Abstract
There has been considerable international study on the etiology of rising mental disorders, such as attention-deficit hyperactivity disorder (ADHD), in human populations. As glyphosate is the most commonly used herbicide in the world, we sought to test the hypothesis that glyphosate use in agriculture may be a contributing environmental factor to the rise of ADHD in human populations. State estimates for glyphosate use and nitrogen fertilizer use were obtained from the U.S. Geological Survey (USGS). We queried the Healthcare Cost and Utilization Project net (HCUPNET) for state-level hospitalization discharge data in all patients for all-listed ADHD from 2007 to 2010. We used rural-urban continuum codes from the USDA-Economic Research Service when exploring the effect of urbanization on the relationship between herbicide use and ADHD. Least squares dummy variable (LSDV) method and within method using two-way fixed effects was used to elucidate the relationship between glyphosate use and all-listed ADHD hospital discharges. We show that a one kilogram increase in glyphosate use, in particular, in one year significantly positively predicts state-level all-listed ADHD discharges, expressed as a percent of total mental disorders, the following year (coefficient = 5.54E-08, p<.01). A study on the effect of urbanization on the relationship between glyphosate and ADHD indicates that the relationship is marginally significantly positive after multiple comparison correction only in urban U.S. counties (p<.025). Furthermore, total glyphosate use is strongly positively associated with total farm use of nitrogen fertilizers from 1992 to 2006 (p<.001). We present evidence from the biomedical research literature of a plausible link among glyphosate, nitrogen dysbiosis and ADHD. Glyphosate use is a significant predictor of state hospitalizations for all-listed ADHD hospital discharges, with the effect concentrated in urban U.S. counties. This effect is seen even after controlling for individual state characteristics, strong correlations over time, and other significant associations with ADHD in the literature. We draw upon the econometric results to propose unique mechanisms, borrowing principles from soil and atmospheric sciences, for how glyphosate-based herbicides may be contributing to the rise of ADHD in all populations.
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is a neurodevelopmental disorder whose incidence worldwide has increased substantially in recent decades. The Center for Disease Control’s (CDC) parent report data on ADHD among U.S. children indicate a sharp rise beginning in 2007 [1]. According to the CDC, symptoms of ADHD include “a persistent pattern of inattention and/or hyperactivity-impulsivity that interferes with functioning or development” [2]. Empirical evidence suggests symptoms of ADHD are often associated with autism [3], further complicating and expanding the disorder profile. Yerys et al. [4] reported that ADHD symptoms in children with autism spectrum disorders (ASD) resulted in a greater autistic trait with more significant impairments in working memory and adaptive behavior. It was shown that deficits in executive function were more severe and persistent in patients with ADHD than with ASD [5]. Similarly, Nydén et al. [6] found adults with ADHD, in comparison to ASD and ADHD/ASD groups, experienced more significant neuropsychological impairments in exercises designed to measure intellectual ability along with attention and executive function.
Much focus has been devoted to identifying etiological factors underlying the disorder. Emerging genetic links to ADHD are promising [7,8] but require replication in diverse populations. Xu et al. [9] have suggested that ADHD is associated with epigenetic aberrations among dopamine receptor and histone-modifying genes, suggesting possible influence of external causes, like secondhand smoke [10,11], on disorder etiology. However, current cigarette smoking among U.S. adults has been decreasing in both genders between 2005 and 2013 [12], suggesting the existence of other external influences. In line with this epigenetic focus, we hypothesized that there may be a link between the rise in ADHD and the parallel rise in glyphosate exposure from agricultural use, whether through air, water, or food sources.
Glyphosate (N-phosphonomethylglycine) has become the most commonly used herbicide in U.S. industrial agriculture [13]. Its use has grown significantly with the development of crops genetically engineered to tolerate the herbicide [14], in part because of the appearance of glyphosate-resistant weeds. “Triple-stacked” corn is a hybrid corn variety that expresses three transgenic events simultaneously in the same plant, including the following: 1) the CP4 EPSPS protein, endowing resistance to the herbicide glyphosate, 2) Cry1Ab protein to protect against European corn borer (Ostrinia nubilalis), and 3) Cry3Bb1 protein to protect against corn rootworm (Diabrotica spp.) trait [15]. Given the functional intimacy of these two variables, we surmise that genetically modified corn and glyphosate could be external factors contributing to the increase in ADHD worldwide.
The adoption of stacked gene varieties in the food supply has grown nearly 68%, as a percentage of total corn planted, in the study period alone [16], although it is very difficult to estimate the percentage of these crops that are being consumed by human populations in the various states. The stacked gene crops contain glyphosate resistance but other pest management controls, as well, including the aforementioned Bacillus thuringiensis (Bt) proteins. In their work, Ohno et al. [17] found that certain Bt proteins, specifically the Cry1Aa and Cry1Ab, when digested and thus fragmented in simulated gastric fluid, induced histamine release from rat mast cells. With Cry1Aa, this effect was noted to be most pronounced in a low pH environment. Enhanced enteric histamine release could begin the neural-enteric crosstalk cascade that could eventually adversely impact dopaminergic activity [18], and dopamine has been implicated in attention-related neural networking [19]. In addition to Bt protein, glyphosate could also be a contributing factor to the increasing prevalence of ADHD.
Glyphosate has been shown to disrupt cytochrome P450 (CYP) enzymes [20], which, among many other downstream effects, can inhibit activation of vitamin D3, which depends on CYP enzymes in both the liver and the kidney [21]. Vitamin D regulates serotonin synthesis in the brain [22], and reduced central nervous system serotonergic activity has been implicated as a factor in ADHD [23].
Our preliminary investigations led us to consider a complicit role of nitrogen dysbiosis in the disease process in addition to the more direct mechanisms presented previously. We became aware that glyphosate usage on crops, such as maize, necessitates an increased application rate of nitrogen fertilizers, because glyphosate disrupts the uptake of nitrogen by plants [24]. Even in glyphosate resistant soybean crops, glyphosate application has been shown to disrupt nitrogen fixation [25]. We also propose that glyphosate disrupts the soil bacteria, leading to a major change in the way nitrogen is handled in the soil. This can cause an accumulation of various toxic nitrogen oxides in rain water exposed to soil, that then causes ammonia and nitrogen oxides in the soil to seep into the major waterways. The action of microbes in the soil and water can cause release of nitrous oxide (N2O) into the air, or the accumulation of nitrates in the water. Both of these are known to have toxic effects on human physiology: the dopaminergic system was shown to mediate the antinociceptive effects of N2O [26], and levels of central neurotransmitters, dopamine (DA) and norepinephrine (NE), were significantly elevated after repeated N2O exposure in CD-1 mice [27]. Dysregulation of both catecholamine systems has been implicated in ADHD [28]. Furthermore, BTBR mice—an inbred mouse strain that exhibits many behaviors typical of autism [29]—have severe heparan sulfate deficiency in the cerebrospinal fluid [30], a shared feature with autism [31], and the reduction of sulfate by Desulfovibrio may be a contributing factor to sulfate insufficiency [32].
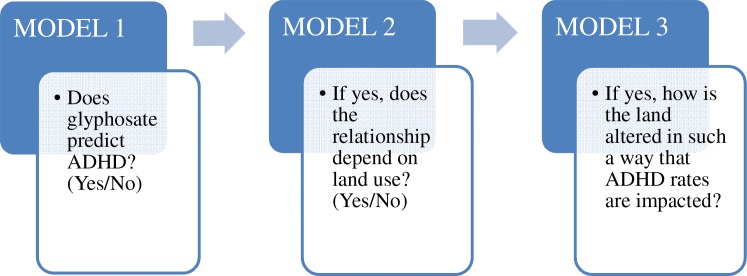
Our analysis has focused on state-provided data on ADHD hospital discharge rates and corresponding information about glyphosate usage, nitrogen fertilizer usage, industrial and food usage of maize and associated N2O emissions. We use herbicide resistant weed event statistics for further clarification on glyphosate use. Our objectives are shown as an empirical schematic in Fig 1. We have been somewhat limited by the lack of available state-level hospital discharge data on ADHD prior to 2007. However, we have been able to make use of data on other mood and behavioral disorders to help inform our analysis.
Fig 1. The empirical schematic for studying the association between glyphosate use and ADHD.
We have uncovered a pattern that is challenging to explain: glyphosate use significantly positively predicts glyphosate resistance events in weeds prior to 2007, but not subsequently. We suspect that a modification in the formulation and the use of maize as a residual cover crop in agriculture caused a widespread suppression of weed resistance development, while at the same time causing an increase in the rate of ADHD in the population. We expect that the two are related, and we propose hypotheses to explain this relationship. We have also investigated the relationship between ADHD and population density, by dividing data into metropolitan, urban, and rural subsets.
Methodology
Herbicide Use
Pesticide use was determined using data from the United States Geological Survey [33]. The USGS uses the estimated pesticide use rate (known as EPest rate) to determine pesticide usage rates for 39 pesticides among 76 crops and in 304 crop reporting districts (CRD) in the United States from 1992–2009. Pesticide usage was defined as pounds applied per harvested-crop acre in the CRD. The proprietary pesticide use survey data for each CRD was obtained from GfK Kynetec, Inc. These data were normalized to the harvested acreage in nearby counties to arrive at county estimates of pesticide use rate. Harvested acreage is non-specific and includes the crops themselves, the soil on which the crops were grown, as well as the air above the acreage. In our analysis, we have summed EPest high county estimates (we also use low county estimates for purposes of replication) to generate state-level pesticide use for each year 2006–2009, a practice recommended by the USGS [34]. The difference between high and low county estimates is that high county estimates include more counties. We have also included use of other herbicides–arbitrarily selecting atrazine, halosulfuron, and clethodim—as referent herbicides from the most prevalent groups of herbicide resistance, including Photosystem II inhibitors, acetolactate synthase (Als) inhibitors, and acetyl-Coenzyme A carboxylase (ACCase) inhibitors, respectively.
In a subset analysis, we have assigned an urbanization score to each of the counties included in the USGS glyphosate usage rate. We have used the 1993 and 2003 urban-rural continuum codes from the USDA Economic Research Services [35]. All counties receiving a code of 0, 1, 2, or 3 were deemed metropolitan counties, and we assigned an urbanization score of 1 (metropolitan) or 2 (fringe metropolitan) to these counties. Counties receiving a USDA continuum code of 4, 5, 6, or 7 were given an urbanization score of 3 (urban). Counties receiving a USDA continuum code of 8 or 9 were given an urbanization score of 4 (rural). We have used the 1993 year codes for all analysis in the years between 1992 and 2002 and the 2003 codes for all analysis in 2003 and later.
Nitrogen Inputs
We used county-level estimates of nitrogen fertilizer use to compile state statistics for farm use of nitrogen fertilizer between the years 1992 and 2006. The selection protocol can be found in [36]. Briefly, the authors gathered annual fertilizer sales data from the Association of American Plant Food Control Officials. This state data was used to determine total farm and non-farm proportions of nitrogen fertilizer use. The farm nitrogen tonnage was then estimated by multiplying the farm nitrogen rate by the nitrogen percentage from N-P-K values and the total nitrogen product tonnage.
Since the county continuum codes changed from 1993 to 2003, we have standardized the codes so that a comparison among counties could be made. To that effort, all counties with a continuum code 0 between 1992 and 2006 were given a code 1. All other codes remained the same.
State-level estimates of total food and industrial use of maize was gathered from data published by the University of Nebraska-Lincoln [37]. The data was derived from the Feed Grain Database: Yearbook Tables, Corn: Food, Seed, and Industrial Use, Table 31 from the Economic Research Service at the USDA [38]. State data were expressed as a percent of the total food and industrial use of maize grain in the U.S.
Nitrous Oxide (N2O) Emissions
Agricultural N2O emissions were obtained from the U.S. Energy Information Administration [39]. Emissions are expressed as million metric tons carbon dioxide equivalent. The EIA classifies emissions according to the following sources: agricultural, energy, industry, and waste management. To account for all sources, we expressed agricultural emissions as a percentage of total N2O emissions.
Herbicide Resistance Events
Statewide data for herbicide-resistant weeds were obtained from the International Survey of Herbicide Resistant Weeds (ISHRW) in August 2014 and replicated in April 2015 [40]. We used four herbicide resistant categories, according to the sites of action resisted. To measure the effect of weeds resistant to EPSP synthase inhibitors (which account for 9.6%, or 14 out of 146, of total herbicide tolerant weeds in the United States at the time of this publication), we chose to include in our model the herbicides that accounted for at least ten percent of the total cumulative resistant weeds in the United States at the time of this publication as reported by ISHRW. Therefore, we included the following herbicide groups, showing the percent of the total resistant weeds in the United States in parentheses: ALS inhibitors (31.5%, or 46 out of 146), Photosystem II inhibitors (17.8%, or 26 out of 146), and ACCase inhibitors (10.3%, or 15 out of 146). However, given that one weed can possess multiple sites of resisted action, we used the number of resisted sites of action for each of these groups in each state. To account for groups with less than ten percent of the total cumulative resistant weeds, we expressed data for herbicide weed resistance in each group of interest as a percentage of the total cumulative weed resistance events in each state for the period 2000–2012. Data before 2000 was incorporated into the cumulative analysis.
