 These preliminary data raise the possibility that serum SNTF might be elevated chronically in a subset of highly experienced contact sports participants. The robustness of this finding and its bearing on the vulnerability of concussed athletes to developing a progressive neurodegenerative condition in the chronic postinjury time period38–41 will require further investigation. The current study has additional limitations. The sample sizes are relatively small, and there is incomplete assessment of the relationship of serum SNTF concentrations with brain structural and long-term functional changes after sports-related concussion. Preseason blood samples were not available for all of the players, precluding direct comparisons of baseline and postconcussion biomarker levels in the same professional athletes. More research will be required to define the potential utilities of blood measures of SNTF, both alone and with other neuronal injury biomarkers, and in combination with neuroradiological, physiometric, and behavioral assessments, for comprehensive evaluation of contact sports participants suspected of suffering brain injuries.
It has been challenging thus far to develop a simple, objective blood biochemical test useful for the prognosis and management of mTBI, including for sports-related concussions. In a study using the same sera evaluated herein, Shahim and colleagues reported that levels of tau and S100β increase in concussed professional hockey players, and the serum tau concentration at 1 h relates to the persistence of PCS.22 Here, we demonstrate that serum SNTF relates with PCS severity in a temporally prolonged manner, suggesting that it may offer greater practical utility than a measure confined to the first hour postconcussion. We also found that serum SNTF and the combined measure of serum SNTF and tau relate more strongly to the severity of PCS than does serum tau alone. A number of additional nervous-system–enriched proteins have been investigated as candidate blood biomarkers for mTBI, including glial fibrillary acidic protein and S100β, as well as neuron-enriched ubiquitin C-terminal hydrolase L1, neuron-specific enolase, and C-terminal αII-spectrin proteolytic fragment SBDP145.14–21 Unfortunately, these candidates have failed thus far to demonstrate a prognostic relationship with the most common form of TBI, mTBI with negative head CT findings, and have yet to show value in sports-related concussions. Analogous to the success of a panel of cerebrospinal fluid markers for early diagnosis of pre-clinical Alzheimer's disease,42 the inclusion of serum SNTF in a panel of biomarkers for mTBI might have greater utility than SNTF alone. In the current study, multi-variate measures of serum SNTF coupled with tau or S100β did not lead to improvements in diagnostic accuracy or prognostic strength beyond SNTF by itself, and it remains to be determined whether linking SNTF with other biochemical markers for neuronal injury, such as neurofilament polypeptides, might further improve patient prognosis. Combining blood SNTF with radiological measures of brain structural abnormalities also merits consideration, but has yet to be tested experimentally. The resolution of these issues is important, given that rapid identification of the subset of mTBI cases at risk of developing brain damage and persistent dysfunction can be expected to foster clinical research into the pathophysiology and treatment of functionally deleterious mTBI. For sports-related concussions, decisions on suitability for return to play currently depend on indirect and, in some cases, imprecise assessments of brain functional status13,43,44 and may benefit from an objective, quantitative measure of blood biomarkers tied to the underlying pathogenic mechanisms of mTBI.
Acknowledgments
This work was supported by grants from the Swedish Medical Research Council, the Centrum for Idrottsforskning, and the National Institutes of Health (NINDS P01 NS056202), along with support from the Department of Neurosurgery, University of Pennsylvania School of Medicine. The authors thank the medical staff of the hockey teams: Bengt Gustafsson, MD, Torsten Johansson, MD, Örjan Fröjd, MD, Henrik Wretling, MD, Karin Runblad, MD, Göran Thoren, MD, Christer Andersson, MD, Ulf Nordström, MD, Stefan Serenius, MD, Mattias Hell, Jonas Kalman, Sina Hedin, Patrik Johansson, and Billy Nilsson for their valuable contributions to the participant enrollment and assessments of PCS. The authors thank Dr. Sean Ren and Samir Sayed of Children's Hospital of Philadelphia for assistance with the electrochemiluminescence-based SNTF immunoassay and Ryan Cocca for outstanding technical assistance.
