Abstract
Background
Hypoxic-ischemic encephalopathy (HIE) is a major cause of morbidity in survivors. Therapeutic hypothermia (TH) is the only available intervention, but the protection is incomplete. Preclinical studies of HIE/TH in the rodent have relied on the postnatal day (P) 7 rat whose brain approximates a 32–36 week gestation infant, less relevant for these studies. We propose that HIE and TH in the term-equivalent P10 rat will be more translational.
Methods
P10–11 rat pups were subjected to unilateral hypoxia-ischemia (HI) and 4 hours recovery in normothermic (N) or hypothermic (TH) conditions. Brain damage was assessed longitudinally at 24 hours, 2 and 12 weeks. Motor function was assessed with the beam walk; recognition memory was measured by novel object recognition.
Results
Neuroprotection with TH was apparent at 2 and 12 weeks in both moderately and severely damaged animals. TH improved motor function in moderate, but not severe damage. Impaired object recognition occurred with severe damage with no evidence of protection of TH.
Conclusion
This adaptation of the immature rat model of HI provides a reproducible platform to further study HIE/TH in which individual animals are followed longitudinally to provide a useful translational preclinical model.
INTRODUCTION
Perinatal asphyxial brain damage resulting in hypoxic-ischemic encephalopathy (HIE) is a major cause of acute mortality and chronic neurological morbidity in infants and children (1, 2); 20–50 % of infants with HIE die within the newborn period; up to 25% of survivors exhibit permanent neuropsychological handicaps. As there are no interventions to prevent perinatal asphyxia the focus is on developing therapeutics to limit cerebral injury progression and promote normal brain growth and development (3).
Therapeutic hypothermia (TH) has become standard therapy for full-term neonates with moderate to severe HIE, with significant neuroprotection supported by multiple randomized clinical trials demonstrating reduced risk of death or neurologic disability at 18- to 24 months of age (4–6). However, protection is incomplete and long-term outcome is not well defined. TH may not improve outcome in neonates with very severe HIE (1, 5). Additionally, at 6 to 7 year follow-up there was no significant difference in IQ scores between the TH and control groups suggesting a failure to support cognitive function (4). Thus, additional preclinical studies are needed to explore the benefits and limitations of TH, and define adjunct therapies to enhance long-term outcome.
TH safety studies and clinical trials in the neonate were derived from experimental studies in newborn piglets (7) and fetal sheep (8), which demonstrated histopathological neuroprotection with moderate hypothermia. The immature rodent model of unilateral hypoxia-ischemia (HI) has been used extensively since a larger number of animals can be studied, and longitudinal assessments and functional outcome can be measured (9). There have been multiple investigations into TH, alone and with other therapies, in neonatal rats and mice, with quite variable results. Studies in the rat have used the P7 animal as originally described (10), however the P7 rat brain more closely approximates a 32–36 week gestational age (GA) infant, potentially making it less relevant to the full-term newborn (11).
The purpose of this study was to define and characterize a reproducible, preclinical model of HI plus TH in the immature rat. We utilize rats of both sexes at P10–11, as representative of the term infant brain, and introduce a standard period of hypothermia following HI. Each pup is followed longitudinally with magnetic resonance imaging (MRI) and behavioral analysis to assess short- and long-term effects. We demonstrate here significant reduction of brain damage and improved motor, but not object recognition, function with this TH paradigm.
RESULTS
Hypothermic protection is temperature dependent
Following unilateral HI, pups were recovered in either a normothermic (35.5°C) or hypothermic (30°C) environment for 4 hours. Hypothermic chamber temperature was chosen to achieve a target rectal temperature of 32°C; rectal temperatures were monitored hourly. Temperatures in normothermic (N) pups ranged from 36.5°C – 38.1°C; hypothermic (TH) pups were 31°C–34°C. In the initial studies, comparison of temperature with damage at 2 weeks showed that TH was most effective at <33.0°C; pups that remained > 33°C were significantly more damaged (p ≤ 0.01) (Supplemental Figure 1, online). Pups that did not reach 33°C were excluded from subsequent analysis.
Hypothermia provides long-term protection in moderate and severe injury
Preliminary studies assessed damage at 24 hours in H&E stained sections; we did not observe a significant effect of cooling (Figure 1). However, cell death following HI can continue for days to months; long-term outcome in a developing brain represents a balance between cell death and growth of viable tissue. Subsequent experiments included longitudinal analysis, with MRI and behavior through 12 weeks.
Figure 1. TH is not protective at 24 hours after HI.