Hospitalization Data
The Healthcare Cost and Utilization Project (HCUPNET) State Inpatient Database was queried to identify state-level hospital discharge data trends for all-listed diagnoses of mental disorders [41]. The number of states reporting such data to HCUPNET was 37, although not all states reporting provided data for every year. The time period of interest was 2007 to 2010. Records for attention-deficit, conduct, and disruptive behavior disorders in HCUPNET were begun in 2007. For the all-listed diagnostic category, we searched for the number of discharges that received a diagnosis of attention-deficit, conduct, and disruptive behavior disorder (ADHD). HCUPNET defines all-listed diagnoses as being “…the principal diagnosis plus additional conditions that coexist at the time of admission, or that develop during the stay, and which have an effect on the treatment or length of stay in the hospital.” All diagnoses that patients receive while admitted to the hospital are assigned to an ICD-9 (International Classification of Disease, 9th Revision–Clinical Modification) code. The ICD-9 codes that are included in the clinical classification software (CCS) diagnosis of attention-deficit, conduct, and disruptive behavior disorder are shown in S1 Table, along with a translation to respective codes in the Diagnostic and Statistical Manual for Mental Disorders IV, Text Revision. Because we have previously hypothesized that glyphosate may exert a deleterious effect on many mental conditions, we have normalized the number of all-listed ADHD hospital discharges to a percentage of total discharges for all-listed mental disorders recorded in HCUPNET.
Hospitalization discharge data for all-listed ADHD diagnoses were also categorized according to the location of the patients’ residence, as a percentage of the total all-listed ADHD hospital discharges. HCUPNET provided four categories in this respect: large central metro, large fringe metro (suburbs), medium and small metro, micropolitan and noncore (rural). We have created four categories, called metropolitan, fringe metropolitan, urban, and rural, to mirror the glyphosate and nitrogen fertilizer usage urbanization coding system mentioned previously. Our metropolitan category included the large central metro heading in HCUPNET. Fringe metro was the second category. Medium/small metro defined our urban category, and our rural category included the micropolitan and noncore (rural) data in HCUPNET. Hospitalization data for each of these categories were expressed in HCUPNET as a percentage of the total hospital discharges for all-listed ADHD diagnoses in each state, and we retained this unit of measure. We also gathered data on age and gender of patients with an all-listed ADHD discharge.
To verify the discharge data among the reporting states in HCUPNET, we have also gathered CDC data on the number of hospital discharges for all-listed ADHD (ICD-9-CM code: 314.01) between 1993 to 2005 [42]. We compared these data to prior year’s estimated glyphosate using ordinary least squares regression.
Least Squares Dummy Variable (LSDV) Regression
Panel regressions were performed using the LSDV in plm package in R, version 3.1.3 [43]. Two-ways within estimation was also used to confirm the LSDV estimations and all model diagnostics and was used solely when time (T) for each subject was greater than 4 years. The LSDV method creates dummy variables for both state and time, while the two-ways within effect estimations use deviations from group means. When noted, simple ordinary least squares regression modeling was performed in R using the fit argument. Annual county input data from the USGS was summed and organized into study categories by Microsoft Access 2010. Fixed effects data were checked for heteroskedasticity in R using the Breusch-Pagan (BP) test (package lmtest) [44]. Heteroskedasticity is the phenomenon that a dependent variable (such as hospitalization discharges) shows variability during the time period in which the independent variable (such as herbicide use) predicts its occurrence. Data were also tested for serial correlation using the Durbin-Watson (DW) statistic [43,44]. We chose the Fisher-type test in Stata 11.1 [45] to check for unit roots in unbalanced panels for each model since the test uses Newey-West standard errors to account for any serial correlation present among the residuals in each model. To our knowledge, an equivalent test was not available in R. The Augmented Dickey-Fuller (ADF) test (package tseries) [46] was used to test for unit roots in Model 3 panel sets. We used the Arellano variance covariance (vcov) HC function covariance estimator [47] to control for heteroskedasticity and serial correlation in all models. We used student’s paired t-test when making comparisons between age and urban subsets of ADHD hospital discharge among available HCUPNET states during our study period (2007–2010). Data were first tested for normality using Shapiro-Wilk test. Unless otherwise specified, we define significance at the .05 level for all statistical testing performed, while a .10 level was used for model diagnostic testing (i.e., BP, DW, ADF, Fisher test). Graphical output was performed using R and GraphPad Prism 6 Demo for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com.
Model 1
Our first model tested our hypothesis that glyphosate use in one year predicts all-listed ADHD hospital discharges the following year. In addition to glyphosate and maize use, we have identified four time-variant covariates, or regressors, including other societal and health care related variables that have been associated with the disorder and could explain the increase in ADHD diagnoses. Furthermore, we have included glyphosate resistance to clarify the herbicide’s effect on human health. We subjected each covariate, excluding our variables of interest, glyphosate use and food/industrial maize use, to a single linear ordinary least squares model for each year from 2007 to 2010, inclusive. If a significant relationship was noted in any year at the .05 alpha level, we included the covariate in the final fixed effects model. Model 1 is expressed below
where Y it is the proportion of all mental disorders for which ADHD was a discharging condition in state i and year t, c 1 is a constant, α i represents a state fixed effect to control for permanent differences between states and v t denotes a time fixed effect, is a (N 1 x 1) column vector representing an individual lagged covariate m, with m = 1,…, M, and N 1 is the total number of state-time observations, is a (N 1 x 1) column vector representing an individual contemporaneous covariate p, with p = 1,…, P, and ε it captures unobserved heterogeneity in state i and year t. The parameters to be estimated include and . The states of Nevada and Hawaii were excluded in this model since there were no weed resistance or glyphosate use data, respectively, for the years of interest. All covariates in model 1 are explained in Table 1.
Table 1. Description of the covariate used, with a citation to explain the inclusion, in both the initial OLS screening and the fixed effects regression analysis for Models 1 and 2.
| Model Notation | Covariate | Description | Citation |
|---|---|---|---|
| Contemporaneous | |||
| p = 1 | Health care access [48] | defined by the number of hospitals in each state, expressed as a percentage of total hospitals in the United States | [49] |
| p = 2 | Economic [50] | defined by the annual home price index in each state, expressed as an average of the index over the four quarters of each year | [51] |
| p = 3 | Population [52] | defined by the percent of the total United States population that resides in each state in each year | [53] |
| Lagged | |||
| m = 1 | Precipitation Lag [54] | defined as the deviation from the average annual precipitation in each state. For example, a reading of .88 would indicate that the precipitation in a given state during a given year was 88% of its average precipitation, with the average being defined by the base period, 1901–2000; (Parameter: Precipitation, Time Scale = 12 months, Month = December) | [55] |
| — | Herbicide resistance | See Text | [40] |
| m = 2, m = 3 | Herbicide use/Maize Use | See Text | [33,37] |
Model 2
Our second model–an ad hoc analysis—sought to test the hypothesis that, if glyphosate is significantly contributing to ADHD, there would be regional influences underpinning this relationship. That is to say that glyphosate is a main input in the agricultural industry, which largely has existed in rural (i.e., less populated) parts of the country, although inroads have been made in sustaining and expanding adaptive metropolitan agriculture systems [56]. To study this, we have categorized all counties from the USGS glyphosate use estimates into four codes (1 = metropolitan, 2 = fringe metropolitan, 3 = urban, 4 = rural) to generate four data points for each HCUPNET state in each year. The state of Hawaii was excluded since no estimates of glyphosate use were available. These data were then matched to the ADHD data in HCUPNET, according to the model specifications in Table 2. The other three predictors were unchanged from Model 1. Hospitalization data by location of patient residence and patient age was masked (*) in HCUPNET if less than or equal to ten discharges or fewer than two hospitals were reported, and these data were removed from the analysis. Beginning in 2007, HCUPNET began revising data on location of patients’ residence to accommodate the specifications provided by the Economic Research Service at the USDA. Our four models were therefore
where each model corresponds to one of four unique k values, is the proportion of total all-listed ADHD disorders in state i and year t that occurred among patients residing in a particular county classification code (k = 1, 2, 3, 4), c 2, α i and v t are as defined in the previous sub-section for each model k, X it is a contemporaneous continuous variable (i.e., population percent, hence the p superscript equals 1 and is therefore omitted for simplicity) in state i and year t as in model 1, denotes one of two lagged variables predicting ADHD discharges and captures unobserved heterogeneity in state i and year t for model k. Our main predictor of interest, glyphosate use (), is expressed in kilograms as the previous year’s total use in counties categorized only by urbanization code k in state i. The sixteen parameters to be estimated include , , and for k = 1, 2, 3, and 4.
Table 2. The coding system that was used in Models 2 and 3.
| USDA Economic Research Service Rural-Urban Continuum Codes (RUCC) a | HCUPNET ADHD (patient residence) | ||
|---|---|---|---|
| Model Set | Code | Description | |
| k = 1 | j = 0 | Central counties in metro areas of 1 million population or more (1993 only) | Large central metro |
| j = 0 | |||
| j = 1 | (Fringe, only 1993) counties in metro areas of 1 million population or more | Large central metro | |
| j = 1 | |||
| k = 2 | j = 2 | Counties in metro areas of 250,000 to 1 million population | Large fringe metro |
| j = 3 | Counties in metro areas of fewer than 250,000 population | Large fringe metro | |
| k = 3 | j = 4 | Urban population of 20,000 or more, adjacent to a metro area | Medium/Small Metro |
| j = 4 | |||
| j = 5 | Urban population of 20,000 or more, not adjacent to a metro area | Medium/Small Metro | |
| j = 5 | |||
| j = 6 | Urban population of 2,500 to 19,999, adjacent to a metro area | Medium/Small Metro | |
| j = 6 | |||
| j = 7 | Urban population of 2,500 to 19,999, not adjacent to a metro area | Medium/Small Metro | |
| j = 7 | |||
| k = 4 | j = 8 | Completely rural or less than 2,500 urban population, adjacent to a metro area | Micropolitan and noncore (rural) |
| j = 8 | |||
| j = 9 | Completely rural or less than 2,500 urban population, not adjacent to a metro area | Micropolitan and noncore (rural) | |
| j = 9 | |||
a Continuum codes changed from 1993 to 2003.
Model 3
Third, we tested our hypothesis that glyphosate use could be indirectly altering the land biota to impact the rise of ADHD. We assessed the influence of county level estimates of glyphosate application on county level estimates of farm nitrogen fertilizer use between 1992 and 2006. The hypothesis is that increasing amounts of glyphosate use would perturb soil such that greater farm use of nitrogen fertilizer would be needed. We summed glyphosate and farm nitrogen use for all USDA county continuum codes between 1992 and 2006. We then organized our panel sets by study categories set forth in Table 2. We focused our attention to glyphosate use in urban counties given our results with the Model 2 set. For comparison, we also analyzed results for fringe metro counties. Our two models were
where each model corresponds to one of two unique k values, is a (N 3 x 1) column vector (n is the number of RUCC, and N 3 is represented by (n*t)) and denotes the farm use of nitrogen-based fertilizers (in kilograms) in the RUCC county code j with classification category k in year t, is a constant that varies by model, is a RUCC fixed effect, and denotes a time fixed effect, indicates the glyphosate use estimate (in kilograms) in the RUCC county code j with category k in year t, and captures unobserved heterogeneity in county code j in year t. The two parameters to be estimated and reported include , where k = 2 and 3. These estimates generate a comparison of the effect of glyphosate’s use in fringe metro and urban counties on application of nitrogen-based fertilizers.
Results
Herbicide Use
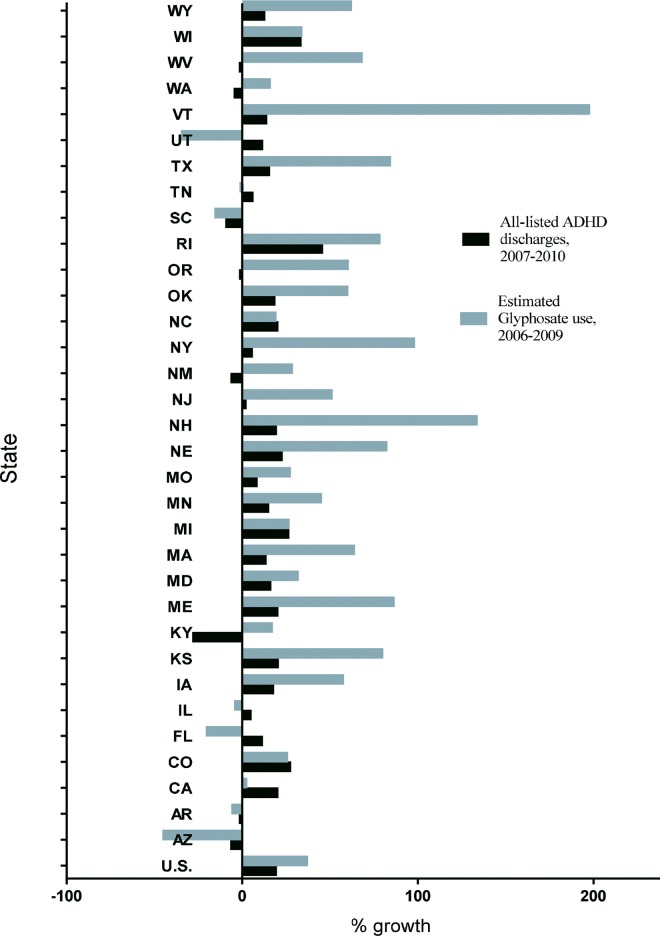
Estimated national glyphosate use increased relatively 36.2%, or 26,795,398 kilograms, from 2006 to 2009, whereas use increased relatively 59.6%, or 26,668,369.50 kilograms, in all available HCUPNET states reporting data on ADHD hospital discharges for available years between 2007 and 2010. Estimated national halosulfuron use relatively decreased 0.69%, or 210.40 kilograms, from 2006 to 2009, and use relatively decreased 2.55%, or 586.20 kilograms, in all available HCUPNET states reporting data on ADHD hospital discharges for all available years between 2007 and 2010. Estimated national atrazine use relatively decreased .049%, 15,016 kilograms, from 2006 to 2009, and use relatively increased 26%, or 4,721,370.7 kilograms in HCUPNET states reporting data on ADHD hospital discharges for all available years between 2007 and 2010. This anomaly can be explained by the inclusion of the state of Illinois, which did not report hospitalization data in 2007 or 2008, but did report in 2009 and 2010. The estimated annual use of atrazine in 2009 in the state of Illinois was 4,561,841.3 kilograms, making it the state with the highest estimated atrazine usage in that year. Estimated national clethodim use increased relatively 83.9%, or 197,257 kilograms, from 2006 to 2009, and use relatively increased 70.7%, or 105,888.10 kilograms, in HCUPNET states reporting data on ADHD hospital discharges for all available years between 2007 and 2010. A state level breakdown on the percent increase in estimated glyphosate use is shown in Fig 2, and the average estimated glyphosate use over the HCUPNET reporting states is shown in Fig 3A. Some HCUPNET states did not have data for all years of interest (2007–2010): Illinois and New Mexico (2009, 2010) and New Hampshire (2007–2009).