Author Disclosure Statement
Dr. Siman is listed as inventor on a U.S. patent application for SNTF as a prognostic blood biomarker for mTBI. Drs. Zetterberg and Blennow are listed as coinventors on a U.S. patent application for plasma tau as a brain injury marker. Dr. Blennow has served on advisory boards for Eli Lilly, Kyowa Kirin Pharma, Pfizer, and Roche.
References
- 1.Rutherford W.H., Merrett J.D., and McDonald J.R. (1979). Symptoms at one year following concussion from minor head injuries. Injury 10, 225–230 [DOI] [PubMed] [Google Scholar]
- 2.Ingebrigtsen T., Waterloo K., Marup-Jensen S., Attner E., and Romner B. (1998). Quantification of post-concussion symptoms 3 months after minor head injury in 100 consecutive patients. J. Neurol. 245, 609–612 [DOI] [PubMed] [Google Scholar]
- 3.Lau B.C., Collins M.W., and Lovel M.R. (2011). Sensitivity and specificity of subacute computerized neurocognitive testing and symptom evaluation in predicting outcomes after sports-related concussion. Am. J. Sports Med. 39, 1209–1216 [DOI] [PubMed] [Google Scholar]
- 4.Rabinowitz A.R., and Levin H.S. (2014). Cognitive sequelae of traumatic brain injury. Psychiatr. Clin. N. Am. 37, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Povlishock J.T., Becker D.P., Cheng C.L., and Vaughan G.W. (1983). Axonal change in minor head injury. J. Neuropathol. Exp. Neurol. 42, 225–242 [DOI] [PubMed] [Google Scholar]
- 6.Huisman T.A., Schwamm L.H., Schaefer P.W., Koroshetz W.J., Shetty-Alva N., Ozsunar Y., Wu O., and Sorensen A.G. (2004). Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am. J. Neuroradiol. 25, 370–376 [PMC free article] [PubMed] [Google Scholar]
- 7.Shenton M.E., Hamoda H.M., Schneiderman J.S., Bouix S., Pasternak O., Rathi Y., Vu M.A., Purohit M.P., Helmer K., Koerte I., Lin A.P., Westin C.F., Kikinis R., Kubicki M., Stern R.A., and Zafonte R. (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 6, 137–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson V.E., Stewart W., and Smith D.H. (2013). Axonal pathology in traumatic brain injury. Exp. Neurol. 246, 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz-Arrastia R., Kochanek P.M., Bergold P., Kenney K., Marx C.E., Grimes C.J., Loh L.T., Adam L.T., Oskvig D., Curley K.C., and Salzer W. (2013). Pharmacotherapy of traumatic brain injury: state of the science and the road forward. J. Neurotrauma 31, 135–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makdissi M., Cantu R.C., Johnston K.M., McCrory P., and Meeuwisse W.H. (2013). The difficult concussion patient: what is the best approach to investigation and management of persistent postconcussive symptoms? Br. J. Sports Med. 47, 308–313 [DOI] [PubMed] [Google Scholar]
- 11.Smith D.H., Hicks R.R., and Povlishock J.T. (2013). Therapy development for diffuse axonal injury. J. Neurotrauma 30, 307–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zetterberg H., Smith D.H., and Blennow K. (2013). Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat. Rev. Neurol. 9, 201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancelliere C., Hincapie C.A., Keightley M., Godbolt A.K., Cote P., Kristman V.L., Stålnacke B.M., Carroll L.J., Hung R., Borg J., Nygren-de Boussard C., Coronado V.G., Donovan J., and Cassidy J.D. (2014). Systematic review of prognosis and return to play after sport concussion: results of the international collaboration on mild traumatic brain injury prognosis. Arch. Phys. Med. Rehab. 95, S210–S229 [DOI] [PubMed] [Google Scholar]
- 14.Bazarian J.J., Zemlan F.P., Mookerjee S., and Stigbrand T. (2006). Serum S100β and cleaved tau are poor predictors of long-term outcome after mild traumatic brain injury. Brain Inj. 20, 759–765 [DOI] [PubMed] [Google Scholar]