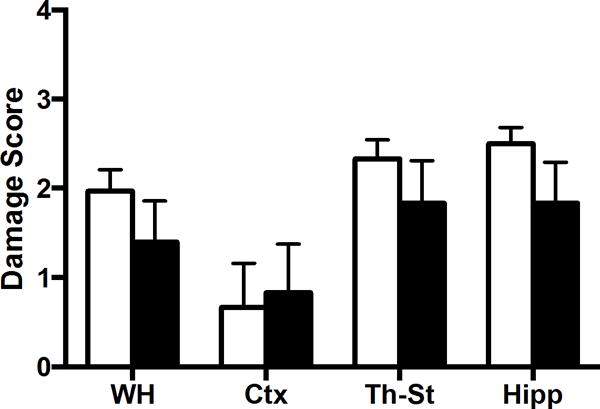
P10–11 rats were subjected to HI and recovered in normothermic (white bars) or hypothermic (black bars) chambers, n=6/group, and sacrificed at 24 hours later. H&E stained cryosections were analyzed for DS (ipsilateral) by 2 reviewers for whole hemisphere (WH), cortex (CTX), thalamus-striatum (Th-St), hippocampus (Hipp).
We performed 10 experiments of HI plus TH; animals were evaluated with MRI at 2 weeks. Damage was assessed with an ordinal damage score (DS) and calculation of regional volumes, as described in Methods (Supplemental Figure 2 A & B, online). There was a tight correlation between these 2 measures (Supplemental Figure 2C, online). When all of the data were considered together there was a range of damage in both N and TH groups, with a trend toward lower DS with TH (Figure 2). On further examination, we observed that the experiments fell into three significantly different outcome groups based on the mean DS in the normothermic pups, reflecting mild (n=3), moderate (N-M, n=4), severe (N-S, n=3) injury (Figure 3). TH did not significantly reduce damage in the mild group and these animals were not followed further. When moderate and severe experiments were analyzed separately, TH resulted in a significant degree of protection in both groups (TH-M, TH-S, p ≤ 0.01).
Figure 2. Effect of TH at 2 weeks.
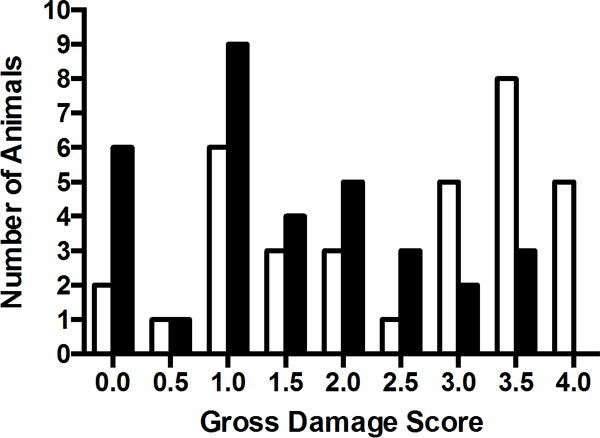
DS was assessed in all animals from T2 images demonstrating more animals with lower DS following TH (black bars) relative to normothermic (white bars) recovery.
Figure 3. Effect of TH on Mild, Moderate, and Severe Damage.
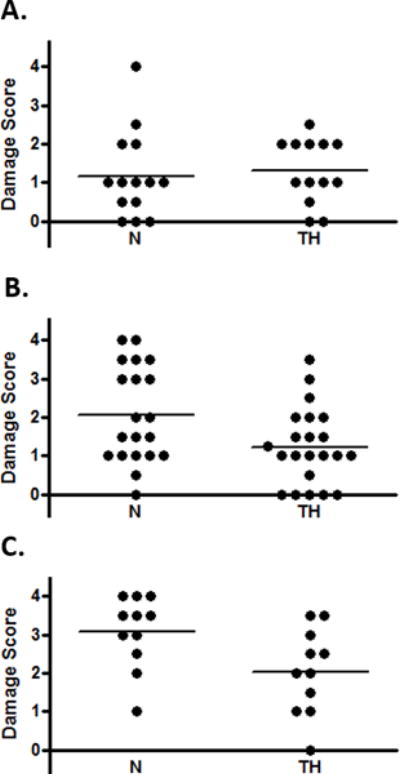
Experiments fell into 3 groups based on mean DS in normothermic (N) animals: A, Mild; B, Moderate; C, Severe p<0.01, ANOVA. DS in the moderate and severe groups was reduced with TH (p< 0.01 for both, unpaired t-test).
Regional volumes were calculated for ipsilateral and contralateral hemispheres (Figures 4A & 4B). TH was protective of neocortex and thalamus/striatum, but not hippocampus in both cohorts. Examination of individual ipsilateral/contralateral ratios (Figure 4C) is a measure of extent of growth of the injured, relative to the contralateral, hemisphere; an abnormality in the contralateral hemisphere is determined by comparison with control. Contralateral volumes were not different from controls, except for a significant reduction in hippocampal volume in the severe cohort (Supplemental Figure 3, online), possibly indicating a heightened vulnerability of the hippocampus to HI that is resistant to TH. Figure 5 depicts the evolution vs. resolution of damage in 2 animals (N, TH), from the severe cohort: 24 hour and 2 week T2-MRI images, and histology of corresponding sections. Interestingly, despite little apparent difference at 24 hr, the injury in the N pup evolves into infarction and large cavitation at 2 and 12 weeks; this process is reduced in the TH pup. Final DS derived from histological analysis of all brains at 12 weeks demonstrated both the evolution of injury and confirmed long-term protection with TH (Figure 6 A&B). Calculated change in DS for each animal between 2 (Supplemental Figure 4, online) and 12 weeks demonstrated minor evolution of damage in the N-M animals (Figure 6C), with more striking changes in the severe cohort (Figure 6D). In both cohorts, DS in the TH groups were either little changed or actually improved, except for the hippocampus. In these studies we did not observe a significant effect of gender on extent of damage or degree of protection by TH (Supplemental Figure 5, online).