Fig 2. Percent change in estimated glyphosate use (2006–2009) and all-listed ADHD hospital discharges among HCUPNET available states (2007–2010).
Fig 3.
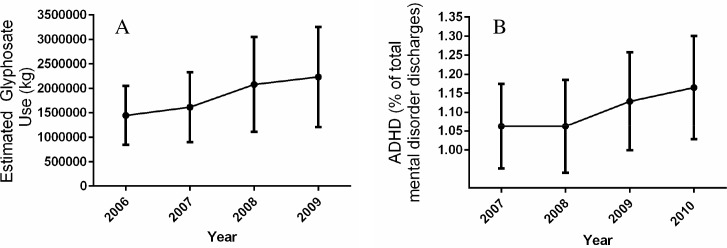
(A) Average glyphosate use (in kilograms) in the HCUPNET states in Model 1 (Nevada and Hawaii excluded). Data are presented as average percentages with 95% confidence interval across all reporting states for each year indicated (2006–2009). (B) Average all-listed hospital discharges for ADHD, as a percent of total all-listed mental health discharges, in HCUPNET reporting states (excluding Nevada and Hawaii) for years 2007–2010 used in Model 1.
Data are presented as average percentages with 95% confidence interval across all reporting states for each year indicated.
Data on estimated glyphosate use by USDA rural-urban continuum code between 1992 and 2006 indicate that the greatest relative increase in use occurred in county codes 4 and 5 (1523 and 1240%, respectively). The largest relative increase in estimated glyphosate use during the period of study (2006–2009) occurred in county code 9 (55%).
Nitrogen inputs
The states with the largest relative increase in farm use of nitrogen fertilizers between 1992 and 2006 were California (65%), Connecticut (76%), Nevada (209%), New Hampshire (34%), North Dakota (57%), South Dakota (205%), Vermont (33%), West Virginia (162%), and Wyoming (48%). The states with the largest relative decrease in estimated farm use of nitrogen fertilizers between 1992 and 2006 were Delaware (-31%), Maryland (-38%), Mississippi (-32%), Utah (-47%), and Virginia (-34%).
USGS county sums of farm use of nitrogen fertilizer show that the greatest relative percentage increase occurred in those counties with a USDA rural-urban continuum code of either 3 or 4 (64 and 49%, respectively).
Total U.S. food and industrial use of maize grain increased by 2 billion bushels, or roughly 67%, between 2006 and 2009. The states showing the largest relative increases include Georgia (516.65%), Texas (294.35%), Mississippi (275.62%), and North Dakota (108.89%). The states showing the largest relative decreases include Pennsylvania (-51.21%), Kentucky (-49.90%), Florida (-49.09%), and New Mexico (-46.88%).
Agricultural N2O Emissions
According to the United States Environmental Protection Agency, nitrous oxide (N2O) accounted for 6% of all U.S. greenhouse gas emissions. Man-made sources of N2O include agriculture, energy, industry, and waste management practices, with agricultural soil management accounting for 75% of U.S. N2O emissions. Because of its long half-life, N2O is thought to be a source with a global warming potential over 300 times that of other greenhouse gases, like carbon dioxide [57].
Total N2O agricultural emissions have been increasing since 1990. Total agricultural emissions have increased, as a percentage of total N2O emissions, 9.20% or about 12.3 million metric tons from the period 1990–2009 [39].
Direct agricultural soil N2O emissions, including those from the use of synthetic nitrogen fertilizers, animal manure, sewage sludge and other non-manure organics, above and below ground crop residues, and soil mineralization, increased 9.1% as a percentage of total N2O emissions, or about 9 million metric tons carbon dioxide equivalent, from 1990–2009. Almost half of that normalized increase (48.77%) came during the period of the current study (2006–2009). On an absolute basis, direct agricultural nitrous oxide emissions have increased 8.23% between 1990 and 2009, with almost a third (30.50%) of that increase occurring between 2006 and 2009. Most of that increase between 2006 and 2009 was attributable to above and below ground crop residues. Use of high residue crops in soil management is a growing trend in agriculture [58].
Indirect agricultural soil N2O emissions, including those from soil leaching, runoff and atmospheric deposition, increased 11.28% as a percentage of total N2O emissions, or about 2 million metric tons carbon dioxide equivalent, between 1990 and 2009. Most of this increase is attributable to soil leaching and runoff. It should also be noted that significant N2O emissions may be coming from indirect sources such as bodies of freshwater [59]. Although formal estimations of N2O emissions from bodies of water are limited, there is growing evidence to suggest that these comprise a significant source of emissions and must be included in future estimates, especially considering the increasing agricultural runoff into outlying drainage channels [60].
Herbicide-Resistance Events
Since it is the policy of the ISHRW that data derived from their database shall not be used in a significant way in a scientific paper without the expressed permission of the director, we have chosen to include the results of this analysis as an unpublished supplement to this paper that can be viewed at the non-profit’s website [61]. Our data leads us to believe that the development of herbicide weed resistance is not a significant component in our hypothesized relationship between glyphosate use and all-listed ADHD hospital discharges.
We can validate, using two-way fixed effects, that use of herbicides with contact modalities of action does significantly predict class resistance between 2000 and 2010. Glyphosate use in states (N = 470), as a lag indicator, does significantly positively predict EPSP synthase inhibitor weed resistance (adjusted estimate: 3.05e-08±1.14e-08, p-value < .01). That is, for a one kilogram increase in glyphosate used, there is a 3.05e-08% increase in glyphosate weed resistance, as a percentage of total weed resistance events in each state, the following year. Similarly, halosulfuron use in states (N = 470) in one year significantly positively predicts acetolactate synthase (ALS) inhibitor weed resistance, as a percentage of total resistance events, the following year (adjusted estimate: 2.09e-05±8.42e-06, p-value: 01). Atrazine use in states (N = 470) in one year also does significantly positively predict photosystem inhibitor weed resistance the following year as a percentage of all resistance events (adjusted estimate: 3.62e-08 ±1.75e-08, p-value: 0.04).
However, during our study period from 2006 to 2009, glyphosate use does not significantly predict EPSP synthase inhibitor resistance events the following year in states (N = 188) (unadjusted estimate: 4.29e-09±7.04e-09, p-value: 0.54). Similarly, halosulfuron use does not significantly predict Als inhibitor resistance events the following year (adjusted estimate: 5.07e-06±6.19e-06, p-value: 0.41). Atrazine use does not significantly positively predict photosystem inhibitor resistance events either (adjusted estimate: 1.11e-08±2.04e-08, p-value: 0.59).
We, therefore, feel confident that, if use predicts resistance, the phenomenon of weed resistance can be a possible mechanism (through consumption of treated vegetation) to explain the hypothesized health effects from the use of similarly purposed herbicides (i.e., same site of targeted action). However, in the current study period, glyphosate use does not significantly positively predict EPSP synthase inhibitor weed resistance in HCUPNET states (adjusted estimate: 7.55e-09±1.24e-08, p-value: 0.54) (S1 Fig). Similarly, halosulfuron use does not significantly positively predict acetolactate synthase (Als) inhibitor weed resistance (adjusted estimate: 6.83e-06±7.27e-06, p-value: 0.35). Atrazine does not significantly positively predict photosystem II weed resistance events either (adjusted estimate: 1.20e-08±3.44e-08, p-value: 0.73). Therefore, the development of herbicide resistance (i.e., herbicide application on vegetation) among contact herbicides, like glyphosate, is insignificant and unrelated to any link between use and disorders of interest between 2007 and 2010, and this data is confirmed by other reports [62].
Hospitalization Data
Fig 3B shows the increase in ADHD discharges across HCUPNET reporting states (excluding Nevada and Hawaii, as these states had no weed statistics or glyphosate use data, respectively) from 2007–2010. Data are expressed as ADHD discharges as a percent of all-listed hospitalization discharge for all mental disorders. The states of Nebraska and Minnesota maintained the highest percentage of all-listed ADHD hospitalization during the study period (pooled averages between 2007 and 2010, inclusive: 2.13 and 1.97, respectively). A state-level breakdown on the increase in all-listed ADHD hospital discharges from 2007 to 2010 in HCUPNET available states is shown in Fig 2.
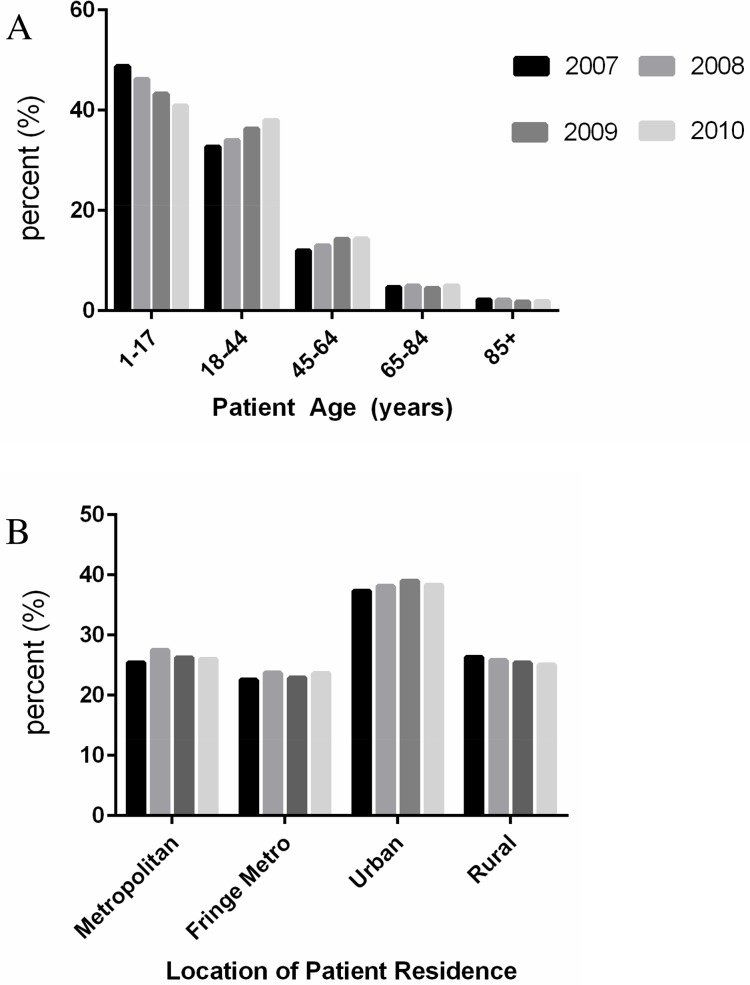
We find distinct trends emerging from a breakdown of all-listed ADHD discharges by patient age in HCUPNET reporting states from 2007 to 2010. Persons aged 1–17 years accounted for an average of 48.8% of the all-listed ADHD discharges in 2007 but that percentage decreased to 40.9% by 2010, a statistically significant drop (p-value = 0.01, 95% CI: 1.67–14.01), while adults aged 18–44 gained as a percentage of total all-listed ADHD discharges, climbing from 32.7% to 38.0% across all HCUPNET reporting states (p-value < 0.05, 95% CI: -9.90–-0.74) (Fig 4).
Fig 4. The breakdown of hospital discharges for attention-deficit, conduct, and disruptive behavior (CCS diagnostic category– 652) by (A) patient age and (B) location of patient residence.
Data are presented as the average percent for available HCUPNET reporting states for each year indicated and may therefore not add to 100%. Masked data (*) in HCUPNET, or data for which there were 10 or fewer patient discharges or fewer than 2 hospitals, were excluded from analysis. The state of Hawaii is excluded from the descriptive statistics reported for the ADHD population.
The state-level ADHD data by patient age comports with national hospital discharge statistics in HCUPNET. There were 174,746±11,765 discharges with a diagnosis of ADHD in 2007 with the patient age breakdown as follows: persons aged 1–17 years (94,168±10,404 or 53.89%), adults aged 18–44 (49,659±2,340 or 28.42%), adults 45–64 (19,964±1,073 or 11.42%), seniors aged 65–84 (7,831±1,107 or 4.48%), and seniors 85 and older (2,943±467 or 1.68%). There were 258,098±14,781 discharges with a diagnosis of ADHD in 2010 with the patient age breakdown as follows: persons aged 1–17 years (120,602±12,225 or 46.73%), adults aged 18–44 (89,659±3,817 or 34.74%), adults 45–64 (31,984±1,408 or 12.39%), seniors aged 65–84 (11,538±1,457 or 4.47%), and seniors 85 and older (3,972±647 or 1.54%).