- 15.Berger R.P., Hayes R.L., Richichi R., Beers S.R., and Wang K.K. (2012). Serum concentrations of ubiquitin C-terminal hydrolase L1 and αII-spectrin breakdown product SBDP145kDa correlate with outcome after pediatric TBI. J. Neurotrauma 29, 162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metting Z., Wilczak N., Rodiger L.A., Schaaf J.M., and van der Naalt J. (2012). GFAP and S100β in the acute phase of mild traumatic brain injury. Neurology 78, 1428–1433 [DOI] [PubMed] [Google Scholar]
- 17.Papa L., Lewis L.M., Falk J.L., Zhang Z., Silvestri S., Giordano P., Brophy G.M., Demery J.A., Dixit N.K., Ferguson I., Liu M.C., Mo J., Akinyi L., Schmid K., Mondello S., Robertson C.S., Tortella F.C., Hayes R.L., and Wang K.K. (2012). Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann. Emerg. Med. 59, 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kou Z., Gattu R., Kobeissy F., Welch R.D., O'Neil B.J., Woodard J.L., Ayaz S.I., Kulek A., Kas-Shamoun R., Mika V., Zuk C., Tomasello F., and Mondello S. (2013). Combining biochemical and imaging markers to improve diagnosis and characterization of mild traumatic brain injury in the acute setting: results from a pilot study. PLoS One 8, e80296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puvenna V., Brennan C., Shaw G., Yang C., Marchi N., Bazarian J.J., Merchant-Borna K., and Janigro D. (2014). Significance of ubiquitin carboxy-terminal hydrolase L1 elevations in athletes after sub-concussive head hits. PLoS One 9, e96296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryb G.E., Dischinger P.C., Auman K.M., Kufera J.A., Cooper C.C., Mackenzie C.F., and Kane R.L. (2014). S-100β does not predict outcome after mild traumatic brain injury. Brain Inj. 9, 1–6 [DOI] [PubMed] [Google Scholar]
- 21.Wolf H., Frantal S., Pajenda G.S., Salameh O., Widhalm H., Hajdu S., and Sarahrudi K. (2013). Predictive value of neuromarkers supported by a set of clinical criteria in patients with mild traumatic brain injury: S100B protein and neuron-specific enolase on trial. J. Neurosurg. 118, 1298–1303 [DOI] [PubMed] [Google Scholar]
- 22.Shahim P., Tegner Y., Wilson D.H., Randall J., Skillback T., Pazooki D., Kallberg B., Blennow K., and Zetterberg H. (2014). Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol. 71, 684–692 [DOI] [PubMed] [Google Scholar]
- 23.Roberts-Lewis J.L., Savage M.J., Marcy V.R., Pinsker L.R., and Siman R. (1994). Immunolocalization of calpainI-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J. Neurosci. 14, 3934–3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buki A., Siman R., Trojanowski J.Q., and Povlishock J.T. (1999). The role of calpain-mediated spectrin proteolysis in traumatically induced axonal injury. J. Neuropathol. Exp. Neurol. 58, 365–375 [DOI] [PubMed] [Google Scholar]
- 25.Saatman K.E., Abai B., Grosvenor A., Vorwerk C.K., Smith D.H., and Meaney D.F. (2003). Traumatic axonal injury results in biphasic calpain activation and retrograde transport impairment in mice. J. Cereb. Blood Flow Metab. 23, 34–42 [DOI] [PubMed] [Google Scholar]
- 26.Siman R., Toraskar N., Dang A., McNeil E., McGarvey M., Plaum J., Maloney E., and Grady M.S. (2009). A panel of neuron-enriched proteins as markers for traumatic brain injury in humans. J. Neurotrauma 26, 1867–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siman R., Giovannone N., Hanten G., Wilde E.A., McCauley S.R., Hunter J.V., Li X., Levin H.S., and Smith D.H. (2013). Evidence that the blood biomarker SNTF predicts brain imaging changes and persistent cognitive dysfunction in mild TBI patients. Front. Neurol. 4, e00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwata A., Stys P.K., Wolf J.A., Chen X.H., Taylor A.G., Meaney D.F., and Smith D.H. (2004). Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J. Neurosci. 