Figure 4. TH improves brain volumes at 2 weeks except for hippocampus.
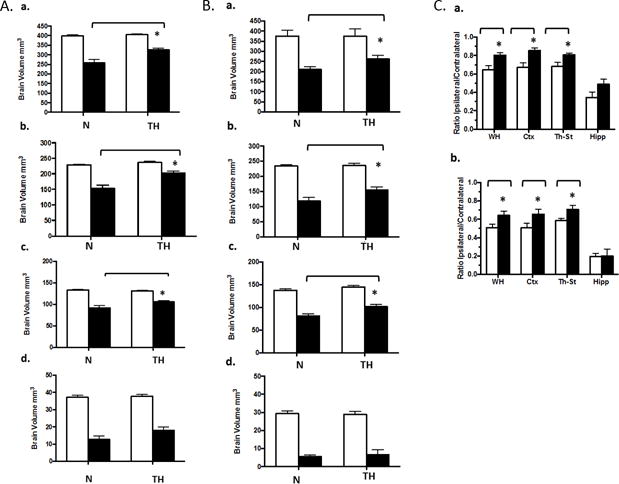
Volumes of healthy brain tissue in contralateral (white bars) and ipsilateral (black bars) hemispheres were calculated from 2 week MRI images as described in Methods. A: Moderate, B: Severe; depicting volumes for: a, whole hemisphere; b, cortex; c, thalamus-striatum; d, hippocampus. TH reduced brain volume loss for all regions except hippocampus, *p<0.05, ANOVA. C: Paired ipsilateral/contralateral ratios, for normothermic (white bars) and TH (black bars) for: a, Moderate; b, Severe cohorts; * p< 0.05.
Figure 5. TH reduces progression of injury following HI: Sequential T2MRI and Histology.
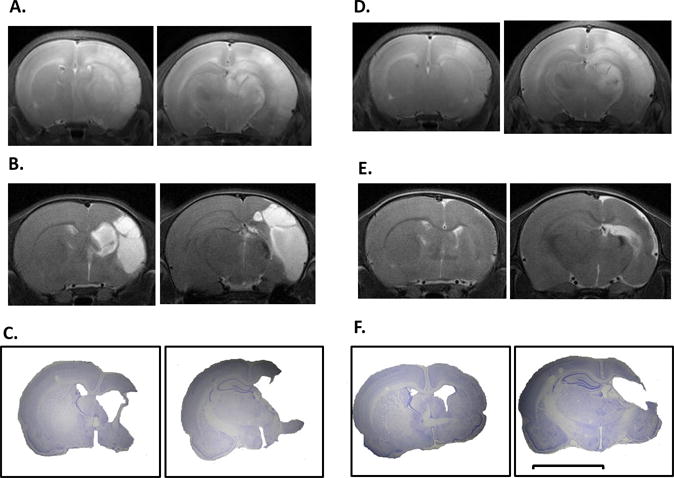
Representative MRI images obtained from 1 N (A–C) and 1 TH (D–F) pup at 24 hours (A,D) and 2 weeks (B, E) are compared with images from final histology of each animal at 12 weeks (C, F). Each panel depicts an anterior and posterior coronal section. Scale bar = 5 mm.
Figure 6. Effects of HI and TH on long term DS.
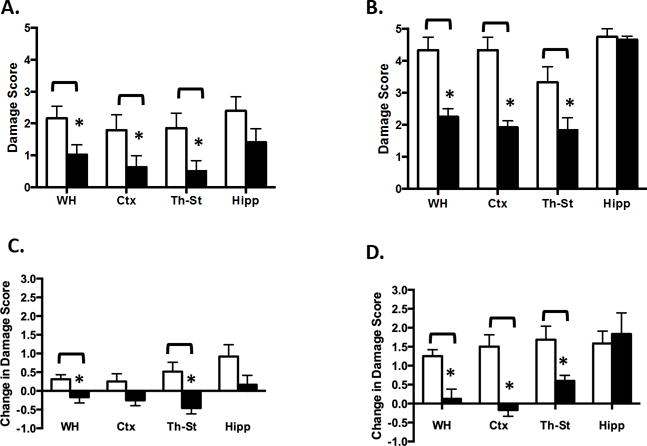
Rats from Moderate (A) and Severe (B) cohorts were sacrificed at 12 weeks post HI. Cryosections were stained with cresyl violet and analyzed for hemispheric and regional DS as described. Change in DS between 2 and 12 weeks was calculated for each animal in Moderate (C) and Severe (D). White bars, normothermic; black bars, TH. * p<0.05, unpaired t-test.