Hospital ADHD discharge diagnoses by gender revealed that majority were males, although there was a significant drop in the percent (58.6% to 56.4%, p-value < 0.01, 95% CI: 0.81–3.50) of discharges among males and a significant rise in the percent (41.4% to 43.6%, p-value < 0.01, 95% CI: -3.51–-0.82) of discharges among females during the study period. This data matches the national HCUPNET statistics on gender and ADHD, with 58.65% of all-listed ADHD cases being male and 41.22% being female in 2007. In 2010, 56.29% of all-listed ADHD discharges diagnoses were male, while 43.55% were female.
Urbanization data among both HCUPNET reporting states and national HCUPNET statistics indicate that the greatest percentage of all-listed ADHD diagnoses occur among persons living in medium and small metro (urban populations) areas. The average percentage of all-listed ADHD hospital discharges occurring in persons living in medium and/or small metro areas (urban) across all HCUPNET reporting states used in this analysis, excluding Hawaii, was significantly higher than other urbanization categories (Fig 4). This finding matches the national HCUPNET discharge statistics, with 32.47% (56,736±7,774 discharges) of total all-listed ADHD hospital discharges occurring in persons residing in urban counties in 2007. In 2010, there were 86,081±9,608 discharges with an all-listed ADHD diagnosis among persons living in urban counties, comprising 33.35% of the national total.
The national all-listed ADHD hospital discharge data from the CDC reaffirms the normalized increase seen in HCUPNET reporting states. The all-listed ADHD hospital discharges (ICD-9-CM code, 314.01) increased 84,000 from 21,000 in 1993 to over 100,000 in 2005, representing a 400% increase.
Fixed Effects
Model 1
The results from the simple ordinary least squares model screening process are presented in Table 3. For year 2007, the only covariate to pass the screening at the .05 level was population (estimate: -0.053±0.02, p-value: 0.01). Population retained a significant association with all-listed ADHD hospital discharges in all subsequent years. Precipitation as a lagged indicator was correlated with all-listed ADHD in 2008. Therefore, population and precipitation were added to the two-way fixed effects final model. Specifically, P = 1 and M = 3 given these screening results. None of the arbitrarily selected herbicides, including halosulfuron, clethodim, and atrazine, passed the initial screening at either USGS estimate.
Table 3. The effect of herbicide use and other covariates on ADHD hospital discharges, showing the initial OLS screening for each predictor for 2008 and 2010 and all significant predictors in a two ways effects within model in R, adjusting for heteroskedasticity and serial correlation.
| Covariate | Linear Model (criteria variable for inclusion, p < .05) | Time and State Fixed Effects (2007–2010) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2008 | 2010 | |||||||||||
| N | r 2 | Estimate | Std Error | N | r 2 | Estimate | Std Error | N 1 a | Estimate | Std Error | ||
| Food/Industrial Use—Maize | — | — | — | — | — | — | — | — | 127 | 2.70E-02 | 0.013 * | |
| Pesticide | ||||||||||||
| Glyphosate | Amino Acid synthesis inhibitor | — | — | — | — | — | — | — | — | 127 | 5.54E-08 | 1.68e-08 ** |
| Atrazine | Photosystem II inhibitor | 31 | 0.04 | 6.98E-08 | 6.28E-08 | 32 | 0.04 | 6.92E-08 | 5.93E-08 | — | — | — |
| Clethodim | ACCase inhibitor | 31 | 0.03 | 1.11E-05 | 1.11E-05 | 32 | 0.02 | 5.96E-06 | 7.35E-06 | — | — | — |
| Halosulfuron | Acetolactate synthase inhibitor | 31 | 0.03 | -7.75E-05 | 7.82E-05 | 32 | 0.01 | -3.18E-05 | 6.12E-05 | — | — | — |
| Home Price Index | 31 | 0.09 | -4.41E-01 | 2.66E-01 | 32 | 0.02 | -2.84E-01 | 3.97E-01 | — | — | — | |
| Population | 31 | 0.17 | -5.41E-02 | 0.022* | 32 | 0.14 | -5.53E-02 | 0.02499* | 127 | -4.11e-01 | 2.81e-01 | |
| Health Care Access | 31 | 0.07 | -5.03E-02 | 3.35E-02 | 32 | 0.05 | -4.82E-02 | 3.81E-02 | — | — | — | |
| Precipitation Lag | 31 | 0.14 | 6.71E-01 | 0.3109* | 32 | 0.01 | 1.96E-01 | 3.96E-01 | 127 | 1.13e-02 | 4.50e-02 | |
** p < .01
* p < .05
a N 1 is the total number of state-time observations
The Durbin-Watson test for panel data indicates marginal serial correlation (DW = 1.721, p-value = 0.067) among the residuals. The Breusch-Pagan test shows the presence of marked heteroskedasticity in this model (BP = 51.95, df = 4, p-value = 1.41e-10). The Fisher type test indicated the presence of stationarity in at least one panel for all covariates.
For every kilogram increase in glyphosate use (high estimate) the prior year, there is a 5.54E-08% increase (p<0.01) in all-listed ADHD hospital discharges as a percent of all mental health disorder discharges in each HCUPNET reporting state the next year (2007–2010), and this effect is seen in the absence of glyphosate use significantly predicting EPSP synthase inhibitor resistance events (Table 3). Fig 5 shows the heteroskedastic trends in this model. These findings were replicated when low USGS herbicide estimates were used for this model (data not shown): the estimate for glyphosate use jumps 2%.
Fig 5. Diagnostic plots for Model 1 show marked heteroskedasticity within the center of distribution.
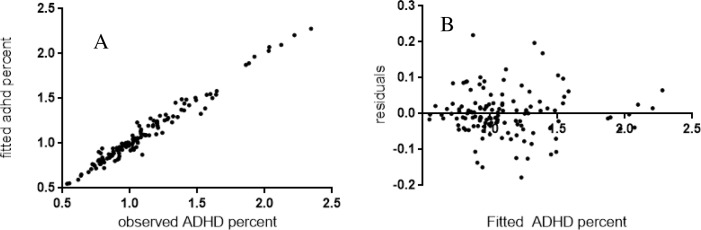
(A) Observed all-listed ADHD percent discharges (N 1 = 127) plotted against predicted percentages (B) Model residuals were plotted against fitted values. Fitted values were tabulated in R by subtracting residuals from observed values.
To put this result in context, the state of Nebraska increased glyphosate use by 3,252,428 kilograms between 2006 and 2009. That translates to a 0.18% increase in all-listed ADHD hospital discharges, representing 42.9% of the total increase in all-listed ADHD hospital discharges between 2007 and 2010 in that state.
For every percentage increase in maize for food and /or industrial use in the prior year, as a percentage of total U.S. maize for food and/or industrial use, there is a 0.027% increase (p<0.05) in all-listed ADHD hospital discharges as a percent of all mental health disorder discharges in each HCUPNET reporting state the following year (2007–2010).
To further help inform our analysis on glyphosate as a single predictor for other mental disorders, we used the same HCUPNET states and years and found a marginally significantly negative relationship after data adjustment between glyphosate use and mood disorders as a percent of total all-listed mental health disorder discharges (adjusted estimate:-1.32e-07±7.37e-08, p-value: 0.076). During the period from 2001 to 2006, however, we find the estimate to be positive, although the estimate is not statistically significant after data adjustment: glyphosate use predicts affective disorders (adjusted estimate: 1.69e-07±1.07e-07, p-value: 0.12), and this occurs during a period when glyphosate use significantly predicts EPSP synthase inhibitor resistance events (adjusted estimate: 4.58e-08±2.30e-08, p-value: .049).
Similarly, glyphosate use does not significantly predict schizophrenia and other psychotic disorders during the earlier period in the decade when use predicts resistance (adjusted estimate: 2.00e-08±5.65e-08, p-value: 0.72) but does strongly predict them in the latter period when use does not predict resistance (2007–2010) (unadjusted estimate: 9.18e-08±3.12e-08, p-value < 0.01).
Although patient age was not a focus in this model, we did find unique associations between glyphosate and the ADHD patient age. When we replace the dependent variable with the percentage of total all-listed ADHD diagnoses that occurred within a certain age cohort (see Fig 4), we found the association to be only positive, although not significant, in young adults. Interestingly, there was a strong negative association between glyphosate use and middle-aged adults, 45–64, (unadjusted estimate: -9.34e-07±2.50e-07, p-value: 0.0003) and seniors (not significant). With respect to patient gender, when we replace the dependent variable with the percent of total all-listed ADHD diagnoses that occurred within a gender (male or female), we found the association to be positive, although not significant (p < .05), with males and negative, although not significant, with females (data not shown).
As an additional supplemental note, we have been able to replicate this model in a simple linear regression with USGS estimated national glyphosate use as the predictor variable and non-normalized national all-listed ADHD hospital discharges from the CDC Vital and Health Statistics Reports (from 1993 to 2005) (unadjusted estimate: 1.31E-06±1.04E-07, p-value: 7.23E-08).
Model 2`
The study significance was adjusted for multiple comparisons across model categories; the revised p-value of 0.0125 was used to determine significance. The urbanization data suggests that glyphosate’s significantly positive relationship with all-listed ADHD hospital discharges is marginally significant in urban counties. As shown in Table 4, for every kilogram increase in glyphosate used in urban counties, the model predicts a 2.40e-06 percent increase in the percentage of all-listed ADHD hospital discharges that are attributable to patients living in these areas. The Durbin-Watson test indicated no serial correlation among the residuals in this sample (DW = 2.30, p-value = 0.96), and the data was determined to be heteroskedastic using the Breusch-Pagan test (BP = 9.90, df = 4, p-value = 0.042). The Fisher type test indicated the presence of stationarity in at least one panel for all covariates. S2, S3, S4, and S5 Figs provide model diagnostics for each panel set included in Model 2.
Table 4. The effect of urbanization on the relationships between glyphosate use and ADHD hospital discharges, using two ways fixed effects within model in R and adjusting for heteroskedasticity and serial correlation and including all significant covariates that passed screening in Model 1.
| Glyphosate use (2006–2009) and ADHD discharges (2007–2010) | ||||
|---|---|---|---|---|
| Category | r 2 | Estimate | Standard Error | |
| Metropolitan (k = 1) | 88 | 0.01 | -1.92e-06 | 4.00e-06 |
| Fringe Metro (k = 2) | 104 | 0.02 | -6.94e-07 | 2.52e-06 |
| Urban (k = 3) | 123 | 0.08 | 2.40e-06 | 9.95e-07 § |
| Rural (k = 4) | 111 | 0.12 | -4.17e-06 | 2.40e-06 |
§ corrected p < .025
In urban counties in Missouri, for example, there was an increase of 372,246.10 kilograms of glyphosate used (representing an 18.7% increase) between 2006 and 2009. The percentage of the total all-listed ADHD diagnoses among patients living in urban areas, as a percent of total all-listed ADHD discharges in the state, rose 3.18% from 2007 to 2010. Using this econometric output, about 28% of the increase in ADHD discharges can be attributed to the increase in glyphosate used in these areas in the prior year.
We have performed additional iterations of this model to accommodate the flexible interpretations of the rural and urban continuum codes among government agencies. For example, the USDA ERS categorizes all counties in the state of Rhode Island as major metropolitan (code 1), while HCUPNET provides data for both metropolitan and fringe metropolitan (codes 2 and 3). We, therefore, combined these categories in an additional analysis and found the association between estimated glyphosate use and all-listed ADHD discharges to be negative in the combined metropolitan code, although it was not statistically significant. In a second iteration, we revised our urban category to codes 4 and 5 only and relocated codes 6 and 7 to the rural category. The same results were noted. These results confirm the first model iteration and suggest that the model is robust to flexible continuum interpretations.
Model 3
The study significance was adjusted for two comparisons across model categories; the revised p-value of 0.025 was used to determine significance for the glyphosate estimates. The two-way within model fixed effects indicate that glyphosate use is associated with farm use of nitrogen fertilizers. In fringe metropolitan counties, the association is significantly positive (unadjusted estimate: 188.38±16.52, p-value < .001). Serial correlation was not present among the residuals (DW = 2.78, p-value = 0.99), and the data was homoskedastic (BP = 0.25, df = 1, p-value = 0.62). We used second-order differencing of both variables and confirmed no unit roots with the ADF test in this longer time series.
In the urban counties as we have defined them, the association is significantly positive. (adjusted estimate: 150.53±24.91, p-value < .001). Serial correlation was not present (DW = 3.41, p-value = 1) and data were homoskedastic (BP = 0.21, df = 1, p-value = 0.64). We differenced both variables twice to ensure stationarity in the urban time series. S6 and S7 Figs present model diagnostics for the panel sets used in this model.
Discussion
The current study suggests that glyphosate use may be contributing to all-listed ADHD hospital discharges in the United States. The main findings of the study include the following:
Normalized glyphosate use, as estimated by the USGS and used as a lag indicator, significantly positively predicts normalized ADHD in the 33 HCUPNET reporting states from 2007–2010. Normalized total food and industrial use of maize as a lagged indicator also significantly positively predicts normalized ADHD in the same data sample. These findings are highly statistically significant even after controlling for individual state differences, strong correlations over time, and other significant associations reported in the ADHD literature.
Glyphosate use strongly positively predicts glyphosate weed resistance events in states from 2001 to 2010 but not in the 33 HCUPNET reporting states or all available states (N = 47) from 2007 to 2010. Therefore, the development of weed resistance is not a significant component in the relationship between glyphosate and ADHD during our study period. Our working hypothesis is that the formulation was altered significantly and marketed heavily around the 2006 time frame and maize was increasingly used as a residual cover crop in agriculture. The data on glyphosate and other mental disorders further supports this hypothesis. The changes in these agricultural inputs and perhaps others contributed to the impairment of weeds to develop resistance to herbicide use in the subsequent years. We further hypothesize that these input changes may be contributing to ADHD in humans—especially young adults who tend to be more adventurous outdoors and therefore exposed—through the release of N2O from altered microbial constitutions in soil and water systems. Since the ingredients in the herbicide are under patent control, they are not required to be listed, and thus it is difficult to determine the contributing factors.