24, 4605–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Reyn C.R., Mott R.E., Siman R., Smith D.H., and Meaney D.F. (2012). Mechanisms of calpain mediated proteolysis of voltage gated sodium channel α-subunits following in vitro dynamic stretch injury. J. Neurochem. 121, 793–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCrory P., Meeuwisse W.H., Aubry M., Cantu B., Dvorák J., Echemendia R.J., Engebretsen L., Johnston K., Kutcher J.S., Raftery M., Sills A., Benson B.W., Davis G.A., Ellenbogen R.G., Guskiewicz K., Herring S.A., Iverson G.L., Jordan B.D., Kissick J., McCrea M., McIntosh A.S., Maddocks D., Makdissi M., Purcell L., Putukian M., Schneider K., Tator C.H., and Turner M. (2013). Consensus statement on concussion in sport: the 4th International Conference on Concussion in sport held in Zurich, November 2012. Br. J. Sports Med. 47: 250–258 [DOI] [PubMed] [Google Scholar]
- 31.Xu J., Ziemnicka D., Scalia J., and Kotula L. (2001). Monoclonal antibodies to alpha spectrin Src homology 3 domain associate with macropinocytic vesicles in nonerythroid cells. Brain Res. 898, 171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siman R, Baudry M, and Lynch G. (1984). Brain fodrin [spectrin]: substrate for calpain I, an endogenous calcium-activated protease. Proc. Natl. Acad. Sci. U. S. A. 81, 3572–3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goedert M., Spillantini M.G., and Crowther R.A. (1991). Tau proteins and neurofibrillary degeneration. Brain Pathol. 1, 279–286 [DOI] [PubMed] [Google Scholar]
- 34.Johnson V., Siman R., Weber M.T., Hay J., Stewart W., and Smith D.H. (2014). SNTF immunohistochemistry identifies a previously undetected population of degenerating axons following traumatic brain injury. J. Neurotrauma Abstr. 31, C3–C19 [Google Scholar]
- 35.Harris A.S., Croall D.E., and Morrow J.S. (1988). The calmodulin binding site in α-fodrin [-spectrin] is near the calcium-dependent protease-I cleavage site. J. Biol. Chem. 263, 15754–15761 [PubMed] [Google Scholar]
- 36.Siman R., Noszek J.C., and Kegerise C.M. (1989). Calpain I activation is specifically related to excitatory amino acid induction of hippocampal damage. J. Neurosci. 9, 1579–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf J.A., Stys P.K., Lusardi T., Meaney D., and Smith D.H. (2001). Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J. Neurosci. 21, 1923–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corsellis J.A., Bruton C.J., and Freeman-Browne D. (1973). The aftermath of boxing. Psychol. Med. 3, 270–303 [DOI] [PubMed] [Google Scholar]
- 39.McKee A.C., Cantu R.C., Nowinski C.J., Hedley-Whyte E.T., Gavett B.E., Budson A.E., Santini V.E., Lee H.S., Kubilus C.A., and Stern R.A. (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 68, 709–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blennow K., Hardy J., and Zetterberg H. (2012). The neuropathology and neurobiology of traumatic brain injury. Neuron 76, 886–899 [DOI] [PubMed] [Google Scholar]
- 41.Smith D.H., Johnson V.E., and Stewart W. (2013). Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat. Rev. Neurol. 9, 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blennow K., Hampel H., Weiner M., and Zetterberg H. (2010). Cerebrospinal fluid and plasma biomarkers in Alzheimer's disease. Nat. Rev. Neurol. 6, 131–144 [DOI] [PubMed] [Google Scholar]
- 43.Harmon K.G., Drezner J.A., Gammons M., Guskiewicz K.M., Halstead M., Herring S.A., Kutcher J.S., Pana A., Putukian M., and Roberts W.O. (2013). American Medical Society for Sports Medicine position statement: concussion in sport. Br. J. Sports Med. 47, 15–26 [DOI] [PubMed] [Google Scholar]
- 44.McCrea M., and Guskiewicz K. (2014). Evidence-based management of sport-related concussion. Prog. Neurol. Surg. 28, 112–127 [DOI] [PubMed] [Google Scholar]