Hypothermia protects motor learning and dexterity but not object encoding and recognition following HIE
To characterize protection of sensorimotor function conferred by TH, rats were tested as young adults (P50–70) on the beam walk test. This measurement of a rodent’s ability to traverse an elevated beam is a robust test of locomotor control and is particularly sensitive to hindlimb deficits (12). Rats were tested on 2 beams of increasing difficulty. Successfully traversing the beam on at least 3 of 5 attempts was considered a “pass”. The highest difficulty level passed differed as a function of group (Kruskal-Wallis test, p < 0.001, Figure 7A). All of the N and TH animals in the moderate cohort, and all but one of the controls, passed both levels successfully. By contrast, none of the rats in the severe cohort passed both levels and several did not pass either (Figure 7A). Overall, the performance of this group was significantly worse than either controls or moderate groups (all p’s < 0.05).
Figure 7. Motor and Cognitive Function in TH- and N-treated HI rats.
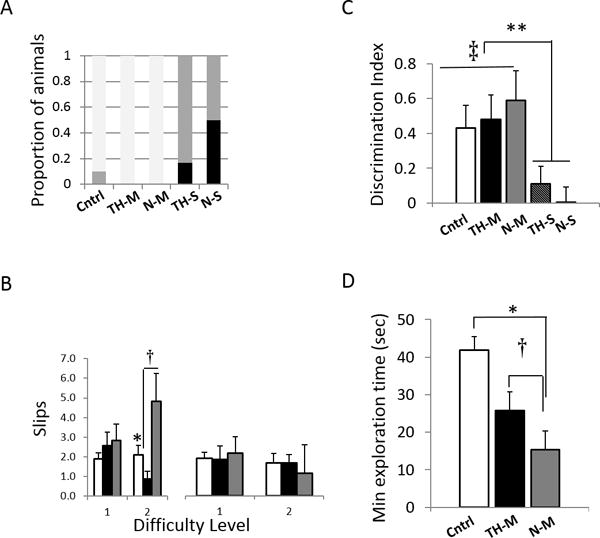
Therapeutic hypothermia (TH) improves motor performance on the beam test. (A) Beam difficulty at first failure. Most rats in control (Cntrl) and moderate (-M) groups pass to level 2 (white bars); severe (-S) groups fail at (grey bars)/before (black bars) level 1. (B) Contralateral hindlimb slips (left panel) on the difficult beam are increased in rats with moderate HIE with normothermic recovery (*p < 0.05, N-M, grey bars > Cntrl, white bars); TH-M (black bars) rats are less impaired († p< 0.05, TH-M < N-M). No difference in ipsilateral (right) hindlimb slips (right panel). Object encoding and recognition is impaired by HI. (C) Cntrl (white bars) and Moderate HIE rats (TH-M, black bars; N-M, grey bars) show above-chance recognition of the novel object (‡ p< 0.05 DINOR > 0); severe HIE rats do not (** p< 0.05, severe HIE:N-S (hatched bar) and TH-S (dark grey bar) vs all other groups). (D) Object contact time for Moderate HIE rats is less than for Cntrls (white bar) during the sample phase. There is a trend for TH (black bar) to mitigate this effect. * p < 0.05, moderate HIE vs Cntrl; † p< 0.1,TH-M > N-M (grey bar).
For rats that passed Level 2, videos were analyzed for left (affected hindlimb), and right foot slips (Figure 7B). For left foot slips the mixed ANOVA revealed an effect of group (F[2,19] = 3.7, p < 0.05) and an interaction between group and beam difficulty level (F[2,19] = 4.7, p < 0.05). Planned comparisons at each difficulty level revealed an effect of TH on motor control at the more difficult level (F[2,19] = 5.9, p < 0.05). N-M rats performed worse than both control and TH-M; the performance of the TH-M rats was significantly better than their non-cooled littermates (Tukey HSD, p < 0.05) and not different from controls (Tukey HSD p > 0.4). There were no group differences for right foot slips.
Temporal lobe-mediated object memory was investigated with novel object recognition (NOR), a behavioral test of encoding and memory that relies on the animal’s innate exploratory behavior and requires no pre-training, and no added stressors (13, 14). NOR involves an initial exposure to two identical objects followed, after a delay, by presentation of one familiar object (from the previous sample phase) and one new object. Control animals spend significantly longer exploring the novel object; this is expressed by a discrimination index (DINOR) calculated as the difference between time spent with the novel vs familiar object as a percentage of total time.
The DINOR differed across experimental groups (F[4,26] = 3.1, p < 0.05, Figure 7C). As expected, naïve controls spent more time investigating the novel than the familiar object; DINOR significantly greater than 0 (1-sample t-test, t[8] = 3.1, p< 0.05). Performance of moderate-HI animals (N-M and TH-M) was not significantly different than controls (Student’s t’s, p’s > 0.15), and indicated reliable recognition of the novel object (DINOR > 0; N-M: t[4] = 3.5; TH-M: t[5] = 3.5; p’s < 0.05). However, relative to controls, moderate-HI groups showed shorter object exploration times during the sample phase, with a trend toward increased exploration time in the TH-M group relative to N-M (Figure 7D). Object recognition was significantly impaired in the severe HIE groups, with N-S and TH-S rats performing no better than chance (Figure 7C).