Our urbanization coding system revealed that glyphosate’s positive effect on ADHD remained marginally significant only in urban counties between 2007 and 2010, suggesting that urbanization may be a factor in the relationship. Recent historic population migration from nonmetro counties might explain the negative association with glyphosate in rural areas [63].
Glyphosate use is significantly positively associated with farm use of nitrogen fertilizers between 1992 and 2006 in urban and fringe metro counties, suggesting that farm use of nitrogen fertilizers may be contributing significantly to the association between glyphosate use and all-listed ADHD hospital discharges prior to 2007, but there may be other land interactions, such as glyphosate’s direct impact on soil microbiota and nitrification inhibition especially after patent reformulation, that could be driving the marked increase in ADHD seen after 2006.
The link between glyphosate, nitrogen inputs, and soil microbiota
Glyphosate's role as a mineral chelator in soil [64] could cumulatively dampen manganese bioavailability in soil. Manganese is important for nitrogen assimilation in plants. If less manganese is available, less nitrogen assimilation occurs. This would explain the strong positive association we have found between glyphosate and nitrogen-based fertilizers. This strong association could contribute to indirect agricultural N2O emissions from leached anthropogenic nitrogen into urban drainage networks [59,60].
The findings in this paper suggest some kind of modification in the use of glyphosate around 2006. The ability for estimated glyphosate use alone to predict mental health disorders changed significantly from 2001 to 2010. For example, estimated glyphosate use positively predicts affective (i.e., mood) disorders from 2001 to 2006, but the estimate for mood disorders turns negative during 2007 to 2010. Estimated glyphosate use does not significantly predict psychotic disorders early in the decade but then does in the period defined in the current study, 2007 to 2010. ADHD was not a recorded discharge in HCUPNET until 2007, although data is available for national all-listed ADHD hospital discharges. These changes suggest some sort of change in glyphosate use in agriculture, and the inability of estimated glyphosate use to predict EPSP synthase inhibitor resistance events in the latter part of the decade, whereas it had in the earlier part of the decade, is evidence of a formulaic inconsistency [65].
Egamberdiyeva et al. [66] have shown that adding nitrification inhibitors such as oxalates inhibits net nitrification in calcareous soils. Furthermore, Wan et al. [67] demonstrated that ammonia-oxidizing archaea (AOA) were more responsive to a simazine herbicide application to agricultural soils. Denitrifying bacterial populations play a critical role in nitrous oxide emissions from amended soil [68]. Therefore, increasing use of herbicides and adding nitrification inhibitors like oxalates to fertilization programs in the United States would theoretically result in an attenuation of the nitrification process that is the conventionally regarded rate-limiting reaction contributing to N2O release from agricultural soils [69].
However, this inhibition of nitrification could potentially lead to nitrogen mismanagement in the soil, including an accumulation and potential release of soil ammonia (NH3) after urea-based fertilizer application [70]. Compared to ammonia-oxidizing (AO) bacteria (AOB), AOA strains have a much higher substrate affinity for ammonia and oxygen and can therefore thrive in the crust of low nutrient, microaerophilic soils with varying ammonia concentrations [71–73]. The growth of archaea in agricultural soils could, therefore, reasonably serve to remediate soil from intensive agricultural herbicide and fertilization programs worldwide. Microbes, including the documented emergence of archaeal strains in 2007, have been shown to be integral decomposers of residual inputs and biosolids in corn agroecosystems [74–76]. In this process, these archaeal strains are also known to be very potent contributors to soil N2O emissions and may be able to contribute even amid the presence of any nitrification inhibition [77], indicating a widespread genomic adaptability distinct from AOB populations [78–80]. It has also been suggested that N2O is emitted as a spontaneous metabolic AO intermediate [77]. Recent research reveals that ammonia-oxidizing archaea are underestimated contributors to N2O emissions in soil [81], and this discovery is consistent with changing glyphosate dynamics.
N2O and ADHD
N2O has been used as an anesthetic agent in health care settings, primarily dentistry. However, there is a growing reappraisal of the compound’s potential adverse health effects. We propose that N2O may be a potential nexus between estimated glyphosate use and increasing all-listed ADHD hospital discharges, especially during the period of study.
Yagiela [82] explains that many of the adverse effects seen with N2O inhalation are attributable to its reaction with the reduced form of vitamin B12 in the body [83]. Vitamin B12, or cobalamin, is a critical co-factor for methionine synthase, an enzyme necessary for nucleic acid synthesis and methylation reactions. Exposure of infants to N2O during surgery resulted in a significant increase in plasma levels of homocysteine, due to an impaired ability to convert it to methionine [84].
Methionine synthase inactivity interferes with methylation of the homocysteine subunit of the dopamine receptor 4 [85], leading to an inactivation of dopamine-induced phospholipid methylation (PLM) through dopamine receptor 4 (DR4). NE has been shown to activate DR4 in rat lateral habenula [86], so it is plausible for there to be excess NE amid DR4 inactivation. Another mode of action of N2O is thought to be inhibition of NMDA receptors [87,88], which has been implicated in ADHD [89]. Since the United States Food and Drug Administration has recently associated ADHD medications with adverse symptoms of psychosis [90], we propose that environmental exposure to N2O –likely from a potent reshaping of soil and water dynamics, especially in urban areas, attributable to intensive agricultural operations, principally including reliance on glyphosate–is the mechanism behind the positive association between glyphosate and all-listed ADHD and psychotic disorders during the period 2007 to 2010.
N2O and Autism Spectrum Disorders (ASD)
Musser et al. [91] have shown a strong familial transmission of autism spectrum disorder (ASD) and ADHD. Mothers who have ADHD have a significantly higher risk of having a child with ADHD or ASD. Furthermore, Hawi et al. [92] demonstrated a preferential paternal transmission of several ADHD risk genes, and N2O has been implicated in causing genetic damage in occupational workers [93]. This data suggests an etiological link between ADHD and ASD, and we propose that link could be inhalational N2O exposure.
This increase has been mitigated, at least in part, by large-scale fossil fuel replacement efforts. However, Nobel Prize laureate Crutzen and others [94] have noted that increasing N2O from agro-biofuel activities may counter the savings seen from fossil fuel replacement strategies, and this revealing finding did not thoroughly consider mode and effect of pesticide delivery in agricultural systems. The fixed effects analyses and the theoretical implications surrounding it suggest that these data ought to be considered when exploring total agricultural N2O emission burden.
In rats, gestational N2O exposure has been shown to affect growth and reflex responses in pups between postnatal days 14 and 21 [95] as well as body laterality in cultured rat embryos [96], and these are consistent phenotypes found in subjects with high functioning autism and Asperger’s syndrome [97,98]. It has been shown that cardiac parasympathetic activation is dependent upon nitric oxide (NO) synthesis [99], and higher NO production is associated with ASD [100], suggesting parasympathetic dominance in ASD. Inhalational nitrous oxide exposure in human subjects contributed to a suppression in sympathetic cardiac activity [101]. Aerobic exercise, a marker of sympathetic activity, for at least 30 minutes three times a week among pregnant women induced a significantly lower autonomic fetal heart rate (HR) and greater heart rate variability (HRV) and this continued in infancy [102,103], suggesting that parasympathetic domination in utero, perhaps attributable to chronic inhalational N2O exposure during fetal organogenesis, may predispose countering effects that constitute the baseline physiology seen in ASD. Toichi and Kamio [104] found parasympathetic activity remained activated in autistic subjects during a mental task. Similarly, Casanova et al. [105] saw a significant increase in cardiac vagal control in autistic subjects treated with low-frequency (LF) repetitive transcranial magnetic stimulation (rTMS). Daluwatte et al. [106] demonstrated constricted pupillary light reflexes (PLR) in ASD subjects, suggesting a lowered parasympathetic modulation during sensory stimulation. It should also be mentioned that heavy metal exposures may exacerbate parasympathetic dominant states [107,108]; interestingly, it may be the case that levels of these heavy metals may be elevated in ASD subjects out of a consequence of this heightened parasympathetic state induced in utero. In other words, the ASD phenotype, through reduced baseline sympathetic activation, may be better able to tolerate higher endogenous levels of such toxins [109], whereas sympathetic activation in non-ASD subjects may aggravate the toxicity of heavy metal exposures [110,111]. The current N2O hypothesis therefore supports the notion of autonomic dysregulation in ASD and suggests that the origin of such dysregulation may occur during gestation, although more comprehensive intergenerational research is needed.
It is likely that the sympathetic hyperarousal seen in ASD is the physiological manifestation to contain this baseline parasympathetic domination arising from N2O exposure in utero and any other xenobiotic exposure after birth. That is to say that many of the behavioral phenotypes that are noted in ASD, including anxiety, hyperactivity, stereotypical compulsivity and compromised social interactions, may arise out of a compensating, homeostatic preference to curtail the dominating parasympathetic influences and may not be explicitly indicative of any genuine etiological link to the disorder. A few animal studies on the effect of prenatal N2O exposure indicate behavioral differences, although care should be taken to avoid direct human ASD comparisons [112,113].
It is advised that policy makers pass greater funding measures to institute sustained and comprehensive N2O monitoring. Aside from its implications as a potent greenhouse gas, we have presented limited existing evidence, using animal and human models, on the complementarity between the fetal/infant health effects from inhalational N2O exposure in utero and ASD. We note that N2O monitoring from agricultural sources discontinued in 2009 at the EIA. Moreover, there is a worrisome lack of data on N2O emissions from bodies of water, even though government agencies like the United States Geological Survey and others have pointed out the potential significance of such sources in overall environmental N2O burden. Emerging ASD advocacy points to a wandering desire to seek bodies of water in autistic subjects, and this may arise from a low-grade addictive phenotype to N2O developed in utero [114]. Future epidemiological studies must consider the influence of exposure to large bodies of water and risk of ADHD and/or autism, and case studies may be needed to ascertain whether a preference for bodies of water in autistic subjects is a significant underlying feature in ASD or not. As mentioned, studies on soil science need be conducted to clarify the emerging role of archaeal strains in agriculture soils and how modern agricultural practices might be contributing to their emergence. Furthermore, data from HCUPNET suggest that adult ADHD is gaining share as a percentage of total ADHD hospital discharges and given our conjecturing here and the findings of others, this may forewarn a greater increase in ASD is still yet to come.
Study Limitations
There exist several limitations to our study. These limitations include, principally, the data itself. We were limited in the number of states we could include due to HCUPNET availability and the availability of regressor data, although there is strong agreement between data sources used in this study. Normalized estimated lagged glyphosate use does positively predict both national (non-normalized CDC all-listed ADHD hospital discharges: 1993–2005, OLS) and state-level (HCUPNET normalized state-level estimates: 2007–2010, two-way fixed effects) all-listed ADHD hospital discharges. Between 2007 and 2010, national all-listed ADHD hospital discharges in HCUPNET, as a percent of total all-listed mental health discharges increased 18.5%, while the average cross-sectional increase across available HCUPNET states between 2007 and 2010 was more modest at 9.6%, suggesting our findings may be conservative. Furthermore, the USGS data on estimated glyphosate use is normalized according to harvested agricultural acreage within a county and does not overtly account for non-farm use. It is possible that non-farm use of glyphosate and nitrogen fertilizers could account for some of the increase in ADHD hospital discharges, as our data cannot exclude this as a possibility. The USGS report indicates that the ratio of non-farm to total nitrogen input estimates from 1987 to 2006 was higher in several states reporting large increases in all-listed ADHD hospital discharges from 2007 to 2010 (i.e., New Hampshire, Massachusetts, and Rhode Island). Our data, however, suggest that agricultural use of glyphosate is a significant predictor of all-listed ADHD hospital discharges in HCUPNET reporting states from 2007 to 2010, even after controlling for state fixed effects, strong correlations over time, and other significant associations with ADHD. While we have shown that glyphosate herbicide resistance events and farm use of nitrogen fertilizers are strongly associated with estimated glyphosate use, neither phenomenon can fully explain the increase in all-listed ADHD hospital discharges in our time period of study.
A second limitation of our study is the lack of genetic markers in our model. Genetic risk factors are being studied for ADHD disorders, in addition to other mental disorders. More specifically, LaHoste et al. [115] have implicated genetic polymorphisms within the DR4 gene as a potential contributor to ADHD, and there are many other studies that point to a genetic predisposition for substance and alcohol addiction [116,117]. In our analysis of national HCUPNET hospitalization data, we find that, as a percentage of total discharges for all mental disorders, discharges for substance/alcohol abuse have actually plateaued (8.03 percent in 2007 to 7.38 percent in 2010 for substance abuse and 8.36 percent in 2007 to 7.66 percent in 2010 for alcohol-related disorders), suggesting that genetic polymorphisms contributing to these conditions have remained static in the health-care seeking population. A significant increase in genetic polymorphisms associated with the mental conditions under study is therefore probably not a plausible explanation for the results obtained, although we cannot say unequivocally that this is the case.
A third limitation is that we have not fully considered the interaction of other variables. For example, we have included maize grain in the current study and found that it is a significant predictor of all-listed ADHD hospital discharges even after controlling for glyphosate, suggesting the mechanism could be related to the genetic composition of the corn as discussed earlier, its use as an agricultural residual cover crop, or another interaction yet to be identified. There are other environmental toxicants that have been associated with ADHD, as well, and we have not accounted for these variables in our models [118,119], principally because we were unable to obtain reliable annual state-level estimates for the years of interest. Furthermore, our study period was subject to influence by the worldwide economic recession. As socioeconomic status has been implicated in ADHD [50], it is possible that our variables of interest (i.e., home price index) were not adequate enough in capturing the potential effect of the economic recession, and we find this to be a limitation. It should also be mentioned that, while the ADHD population in this study was based on ICD-9 diagnoses, there have been significant diagnostic improvements and greater awareness of mental conditions like ADHD in recent years. It is therefore difficult to know for certain whether such conditions are actually increasing or are the result of major diagnostic advancements and greater public awareness [120]. This is a significant quandary, especially in light of the economic difficulty of the period [121]. Therefore, attempts should be made to replicate these results using a diverse set of patient populations, diagnostic environments, and economic conditions.
Conclusions
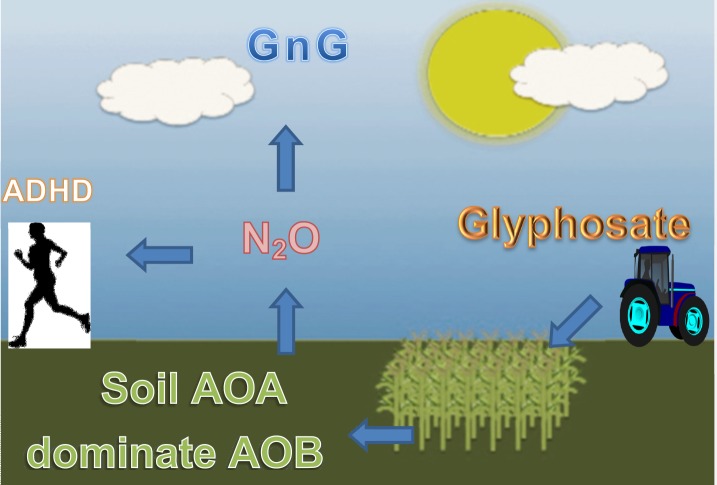
We have shown that agricultural estimated glyphosate use and total food and industrial use of maize grain are significant lagged predictors of all-listed ADHD hospital discharges in HCUPNET reporting states from 2007 to 2010 even after controlling for state fixed effects, strong correlations over time, and other documented associations with ADHD in the literature. The association appears to be most significant in urban counties. Fig 6 is a global schematic that demonstrates the indirect mechanism that may underlie the increase in ADHD seen during our study period.
Fig 6. A global schematic proposing an indirect mechanism for how glyphosate-based herbicides may be contributing to the rise of ADHD during the period, 2007 to 2010.
Abbreviations: N2O –nitrous oxide; GnG–greenhouse gassing; AOB–ammonia-oxidizing bacteria; AOA–ammonia-oxidizing archaea; ADHD–attention-deficit hyperactivity disorder.
These significant associations occur amid the absence of estimated glyphosate use predicting resistance events and marked swings in the ability of estimated glyphosate use to predict other mental health discharges. We propose that a patented reformulation in glyphosate could be enhancing both its herbicidal and bactericidal properties, enabling the dominance of the more genetically adaptable archaea in agricultural soils. Maize residuals may also be used as an herbicide control, and this may be contributing to the increase in direct agricultural N2O emissions, most especially given the changing soil dynamics possibly induced by glyphosate use. Impairment of several physiological mechanisms, including NMDA receptors and methionine synthase, is a likely explanation for how glyphosate may significantly contribute to an increase in all-listed ADHD hospital discharges. The precise mechanisms mentioned here require further empirical support, and this ought to include a systematic reevaluation of the crucial intermediates involved.
Supporting Information
Diagnostics include (A) a histogram of residuals for the panel and (B) residuals plotted against X. Histograms were produced in R using the hist(residual(x)) argument.
(TIF)
Model diagnostics for the metropolitan panel set included in Model 2. Diagnostics include (A) a histogram of residuals and (B) a residual plot against fitted values. Histograms were produced in R using the hist(residual(x)) argument. Residual plots were created in R by subtracting residuals from the observed ADHD percent and plotting these fitted values (yhat) against model residuals. The state of Rhode Island was an extreme outlier in S2 Fig and was removed from analysis.
(TIF)
Model diagnostics for the fringe metropolitan panel set included in Model 2. Diagnostics include (A) a histogram of residuals and (B) a residual plot against fitted values. Histograms were produced in R using the hist(residual(x)) argument. Residual plots were created in R by subtracting residuals from the observed ADHD percent and plotting these fitted values (yhat) against model residuals.
(TIF)
Model diagnostics for the urban panel set included in Model 2. Diagnostics include (A) a histogram of residuals and (B) a residual plot against fitted values. Histograms were produced in R using the hist(residual(x)) argument. Residual plots were created in R by subtracting residuals from the observed ADHD percent and plotting these fitted values (yhat) against model residuals.
(TIF)
Model diagnostics for the rural panel set included in Model 2. Diagnostics include (A) a histogram of residuals and (B) a residual plot against fitted values. Histograms were produced in R using the hist(residual(x)) argument. Residual plots were created in R by subtracting residuals from the observed ADHD percent and plotting these fitted values (yhat) against model residuals.
(TIF)
Model diagnostics for the fringe metropolitan panel set included in Model 3 using second-order stationary data. Diagnostics include (A) a histogram of residuals and (B) a residual plot against fitted values. Histograms were produced in R using the hist(residual(x)) argument. Residual plots were created in R by subtracting residuals from the observed ADHD percent and plotting these fitted values (yhat) against model residuals.
(TIF)
Model diagnostics for the urban panel set included in Model 3 using second-order stationary data. Diagnostics include (A) a histogram of residuals and (B) a residual plot against fitted values. Histograms were produced in R using the hist(residual(x)) argument. Residual plots were created in R by subtracting residuals from the observed ADHD percent and plotting these fitted values (yhat) against model residuals.
(TIF)
(DOCX)
Acknowledgments
The second author was supported by HL007567-29 (T32) from the National Heart, Lung and Blood Institute. The authors thank Stephanie Seneff for her edits and thoughtful discussions. The authors are also extremely grateful to two anonymous reviewers for their helpful comments and suggestions which significantly improved the final manuscript.
Data Availability
Glyphosate data is available from the United States Geological Survey (USGS). All mental health discharge data are available at (HCUPnet). The links to access these public data are contained within the manuscript.
Funding Statement
This research was supported by the NIH National Heart, Lung, and Blood Institute (Grants T32HL007567 and HL007567-29 (T32)).
References
- 1. Visser SN, Bitsko RH, Danielson ML, Perou R, Blumberg SJ. Increasing Prevalence of Parent-Reported Attention-Deficit/Hyperactivity Disorder Among Children—United States, 2003 and 2007. 2010; 59: 1439–1443. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5944a3.htm?s_cid=mm5944a3_w. Accessed October 2014. 21063274 [Google Scholar]
- 2.Centers for Disease Control and Prevention. Attention-Deficit/Hyperactivity Disorder: Symptoms and Diagnosis. Available: http://www.cdc.gov/ncbddd/adhd/diagnosis.html. Accessed 11 April 2015.
- 3. Sinzig J, Walter D, Doepfner M. Attention deficit/hyperactivity disorder in children and adolescents with autism spectrum disorder: symptom or syndrome? J Atten Disord. 2009; 13:117–26. doi: 10.1177/1087054708326261 [DOI] [PubMed] [Google Scholar]
- 4. Yerys BE, Wallace GL, Sokoloff JL, Shook DA, James JD, Kenworthy L. Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Res. 2009; 2:322–33. doi: 10.1002/aur.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Happé F, Booth R, Charlton R, Hughes C. Executive function deficits in autism spectrum disorders and attention-deficit/hyperactivity disorder: examining profiles across domains and ages. Brain Cogn. 2006; 61:25–39. [DOI] [PubMed] [Google Scholar]
- 6. Nydén A, Niklasson L, Stahlberg O, Anckarsater H, Wentz E, Rastam M, et al. Adults with autism spectrum disorders and ADHD neuropsychological aspects. Res Dev Disabil. 2010; 31:1659–68. doi: 10.1016/j.ridd.2010.04.010 [DOI] [PubMed] [Google Scholar]
- 7. Sokolova E, Hoogman M, Groot P, Claassen T, Vasquez AA, Buitelaar JK, et al. Causal discovery in an adult ADHD data set suggests indirect link between DAT1 genetic variants and striatal brain activation during reward processing. Am J Med Genet B Neuropsychiatr Genet. 2015. doi: 10.1002/ajmg.b.32310 [DOI] [PubMed] [Google Scholar]
- 8. Stergiakouli E, Martin J, Hamshere ML, Langley K, Evans DM, St Pourcain B, et al. Shared genetic influences between attention-deficit/hyperactivity disorder (ADHD) traits in children and clinical ADHD. J Am Acad Child Adolesc Psychiatry. 2015; 54: 322–7. doi: 10.1016/j.jaac.2015.01.010 Epub 2015 Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu Y, Chen XT, Luo M, Tang Y, Zhang G, Wu D, et al. Multiple epigenetic factors predict the attention deficit/hyperactivity disorder among the Chinese Han children. J Psychiatr Res. 2015. doi: 10.1016/j.jpsychires.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 10.Padrón A, Galán I, García-Esquinas E, Fernández E, Ballbè M, Rodríguez-Artalejo F. Exposure to secondhand smoke in the home and mental health in children: a population-based study. Tob Control. 2015. doi: 10.1136/tobaccocontrol-2014-052077 [DOI] [PubMed]
- 11. Max W, Sung HY, Shi Y. Attention deficit hyperactivity disorder among children exposed to secondhand smoke: a logistic regression analysis of secondary data. Int J Nurs Stud. 2013; 50: 797–806. doi: 10.1016/j.ijnurstu.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 12. Ahmed J, Agaku IT, O'Connor E, King BA, Kenemer JB, Neff L. Current Cigarette Smoking Among Adults—United States, 2005–2013. Morbidity and Mortality Weekly Report (MMWR). 2014; 63:1108–1112. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6347a4.htm?s_cid=mm6347a4_w. Accessed 12 April 2015. [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Environmental Protection Agency (EPA). CADDIS Volume 2: Sources, Stressors & Responses. Herbicides. Available: http://www.epa.gov/caddis/ssr_herb_int.html. Accessed October 2014.
- 14. Bøhn T, Cuhra M, Traavik T, Sanden M, Fagan J, Primicerio R. Compositional differences in soybeans on the market: glyphosate accumulates in Roundup Ready GM soybeans. Food Chem. 2014; 15: 207–15. [DOI] [PubMed] [Google Scholar]
- 15. Cox WJ, Hanchar J, Shields E. Stacked corn hybrids show inconsistent yield and economic responses in New York. Agronomy Journal. 2009; 101: 1530–37. [Google Scholar]
- 16.U.S. Department of Agriculture. Adoption of genetically engineered crops in the U.S. Available: http://www.ers.usda.gov/data-products/adoption-of-genetically-engineered-crops-in-the-us.aspx. Accessed 23 October 2014.
- 17.Ohno M, Suganuma M, Egawa Y, Tomimoto K, Hara T, Hayakawa T, et al. A comparative study of histamine release from rat mast cells by Cry1Aa, Cry1Ab and Cry1Ac fragmented with simulated gastric fluid (SGF). 6th Pacific Rim Conference on the Biotechnology of Bacillus Thuringiensis and its Environmental Impact. 2005.