DISCUSSION
In this study we present a modification of our original model of HI in the P7 rat to more closely model the clinical situation of therapeutic hypothermia following birth asphyxia in the term infant. The major features of this modification are:
(i) use of the P10–11 rat pup; (ii) temperature monitoring in all pups; (iii) 24 hour MRI analysis to assess initial injury severity; (iv) subsequent 2 week MRI analysis for evaluation of injury progression and; (v) evaluation of sensorimotor and cognitive outcomes as adults prior to sacrifice and histopathology. We propose that this preclinical paradigm will provide a valuable platform to both further understand the benefits and limitations of hypothermia following HI and to evaluate potential adjunct therapeutics to improve outcome.
This model was originally developed in the P7 rat pup, which approximates the cerebral development of a 32–36 week gestation human infant (10). In the past 30 years this model has come to be widely used as a preclinical model of HIE and was the paradigm in which the initial rodent studies of TH were conducted (9, 15, 16). Subsequent studies investigated delayed hypothermia and combination therapies (17-20) with quite variable results and inconsistent protection with TH. To more closely model the full term infant brain we have chosen to investigate the effects of hypothermia in the P10–11 rat pup (11, 21, 22). An important and potentially clinically relevant difference between the responses to HI at the different ages is the increased vulnerability of the hippocampus in the older rat pup, as previously described (23). The extreme vulnerability of the hippocampus to HI is replicated in the studies presented here, in both moderately and severely damaged animals. Further, the hippocampus was the only region that was not protected by TH, as has been recently reported clinically (24). Severe HI injury resulted in nearly complete loss of the ipsilateral hippocampus, as well as a decrease in the contralateral hippocampal volume, which is a new observation in this model and will be investigated in future studies.
The rigorous control of temperature during hypothermia is vital to both the efficacy and reproducibility of the protection. Target temperature in clinical practice of TH in asphyxiated infants is 33.5°C–34.5°C (25, 26) and most rodent studies aim to achieve a rectal temperature of 32°C–33°C, as we did in the current study. Rectal temperature has been shown to correlate well with brain temperature in this model (16). Most studies monitor temperature in sentinel pups that are excluded from further analysis, based on the observation that the presence of the rectal temperature probe affects the outcome (27). We monitored rectal temperatures in all of the pups at hourly intervals and observed a range of post-HI temperatures in both normothermic and hypothermic animals. Importantly we observed that those pups that “resisted” cooling and did not reach the target temperature of < 33°C, demonstrated a variable degree of damage including the most severe, whereas none of the effectively cooled pups were severely damaged. The reason(s) for this variation in response are unknown however post-ischemic hyperthermia is frequently observed consistent with severe damage in preclinical studies and in asphyxiated infants (28). An inability to reach the target temperature during cooling might reflect a more severe insult at the outset, or some other variable in the animal. We employ a cutoff temperature of 33°C as an exclusion criterion in these studies.
The animal-to-animal variability in this model is well known. Since the original publication in 1981, we, and others, have made numerous technical adjustments to minimize the variability, including controlling litter size, reduced time of anesthesia, pre-warming pre-hypoxia. However, as in human infants, there is still a variable response to HI, ranging from none to mild, moderate or severe. The response to hypothermia is also variable with the greatest benefit observed in the context of moderate injury in both clinical trials and experimental studies. Thus in order to evaluate the treatment effect it is important to assess the severity of injury in each animal; MRI analysis provides the tool to stratify experimental animals into mild, moderate and severe injury groups. Of the experiments included in this study, 3 resulted in mild injury in normothermic pups with no added protection with TH (Figure 3); further analysis of the remaining pups showed that 4 hours of TH immediately following HI in the P10–11 rat is protective in both moderate and severe injury.
An effective neuroprotective intervention must include long-term functional improvement, in addition to reduction in brain damage. Defects in locomotor and cognitive function are common in children with HIE and experimental animals following unilateral HI (29). A complete battery of behavioral tests will be needed to evaluate functional outcome. However in this initial study we used two simple tasks to evaluate sensorimotor function (beam walk) and recognition memory (NOR). Injury to the ipsilateral sensorimotor cortex is characteristic of this animal model and the resulting motor impairments can be reliably detected by the beam walk test (12). The results of Figure 7 reinforce the importance of evaluating experimental animals in the context of severity of injury. Impairment in beam walking was significant in N-M and worse in N-S cohorts. TH cohorts showed improvement of locomotor function on the beam walk, relative to their corresponding normothermic animals. Although we did not fully evaluate improvement of performance with time, a retest of the severely damaged cohort did not improve their scores (data not shown). Future studies will initiate testing at a younger age to follow a potential trajectory of recovery of motor function and possible effects of TH.