- 18. Moreno E, Moreno-Delgado D, Navarro G, Hoffmann HM, Fuentes S, Rosell-Vilar S, et al. Cocaine disrupts histamine H3 receptor modulation of dopamine D1 receptor signaling: σ1-D1-H3 receptor complexes as key targets for reducing cocaine's effects. J Neurosci. 2014; 34:3545–58. doi: 10.1523/JNEUROSCI.4147-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dang LC, O'Neil JP, Jagust WJ. Dopamine supports coupling of attention-related networks. J Neurosci. 2012; 32:9582–7. doi: 10.1523/JNEUROSCI.0909-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hietanen E, Linnainmaa K, Vainiok H. Effects of phenoxyherbicides and glyphosate on the hepatic and intestinal biotransformation activities in the rat. Acta Pharmacol. et Toxicol. 1983; 53: 103–112. [DOI] [PubMed] [Google Scholar]
- 21. Tieu EW, Li W, Chen J, Kim TK, Ma D, Slominski AT, et al. Metabolism of 20-hydroxyvitamin D3 and 20,23-dihydroxyvitamin D3 by rat and human CYP24A1. J Steroid Biochem Mol Biol. 2015149:153–65. doi: 10.1016/j.jsbmb.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quist JF, Barr CL, Schachar R, Roberts W, Malone M, Tannock R, et al. Evidence for the serotonin HTR2A receptor gene as a susceptibility factor in attention deficit hyperactivity disorder (ADHD). Molecular Psychiatry 2000; 5: 537–541. [DOI] [PubMed] [Google Scholar]
- 23. Kent L, Doerry U, Hardy E, Parmar R, Gingell K, Hawi Z, et al. Evidence that variation at the serotonin transporter gene influences susceptibility to attention deficit hyperactivity disorder (ADHD): analysis and pooled analysis. Molecular Psychiatry 2002; 7: 908–912. [DOI] [PubMed] [Google Scholar]
- 24. Kremer RJ, Means NE. Glyphosate and glyphosate-resistant crop interactions with rhizosphere microorganisms. European Journal of Agronomy. 2009; 31: 153–161 [Google Scholar]
- 25. Zablotowicz RM, Reddy KN. Nitrogenase activity, nitrogen content, and yield responses to glyphosate in glyphosate-resistant soybean. International Journal of BioMedicine. 2014; 4: 155–158. [Google Scholar]
- 26. Koyanagi S, Himukashi S, Mukaida K, Shichino T, Fukuda K. Dopamine D2-like receptor in the nucleus accumbens is involved in the antinociceptive effect of nitrous oxide. Anesth Analg. 2008; 106:1904–9. doi: 10.1213/ane.0b013e318172b15b [DOI] [PubMed] [Google Scholar]
- 27. Abdul-Kareem HS, Sharma RP, Drown DB. Effects of repeated intermittent exposures to nitrous oxide on central neurotransmitters and hepatic methionine synthetase activity in CD-1 mice. Toxicol Ind Health. 1991. 7:97–108. [PubMed] [Google Scholar]
- 28. Jenson D, Yang K, Acevedo-Rodriguez A, Levine A, Broussard JI, Tang J, et al. Dopamine and norepinephrine receptors participate in methylphenidate enhancement of in vivo hippocampal synaptic plasticity. Neuropharmacology. 201590:23–32. doi: 10.1016/j.neuropharm.2014.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McTighe SM, Neal SJ, Lin Q, Hughes ZA, Smith DG. The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex. PLOS One. 2013. 8:e62189 doi: 10.1371/journal.pone.0062189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blanchard DC, Defensor EB, Meyza KZ, Pobbe RLH, Pearson BL, Bolivar VJ, et al. BTBR T+tf/J mice: Autism-relevant behaviors and reduced fractone associated heparan sulfate. Neurosci Biobehav Rev. 2012; 36:285–96. doi: 10.1016/j.neubiorev.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pearson BL, Corley MJ, Vasconcellos A, Blanchard DC, Blanchard RJ. Heparan sulfate deficiency in autistic postmortem brain tissue from the subventricular zone of the lateral ventricles. Behav Brain Res. 2013; 243: 138–145. doi: 10.1016/j.bbr.2012.12.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dalsgaard T, Bak F. Nitrate Reduction in a sulfate-reducing bacterium, Desulfovibrio desulfuricans, isolated from rice paddy soil: Sulfide inhibition, kinetics, and regulation. Appl Environ Microbiol 1994; 60: 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone WW. Estimated Annual Agricultural Pesticide Use for Counties of the Conterminous United States, 1992–2009. 2013. Available: http://pubs.usgs.gov/ds/752/. Accessed September 2014.
- 34.Thelin GP, Stone WW. Estimation of annual agricultural pesticide use for counties of the conterminous United States, 1992–2009: U.S. Geological Survey Scientific Investigations Report 2013–5009. 2013, 54.
- 35. Parker T. Rural Urban Continuum Codes: documentation U.S. Department of Agriculture; 2013. Available: http://www.ers.usda.gov/data-products/rural-urban-continuum-codes/documentation.aspx. Accessed November 2014. [Google Scholar]
- 36.Gronberg JM, Spahr NE. County-level estimates of nitrogen and phosphorus from commercial fertilizer for the Conterminous United States, 1987–2006: U.S. Geological Survey Scientific Investigations Report 2012–5207, 20.
- 37.Conley DM, Nagesh RGA, Salame EJ. Supply and Utilization of Corn in the United States, by State, 2004–2010. 2012. Available: http://ianrpubs.unl.edu/epublic/pages/publicationD.jsp?publicationId=1523. Accessed October 2014.
- 38.U.S. Department of Agriculture (b). Feed Grain Database: Yearbook Tables, Corn: Food, Seed, and Industrial Use, Table 31, Economic Research Service. 2014. Available: http://www.ers.usda.gov/datafiles/Feed_Grains_Yearbook_Tables/All_tables_in_one_file/fgyearbooktablesrecent.pdf.
- 39.U.S. Energy Information Administration (EIA). Emissions of greenhouse gases in the United States 2009. Available: http://www.eia.gov/environment/emissions/ghg_report/pdf/0573%282009%29.pdf. Accessed 3 December 2014.
- 40.Heap I. International Survey of Herbicide Resistance Weeds (ISHRW). 2014. Available: http://www.weedscience.org/Summary/home.aspx. Accessed October 2014 and April 2015.
- 41.Agency for Healthcare Research and Quality: Healthcare Cost and Utilization Project (HCUPNET). Available: http://HCUPnet.ahrq.gov/HCUPNETnet.app/HCUPnet.jsp?Id=0AA6B9DD7BE6D645&Form=DispTab&GoTo=SelLAY&JS=Y. Accessed October 2014.
- 42.Center for Disease Control and Prevention (CDC). Vital and Health Statistics: National Hospital Discharge Surveys for years 1993–2005. Accessed December 22, 2014.
- 43. R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: Available: http://www.R-project.org/. [Google Scholar]
- 44.Hothorn T, Zeileis A, Farebrother RW, Cummins C, Millo G, Mitchell D. Testing Linear Regression Models. 2014. Version 0.9–33.
- 45.Stata Corp LP. 2014. xtunitroot—Panel-data unit-root tests. Available: http://www.stata.com/manuals13/xtxtunitroot.pdf.
- 46.Trapletti A, Hornik K, LeBaron B. Time Series Analysis and Computational Finance. 2015. Version 0.10–34.
- 47. Arellano M. Computing robust standard errors for within-groups estimators. Oxford Bulletin of Economics and Statistics. 1987. 49, 4. [Google Scholar]
- 48. Bihlar Muld B, Jokinen J, Bölte S, Hirvikoski T. Long-term outcomes of pharmacologically treated versus non-treated adults with ADHD and substance use disorder: a naturalistic study. J Subst Abuse Treat. 2014. doi: 10.1016/j.jsat.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 49.The Henry J. Kaiser Family Foundation. 2014. Available: http://kff.org/other/state-indicator/total-hospitals/. Accessed October 2014.
- 50. Russell G, Ford T, Rosenberg R, Kelly S. The association of attention deficit hyperactivity disorder with socioeconomic disadvantage: alternative explanations and evidence. J Child Psychol Psychiatry. 2014; 55:436–45. doi: 10.1111/jcpp.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Davis MA, Heathcote J. The Price and Quantity of Residential Land in the United States. Journal of Monetary Economics. 2007; 54: 2595–2620; data located at Land and Property Values in the U.S., Lincoln Institute of Land Policy Available: http://www.lincolninst.edu/resources/. [Google Scholar]
- 52. Baumgardner DJ, Schreiber AL, Havlena JA, Bridgewater FD, Steber DL, Lemke MA. Geographic analysis of diagnosis of Attention-Deficit/Hyperactivity Disorder in children: Eastern Wisconsin, USA. Int J Psychiatry Med. 2010; 40:363–82. [DOI] [PubMed] [Google Scholar]
- 53.U.S. Census Bureau, Population Division. Table 1. Intercensal Estimates of the Resident Population for the United States, Regions, States, and Puerto Rico: April 1, 2000 to July 1, 2010. Available: http://www.census.gov/popest/data/historical/2000s/index.html. Accessed October 2014.
- 54. Kuo FE, Taylor AF. A potential natural treatment for attention-deficit/hyperactivity disorder: evidence from a national study. Am J Public Health. 2004; 94:1580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.U.S. National Oceanic and Atmospheric Administration, 2014. Available: http://www.ncdc.noaa.gov/cag/. Accessed October 2014.
- 56. Heimlich RE, Barnard CH. Agricultural adaptation to urbanization: farm types in northeast metropolitan areas. Northeastern Journal of Agricultural and Resource Economics. 1992; 21: 50–60. [Google Scholar]
- 57.U.S. Environmental Protection Agency. 2014. Overview of greenhouse gases: nitrous oxide emissions. Available: http://epa.gov/climatechange/ghgemissions/gases/n2o.html. Accessed October 2014.
- 58.Hofstrand D. Crop residue—a valuable resource. 2009. AgMRC Renewable Energy Newsletter. Available: http://www.agmrc.org/renewable_energy/ethanol/crop-residue-a-valuable-resource/.
- 59. Beaulieu JJ, Tank JL, Hamilton SK, Wollheim WM, Hall RO Jr, Mulholland PJ, et al. Nitrous oxide emission from denitrification in stream and river networks. Proceedings of the National Academy of Sciences of the United States of America. 2011; 108: 214–19. doi: 10.1073/pnas.1011464108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Outram FN, Hiscock KM. Indirect nitrous oxide emissions from surface water bodies in a lowland arable catchment: A significant contribution to agricultural greenhouse gas budgets? Environ. Sci. Technol. 2012; 46: 8156–8163. doi: 10.1021/es3012244 [DOI] [PubMed] [Google Scholar]
- 61.IHER. 2015. State-level averages on cumulative herbicide resistance events since 2000. Available: www.iher.org.
- 62.Hahn RR. Glyphosate-Resistant Weeds Likely in NY. 2014. Available: http://blogs.cornell.edu/whatscroppingup/2014/12/05/glyphosate-resistant-weeds-likely-in-ny/. Accessed 17 December 2014.
- 63. Cromartie J. Population and migration: recent population change 2014. U.S. Department of Agriculture Economic Research Service; Available: http://www.ers.usda.gov/topics/rural-economy-population/population-migration/recent-population-change.aspx. Accessed October 2014. [Google Scholar]
- 64. Eker S, Ozturk L, Yazici A, Erenoglu B, Romheld V, Cakmak I. Foliar-applied glyphosate substantially reduced uptake and transport of iron and manganese in sunflower (Helianthus annuus L.) plants. J Agric Food Chem. 2006; 54:10019–25. [DOI] [PubMed] [Google Scholar]
- 65.Abraham W. Glyphosate Formulations and Their Use for the Inhibition of 5-enolpyruvylshikimate-3-phosphate Synthase. Monsanto Technology Llc, assignee. Patent US7771736 B2. 10 Aug. 2010. Print.
- 66. Egamberdiyeva D, Mamiev M, Poberejskaya SK. The influence of mineral fertilizer combined with a nitrification inhibitor on microbial populations and activities in calcareous Uzbekistanian soil under cotton cultivation. Scientific World Journal. 2001; 1: 108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wan R, Wang Z, Xie S. Dynamics of communities of bacteria and ammonia-oxidizing microorganisms in response to simazine attenuation in agricultural soil. Sci Total Environ. 2014; 15: 502–8. doi: 10.1016/j.scitotenv.2013.11.090 [DOI] [PubMed] [Google Scholar]
- 68. Maeda K, Toyoda S, Hanajima D, Yoshida N. Denitrifiers in the surface zone are primarily responsible for the nitrous oxide emission of dairy manure compost. J Hazard Mater. 2013; 248–49: 329–36. [DOI] [PubMed] [Google Scholar]
- 69. Kyaw KM, Toyota K. Suppression of nitrous oxide production by the herbicides glyphosate and propanil in soils supplied with organic matter. Soil Science & Plant Nutrition. 2007; 53: 441–447. [Google Scholar]
- 70. Kim DG, Saggar S, Roudier P. The effect of nitrification inhibitors on soil ammonia emissions in nitrogen managed soils: a meta-analysis. Nutrient Cycling in Agroecosystems. 2012. doi: 10.1007/s10705-012-9498-9 [Google Scholar]
- 71. Kim JG, Jung MY, Park SJ, Rijpstra WI, Sinninghe Damsté JS, Madsen EL, et al. Cultivation of a highly enriched ammonia-oxidizing archaeon of thaumarchaeotal group I.1b from an agricultural soil. Environ Microbiol. 2012; 14: 1528–43. doi: 10.1111/j.1462-2920.2012.02740.x [DOI] [PubMed] [Google Scholar]
- 72. Verhamme DT, Prosser JI, Nicol GW. Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. The ISME Journal. 2011; 5: 1067–1071. doi: 10.1038/ismej.2010.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stieglmeier M, Mooshammer M, Kitzler B, Wanek W, Zechmeister-Boltenstern S, Richter A, et al. Aerobic nitrous oxide production through N-nitrosating hybrid formation in ammonia-oxidizing archaea. ISME J. 2014; 8:1135–46. doi: 10.1038/ismej.2013.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sheibani S, Yanni SF, Wilhem R, Whalen JK, Whyte LG, Greer CW, et al. Soil bacteria and archaea found in long-term corn (Zea mays L.) agroecosystems in Quebec, Canada. Canadian Journal of Soil Science. 2013; 93: 45–57. [Google Scholar]
- 75.Segal L. Ammonia-oxidizing bacteria and archaea under continuous maize: the influence of tillage, N input and aggregation on abundance and community composition. 2014. Dissertation. The Graduate College at the University of Nebraska–Lincoln. Available: http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1079&context=agronhortdiss.