The NOR test was chosen for an initial evaluation of cognitive outcome as it relies on neuronal connections within the medial temporal lobe; object recognition has been shown to depend on temporal lobe circuits in both rodents and primates (30–32). The test is suitable to rats across a developmental timeline from weaning to old age, requires no pre-training and does not require use of potential stressors (33). Based on the measurement of the DINOR alone, it would appear that there was no effect of moderate HI damage in object recognition. However, HI animals did spend significantly less time with the objects in the familiarization/sample phase. Reduced exploration time has been reported before, both for neonatal HI (34), and traumatic brain injury in the adult rat (35). While preliminary, we note a trend for TH to normalize exploration time in the moderately damaged animals. Further testing will utilize an expanded NOR paradigm to tease apart relationships between object exploration and subsequent recognition, and the effects of HIE and TH. Severely damaged animals were unable to perform this task at all (Figure 7C). The apparent ‘sparing’ of the moderate group might relate to extent of bilateral damage to hippocampal-perirhinal circuits. Recent studies on NOR highlight the importance of the perirhinal cortex (31, 32, 36) and, although there is hippocampal damage in essentially all of the animals post-HI, the perirhinal cortex is more variably involved, especially in severe injury. It is noteworthy that while moderately damaged animals retained contralateral hippocampal volume, severely damaged animals did not, potentially compromising function in hippocampal-perirhinal circuits. It will be important to more fully characterize injury to hippocampal-perirhinal circuitry, and its relationship to NOR and other recognition memory tasks in this model over an extended timeline.
There are limitations to this study. MRI imaging is being increasingly utilized early in asphyxiated newborns to predict neurodevelopmental outcome (37–39). We have not yet analyzed our MRI T2 images to that extent, nor did we conduct a sufficient battery of neurodevelopmental tasks to fully assess outcome. However, this preclinical platform offers the opportunity to pursue such studies as well as include more advanced MRI imaging techniques to further define the trajectory of ongoing neurodegeneration or repair. Thus, we propose that this initial long-term study provides the basis for a useful, reproducible and translationally relevant experimental paradigm to model the effects of therapeutic hypothermia following HIE in the term infant.
METHODS
Animal model of unilateral HI plus hypothermia
Timed pregnant Wistar rats (Charles River Laboratories, Wilmington, MA) in groups of 3 were purchased at embryonic (E) day 15, housed individually, and allowed to deliver vaginally. On the day of birth, pups were randomized and reassigned to dams (10/litter,~ equal male and female).
Unilateral HI was induced in the right hemisphere at P10 according to our standard protocol (10, 36), 8%O2/bal N2, 65 minutes. During hypoxia, the temperature in the chamber was maintained at 34.5 OC to achieve an average rectal temp of 36–36.5 °C (nesting temperature), monitored in 2 non-ligated pups (Physitemp, Clifton, NJ). Pups were randomly assigned to either normothermic or hypothermic recovery for 4 hours. Normothermic recovery was in the same chamber as the hypoxia; hypothermic pups were placed in open jars in a water bath set at 28 to 30°C to maintain a target rectal temperature of 32°C. Pups were rewarmed and placed back with the dam, weaned at P21, and handled every other day. Following MRI and behavioral analysis, animals were deeply anesthetized with a fatal dose of pentobarbital prior to perfusion-fixation with 4% paraformaldehyde. Brains were removed, cryoprotected, and 50 μm sections were cut on a freezing microtome (Microm, Walldorf, Germany). All animal experiments were approved by the Weill Cornell Medical College IACUC.
MRI Analysis
Acquisition of images:T2-weighted images were acquired at 24hr and 2-week post-HI using a RARE sequence on a Bruker Biospec 7.0-T MR scanner with the following acquisition parameters: RARE factor 10, matrix 256 × 256, FOV 20 mm × 20 mm, slice thickness 1 mm, 17 contiguous coronal sections at P11 and 20 contiguous coronal sections at P25; TReff = 2,191.1 ms at P11 and 2,577.8 ms at P25, TEeff 42.9 ms. A 72 mm Bruker linear coil was used for excitation and a quadrature brain coil was used for signal reception. MRI images from Bregma 2.52 to −5.40 (8 images) were given a gross damage score according to the ordinal scale: 0=no damage, 1=atrophy alone, 2=atrophy with ventriculomegaly, 3=10–25% infarct, 4=25–100% infarct. Regions of interest (ROI) were traced (Supplemental Figure 2B, online) using Image J software (NIH, Bethesda, MD). Regional volume measurements were calculated as the product of “inter-section distance (1 mm) x area of normal tissue” for whole hemisphere, cortex, thalamus/striatum plus hypothalamus, and hippocampus.
Histochemical analysis
Coronal brain sections from Bregma 2.52 to −5.40 (1 out of every 6th section) were mounted onto gelatin-coated slides. The sections were stained with a 1% cresyl violet solution (Sigma, Saint Louis, MO) and evaluated with light microscopy (Olympus, Tokyo, Japan). Using the same scoring system used for the MRI, overall and regional damage scores were determined for each section.