- 76. Kelly JJ, Policht K, Grancharova T, Hundal LS. Distinct responses in ammonia-oxidizing archaea and bacteria after addition of biosolids to an agricultural soil. Appl Environ Microbiol. 2011; 77: 6551–8. doi: 10.1128/AEM.02608-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hatzenpichler R. Diversity, physiology, and niche differentiation of ammonia-oxidizing archaea. Appl Environ Microbiol. 2012; 78: 7501–10. doi: 10.1128/AEM.01960-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Qin W, Amin SA, Martens-Habbena W, Walker CB, Urakawa H, Devol AH, et al. Marine ammonia-oxidizing archaeal isolates display obligate mixotrophy and wide ecotypic variation. Proc Natl Acad Sci USA. 2014; 111: 12504–9 doi: 10.1073/pnas.1324115111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lu L, Jia Z. Urease gene-containing Archaea dominate autotrophic ammonia oxidation in two acid soils. Environ Microbiol. 2013; 15: 1795–809. doi: 10.1111/1462-2920.12071 [DOI] [PubMed] [Google Scholar]
- 80. Daugherty M, Vonstein V, Overbeek R, Osterman A. Archaeal shikimate kinase, a new member of the GHMP-kinase family. J Bacteriol. 2001; 183: 292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jung MY, Well R, Min D, Giesemann A, Park SJ, Kim JG, et al. Isotopic signatures of N2O produced by ammonia-oxidizing archaea from soils. ISME J. 2014; 8: 1115–25. doi: 10.1038/ismej.2013.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yagiela JA. Health hazards and nitrous oxide: a time for reappraisal. Anesth Prog. 1991; 38: 1–11. [PMC free article] [PubMed] [Google Scholar]
- 83. Christensen B, Ueland PM. Methionine synthase inactivation by nitrous oxide during methionine loading of normal human fibroblasts. Homocysteine remethylation as determinant of enzyme inactivation and homocysteine export. J Pharmacol Exp Ther. 1993; 267: 1298–303. [PubMed] [Google Scholar]
- 84. Pichardo D, Luginbuehl IA, Shakur Y, Wales PW, El-Sohemy A, O'Connor DL. Effect of nitrous oxide exposure during surgery on the homocysteine concentrations of children. Anesthesiology. 2012; 117:15–21. doi: 10.1097/ALN.0b013e318259a8cc [DOI] [PubMed] [Google Scholar]
- 85. Sharma A, Kramer ML, Wick PF, Liu D, Chari S, Shim S, et al. D4 dopamine receptor-mediated phospholipid methylation and its implications for mental illnesses such as schizophrenia. Mol Psychiatry. 1999; 4: 235–46. [DOI] [PubMed] [Google Scholar]
- 86. Root DH, Hoffman AF, Good CH, Zhang S, Gigante E, Lupica CR, et al. Norepinephrine activates dopamine D4 receptors in the rat lateral habenula. J Neurosci. 2015. 25:3460–9. doi: 10.1523/JNEUROSCI.4525-13.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mennerick S, Jevtovic-Todorovic V, Todorovic SM, Shen W, Olney JW, Zorumski CF. Effect of nitrous oxide on excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci 1998; 18: 9716–9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nagele P, Metz LB, Crowder CM. Nitrous oxide (N2O) requires the N-methyl-D aspartate receptor for its action in Caenorhabditis elegans. PNAS 2004; 101: 8791–8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lehohla M, Kellaway L, Russell VA. NMDA receptor function in the prefrontal cortex of a rat model for attention-deficit hyperactivity disorder. Metab Brain Dis. 2004; 19: 35–42. [DOI] [PubMed] [Google Scholar]
- 90. Schizophrenia.com. FDA warns about ADHD medication connection to psychosis and cardiovascular events. March 21, 2007. Available: http://www.schizophrenia.com/sznews/archives/004802.html#. Accessed 23 December 2014.
- 91. Musser ED, Hawkey E, Kachan-Liu SS, Lees P, Roullet JB, Goddard K, et al. Shared familial transmission of autism spectrum and attention-deficit/hyperactivity disorders. J Child Psychol Psychiatry. 2014. 55:819–27. doi: 10.1111/jcpp.12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hawi Z, Segurado R, Conroy J, Sheehan K, Lowe N, Kirley A, et al. Preferential transmission of paternal alleles at risk genes in attention-deficit/hyperactivity disorder. Am J Hum Genet. 2005. 77: 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wrońska-Nofer T, Palus J, Krajewski W, Jajte J, Kucharska M, Stetkiewicz J, et al. DNA damage induced by nitrous oxide: study in medical personnel of operating rooms. Mutat Res. 2009. 666:39–43. doi: 10.1016/j.mrfmmm.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 94. Crutzen PJ, Mosier AR, Smith KA, Winiwarter W. N2O release from agro-biofuel production negates global warming reduction by replacing fossil fuels. Atmos. Chem. Phys. 2008. 8:389–395. [Google Scholar]
- 95. Tassinari MS, Mullenix PJ, Moore PA. The effects of nitrous oxide after exposure during middle and late gestation. Toxicol Ind Health. 1986. 2:261–71. [DOI] [PubMed] [Google Scholar]
- 96. Baden JM, Fujinaga M. Effects of nitrous oxide on day 9 rat embryos grown in culture. Br J Anaesth. 199166:500–3. [DOI] [PubMed] [Google Scholar]
- 97. Perkins TJ, Stokes MA, McGillivray JA, Mussap AJ, Cox IA, Maller JJ, et al. Increased left hemisphere impairment in high-functioning autism: a tract based spatial statistics study. Psychiatry Res. 2014. 30:119–23. doi: 10.1016/j.pscychresns.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 98. Dissanayake C, Bui QM, Huggins R, Loesch DZ. Growth in stature and head circumference in high-functioning autism and Asperger disorder during the first 3 years of life. Dev Psychopathol. 2006. 18:381–93. [DOI] [PubMed] [Google Scholar]
- 99. Chowdhary S, Ng GA, Nuttall SL, Coote JH, Ross HF, Townend JN. Nitric oxide and cardiac parasympathetic control in human heart failure. Clin Sci (Lond). 2002. 102:397–402. [PubMed] [Google Scholar]
- 100. Sweeten TL, Posey DJ, Shankar S, McDougle CJ. High nitric oxide production in autistic disorder: a possible role for interferon-gamma. Biol Psychiatry. 2004. 15:434–7. [DOI] [PubMed] [Google Scholar]
- 101. Okushima K, Kohjitani A, Asano Y, Sugiyama K. Inhalational conscious sedation with nitrous oxide enhances the cardiac parasympathetic component of heart rate variability. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008. 106:e1–5. doi: 10.1016/j.tripleo.2008.08.020 [DOI] [PubMed] [Google Scholar]
- 102. May LE, Glaros A, Yeh HW, Clapp JF 3rd, Gustafson KM. Aerobic exercise during pregnancy influences fetal cardiac autonomic control of heart rate and heart rate variability. Early Hum Dev. 2010. 86:213–7. doi: 10.1016/j.earlhumdev.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 103. May LE, Scholtz SA, Suminski R, Gustafson KM. Aerobic exercise during pregnancy influences infant heart rate variability at one month of age. Early Hum Dev. 2014. 90:33–8. doi: 10.1016/j.earlhumdev.2013.11.001 Epub 2013 Nov 25. [DOI] [PubMed] [Google Scholar]
- 104. Toichi M, Kamio Y. Paradoxical autonomic response to mental tasks in autism. J Autism Dev Disord. 2003. 33:417–26. [DOI] [PubMed] [Google Scholar]
- 105. Casanova MF, Hensley MK, Sokhadze EM, El-Baz AS, Wang Y, Li X, et al. Effects of weekly low-frequency rTMS on autonomic measures in children with autism spectrum disorder. Front Hum Neurosci. 2014. 21; 8:851 doi: 10.3389/fnhum.2014.00851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Daluwatte C, Miles JH, Sun J, Yao G. Association between pupillary light reflex and sensory behaviors in children with autism spectrum disorders. Res Dev Disabil. 2014. 16; 209–215. doi: 10.1016/j.ridd.2014.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. He SC, Qiao N, Sheng W. Neurobehavioral, autonomic nervous function and lymphocyte subsets among aluminum electrolytic workers. Int J Immunopathol Pharmacol. 2003. 16:139–44. [DOI] [PubMed] [Google Scholar]
- 108. Valera B, Dewailly E, Poirier P, Counil E, Suhas E. Influence of mercury exposure on blood pressure, resting heart rate and heart rate variability in French Polynesians: a cross-sectional study. Environ Health. 2011. 13:99 doi: 10.1186/1476-069X-10-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Blaurock-Busch E, Amin OR, Rabah T. Heavy Metals and Trace Elements in Hair and Urine of a Sample of Arab Children with Autistic Spectrum Disorder Maedica (Buchar). 2011. 6: 247–257. [PMC free article] [PubMed] [Google Scholar]
- 110. Zhang JH, Hu CW, Zhu YZ, Liu SM, Bai CS, Han YF, et al. Effects of norepinephrine on immune functions of cultured splenic lymphocytes exposed to aluminum trichloride. Biol Trace Elem Res. 2013. 154:275–80. doi: 10.1007/s12011-013-9729-1 [DOI] [PubMed] [Google Scholar]
- 111. Yeter D, Deth R, Kuo H-C. Mercury promotes catecholamines which potentiate mercurial autoimmunity and vasodilation: implications for inositol 1,4,5-triphosphate 3-kinase C susceptibility in Kawasaki Syndrome. Korean Circulation Journal. 2013. 43:581–591. doi: 10.4070/kcj.2013.43.9.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mullenix PJ, Moore PA, Tassinari MS. Behavioral toxicity of nitrous oxide in rats following prenatal exposure. Toxicol Ind Health. 1986. 2:273–87. [DOI] [PubMed] [Google Scholar]
- 113. Rice SA. Effect of prenatal N2O exposure on startle reflex reactivity. Teratology. 1990. 42:373–81. [DOI] [PubMed] [Google Scholar]
- 114.Autism Speaks. 2013. Available: http://www.autismspeaks.org/sites/default/files/docs/missing_children_with_special_needs.pdf. Accessed January 2015.
- 115. LaHoste GJ, Swanson JM, Wigal SB, Glabe C, Wigal T, King N, et al. Dopamine D4 receptor gene polymorphism is associated with attention deficit hyperactivity disorder. Mol Psychiatry. 1996; 1: 121–4. [PubMed] [Google Scholar]
- 116. Desrivières S, Lourdusamy A, Müller C, Ducci F, Wong CP, Kaakinen M, et al. Glucocorticoid receptor (NR3C1) gene polymorphisms and onset of alcohol abuse in adolescents Addict Biol. 2011; 16: 510–3. doi: 10.1111/j.1369-1600.2010.00239.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Palmer RH, Brick L, Nugent NR, Bidwell LC, McGeary JE, Knopik VS, et al. Examining the role of common genetic variants on alcohol, tobacco, cannabis, and illicit drug dependence. Addiction. 2014. doi: 10.1111/add.12815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kim BN, Cho SC, Kim Y, Shin MS, Yoo HJ, Kim JW, et al. Phthalates exposure and attention-deficit/hyperactivity disorder in school-age children. Biol Psychiatry. 2009; 15: 958–63. [DOI] [PubMed] [Google Scholar]
- 119. Liu W, Huo X, Liu D, Zeng X, Zhang Y, Xu X. S100β in heavy metal-related child attention-deficit hyperactivity disorder in an informal e-waste recycling area. Neurotoxicology. 2014; 45: 185–91. doi: 10.1016/j.neuro.2014.10.013 [DOI] [PubMed] [Google Scholar]
- 120. Geltman PL, Fried LE, Arsenault LN, Knowles AM, Link DA, Goldstein JN, et al. A Planned Care Approach and Patient Registry to Improve Adherence to Clinical Guidelines for the Diagnosis and Management of Attention-Deficit/Hyperactivity Disorder. Acad Pediatr. 2015. 15:289–96. doi: 10.1016/j.acap.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 121. Simou E, Koutsogeorgou E. Effects of the economic crisis on health and healthcare in Greece in the literature from 2009 to 2013: a systematic review. Health Policy. 2014115:111–9. doi: 10.1016/j.healthpol.2014.02.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diagnostics include (A) a histogram of residuals for the panel and (B) residuals plotted against X. Histograms were produced in R using the hist(residual(x)) argument.
(TIF)
Model diagnostics for the metropolitan panel set included in Model 2. Diagnostics include (A) a histogram of residuals and (B) a residual plot against fitted values. Histograms were produced in R using the hist(residual(x)) argument. Residual plots were created in R by subtracting residuals from the observed ADHD percent and plotting these fitted values (yhat) against model residuals. The state of Rhode Island was an extreme outlier in S2 Fig and was removed from analysis.
(TIF)
Model diagnostics for the fringe metropolitan panel set included in Model 2. Diagnostics include (A) a histogram of residuals and (B) a residual plot against fitted values. Histograms were produced in R using the hist(residual(x)) argument. Residual plots were created in R by subtracting residuals from the observed ADHD percent and plotting these fitted values (yhat) against model residuals.
(TIF)
Model diagnostics for the urban panel set included in Model 2. Diagnostics include (A) a histogram of residuals and (B) a residual plot against fitted values. Histograms were produced in R using the hist(residual(x)) argument. Residual plots were created in R by subtracting residuals from the observed ADHD percent and plotting these fitted values (yhat) against model residuals.
(TIF)
Model diagnostics for the rural panel set included in Model 2. Diagnostics include (A) a histogram of residuals and (B) a residual plot against fitted values. Histograms were produced in R using the hist(residual(x)) argument. Residual plots were created in R by subtracting residuals from the observed ADHD percent and plotting these fitted values (yhat) against model residuals.
(TIF)
Model diagnostics for the fringe metropolitan panel set included in Model 3 using second-order stationary data. Diagnostics include (A) a histogram of residuals and (B) a residual plot against fitted values. Histograms were produced in R using the hist(residual(x)) argument. Residual plots were created in R by subtracting residuals from the observed ADHD percent and plotting these fitted values (yhat) against model residuals.
(TIF)
Model diagnostics for the urban panel set included in Model 3 using second-order stationary data. Diagnostics include (A) a histogram of residuals and (B) a residual plot against fitted values. Histograms were produced in R using the hist(residual(x)) argument. Residual plots were created in R by subtracting residuals from the observed ADHD percent and plotting these fitted values (yhat) against model residuals.
(TIF)
(DOCX)
Data Availability Statement
Glyphosate data is available from the United States Geological Survey (USGS). All mental health discharge data are available at (HCUPnet). The links to access these public data are contained within the manuscript.