Behavioral Analysis
The beam walking test was used to evaluate gross motor function at 6 weeks post-HI. Beams were 40 cm long, elevated 32 cm from the table surface. Level 1 beam was flat and 2.5 cm wide; Level 2 beam was a cylindrical rod of 2.5 cm diameter. A dark box was at the end of the beam; rats instinctively advance into the box when placed on a beam. Rats were placed on the beam 10 cm from the box to learn the test. Then each rat was placed 40 cm from the box and given 5 attempts to cross the beam. Videos were recorded and the fails, i.e. inability to cross, right and left foot slips were recorded by an investigator blinded to the treatment.
The novel object recognition test was performed at 8-week post-HI. The day before the test, rats were allowed to explore the testing chamber, a polycarbonate box (dimensions 40 cm x 40 cm), and allowed to explore for 12 minutes. The sample phase and recognition test were separated by a 6 minutes. During the sample phase, 2 identical objects were placed near the corners of the box and the rat was allowed to explore both objects for 5 minutes. The rat was removed for 6 minutes, wherein the box was cleaned with a chlorine dioxide liquid (Clidox, Pharmacal Research Laboratories, Waterbury, CT) and one of the familiar objects was replaced with a novel object of the same material but a different shape. The rat was returned to the box and allowed to explore for 2 minutes. Any-Maze video tracking system software (Stoelting Co. Wooddale, IL) was used to record the time spent with novel and familiar objects. Object exploration was defined as the rat directing its nose toward the object at a distance of < 2 cm. The videos were reviewed offline by two investigators blinded to the treatment. The Discrimination Index (DINOR) was calculated as [(time with novel object – time with familiar object)]/(total time spent with objects).
Statistics
All values were expressed as the mean ± SEM. Effects of HI and TH were assessed with Kruskal-Wallis and ANOVA tests for non-continuous and continuous variables, respectively. Omnibus tests were followed by planned pairwise comparisons. Post-hoc tests detecting an effect of TH were corrected for multiple comparisons with Tukey’s Honest Significant Difference (HSD) method. Fisher’s exact test and Dunnett’s t were also used for post-hoc analyses for the behavioral studies.
Supplementary Material
Acknowledgments
We would like to thank Dr. Jeffrey Perlman for support of these studies and helpful discussions.
Financial support: This work was supported by a grant from the LeDucq Fondation, France (SJV) and National Institutes of Health, USA (NIH)/ R21NS083425 (SJV). HM receives support from the Sidney R. Baer, Jr. Foundation and the New York State Office of Mental Health.
Footnotes
Disclosure: The authors have nothing to disclose
References
- 1.Wu YW, March WM, Croen LA, et al. Perinatal stroke in children with motor impairment: a population-based study. Pediatrics. 2004;114:612–619. doi: 10.1542/peds.2004-0385. [DOI] [PubMed] [Google Scholar]
- 2.Vannucci RC. Hypoxic-ischemic encephalopathy. Am J Perinatol. 2000;17:113–120. doi: 10.1055/s-2000-9293. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez FF, Ferriero DM. Therapeutics for neonatal brain injury. Pharmacol Ther. 2008;120:43–53. doi: 10.1016/j.pharmthera.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–2092. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 6.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 7.Thoresen M, Penrice J, Lorek A, et al. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res. 1995;37:667–670. doi: 10.1203/00006450-199505000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Gunn AJ, Gunn TR, de Haan HH, et al. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bona E, Hagberg H, Loberg EM, et al. Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: short- and long-term outcome. Pediatr Res. 1998;43:738–745. doi: 10.1203/00006450-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Rice JE, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 11.Patel SD, Pierce L, Ciardiello AJ, et al. Neonatal encephalopathy: pre-clinical studies in neuroprotection. Biochem Soc T. 2014;42:564–568. doi: 10.1042/BST20130247. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein LB. Model of recovery of locomotor ability after sensorimotor cortex injury in rats. ILAR J. 2003;44:125–129. doi: 10.1093/ilar.44.2.125. [DOI] [PubMed] [Google Scholar]
- 13.Ennaceur A, Delacour JA. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 14.Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yager J, Towfighi J, Vannucci RC. Influence of Mild Hypothermia on Hypoxic-Ischemic Brain-Damage in the Immature Rat. Pediatr Res. 1993;34:525–529. doi: 10.1203/00006450-199310000-00029. [DOI] [PubMed] [Google Scholar]
- 16.Thoresen M, Bagenholm R, Loberg EM, et al. Posthypoxic cooling of neonatal rats provides protection against brain injury. Arch Dis Child Fetal Neonatal Ed. 1996;74:F3–9. doi: 10.1136/fn.74.1.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang AY, Gonzalez FF, Sheldon RA, et al. Effects of combination therapy using hypothermia and erythropoietin in a rat model of neonatal hypoxia-ischemia. Pediatr Res. 2013;73:12–17. doi: 10.1038/pr.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabir H, Scull-Brown E, Liu X, et al. Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 hours in neonatal rats. Stroke. 2012;43:3364–3370. doi: 10.1161/STROKEAHA.112.674481. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Barks JD, Xu G, et al. Topiramate extends the therapeutic window for hypothermia-mediated neuroprotection after stroke in neonatal rats. Stroke. 2004;35:1460–1465. doi: 10.1161/01.STR.0000128029.50221.fa. [DOI] [PubMed] [Google Scholar]
- 20.Barks JD, Liu YQ, Shangguan Y, et al. Phenobarbital augments hypothermic neuroprotection. Pediatr Res. 2010;67:532–537. doi: 10.1203/PDR.0b013e3181d4ff4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romijn HJ, Hofman MA, Gramsbergen A. At What Age Is the Developing Cerebral-Cortex of the Rat Comparable to That of the Full-Term Newborn Human Baby. Early Hum Dev. 1991;26:61–67. doi: 10.1016/0378-3782(91)90044-4. [DOI] [PubMed] [Google Scholar]
- 22.Tucker AM, Aquilina K, Chakkarapani E, et al. Development of amplitude-integrated electroencephalography and interburst interval in the rat. Pediatr Res. 2009;65:62–66. doi: 10.1203/PDR.0b013e3181891316. [DOI] [PubMed] [Google Scholar]
- 23.Towfighi J, Mauger D, Vannucci RC, et al. Influence of age on the cerebral lesions in an immature ratmodel of cerebral hypoxia-ischemia: A light microscopic study. Dev Brain Res. 1997;100:149–160. doi: 10.1016/s0165-3806(97)00036-9. [DOI] [PubMed] [Google Scholar]
- 24.Kasdorf E, Engel M, Heier L, et al. Therapeutic Hypothermia in Neonates and Selective Hippocampal Injury on Diffusion-Weighted Magnetic Resonance Imaging. Pediatr Neurol. 2014;51:104–108. doi: 10.1016/j.pediatrneurol.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Azzopardi DV, Strohm B, Edwards AD, et al. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child-Fetal. 2009;94:F260–F264. doi: 10.1136/adc.2008.146977. [DOI] [PubMed] [Google Scholar]
- 26.Papile LA, Baley JE, Benitz W, et al. Hypothermia and Neonatal Encephalopathy. Pediatrics. 2014;133:1146–1150. doi: 10.1542/peds.2014-0899. [DOI] [PubMed] [Google Scholar]
- 27.Thoresen M, Bagenholm R, Loberg EM, et al. The stress of being restrained reduces brain damage after a hypoxic-ischaemic insult in the 7-day-old rat. Neuroreport. 1996;7:481–484. doi: 10.1097/00001756-199601310-00025. [DOI] [PubMed] [Google Scholar]
- 28.Laptook A, Tyson J, Shankaran S, et al. Elevated temperature after hypoxic-ischemic encephalopathy: Risk factor for adverse outcomes. Pediatrics. 2008;122:491–499. doi: 10.1542/peds.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schallert T, Fleming SM, Leasure JL, et al. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 30.Alvarado MC, Bachevalier J. Revisiting the maturation of medial temporal lobe memory functions in primates. Learn Memory. 2000;7:244–256. doi: 10.1101/lm.35100. [DOI] [PubMed] [Google Scholar]
- 31.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winters BD, Forwood SE, Cowell RA, et al. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci. 2004;24:5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reger ML, Hovda DA, Giza CC. Ontogeny of Rat Recognition Memory measured by the novel object recognition task. Dev Psychobiol. 2009;51:672–678. doi: 10.1002/dev.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rojas JJ, Deniz BF, Miguel PM, et al. Effects of daily environmental enrichment on behavior and dendritic spine density in hippocampus following neonatal hypoxia-ischemia in the rat. Exp Neurol. 2013;241:25–33. doi: 10.1016/j.expneurol.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 35.Davis AR, Shear DA, Chen Z, et al. A comparison of two cognitive test paradigms in a penetrating brain injury model. J Neurosci Methods. 2010;189:84–87. doi: 10.1016/j.jneumeth.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Albasser MM, Davies M, Futter JE. Magnitude of the object recognition deficit associated with perirhinal cortex damage in rats: Effects of varying the lesion extent and the duration of the sample period. Behav Neurosci. 2009;123:115–124. doi: 10.1037/a0013829. [DOI] [PubMed] [Google Scholar]
- 37.Rutherford MA, Azzopardi D, Whitelaw A, et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics. 2005;116:1001–1006. doi: 10.1542/peds.2005-0328. [DOI] [PubMed] [Google Scholar]
- 38.Rutherford M, Pennock J, Schwieso J, et al. Hypoxic-ischaemic encephalopathy: early and late magnetic resonance imaging findings in relation to outcome. Arch Dis Child Fetal Neonatal Ed. 1996;75:F145–151. doi: 10.1136/fn.75.3.f145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19:143–149. [PMC free article] [PubMed] [Google Scholar]
- 40.Vannucci SJ, Seaman LB, Vannucci RC. Effects of hypoxia-ischemia on GLUT1 and GLUT3 glucose transporters in immature rat brain. J Cereb Blood Flow Metab. 1996;16:77–81. doi: 10.1097/00004647-199601000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


